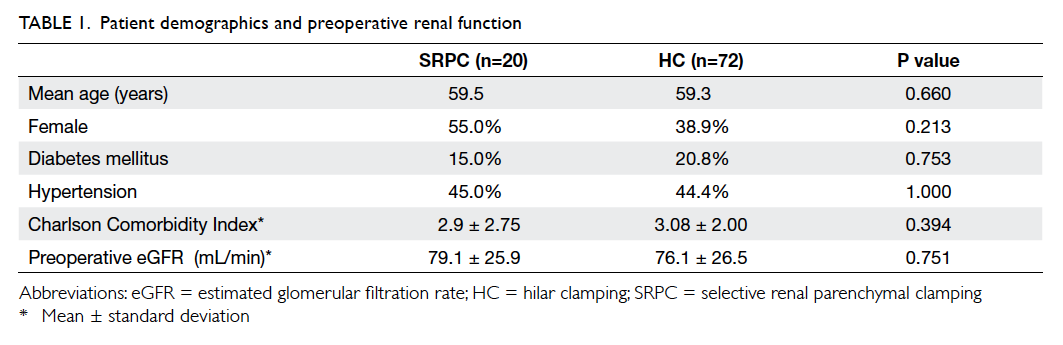
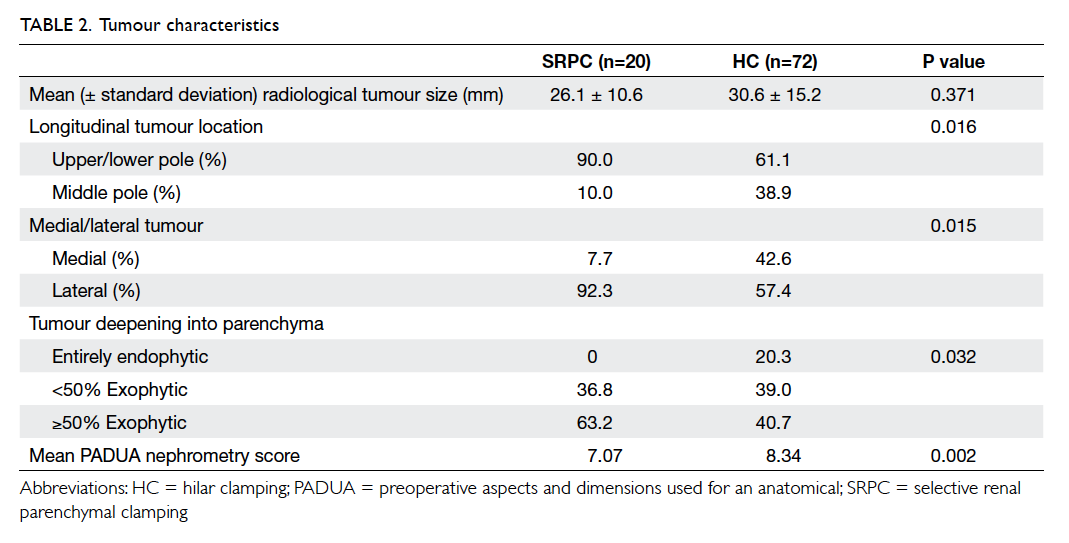
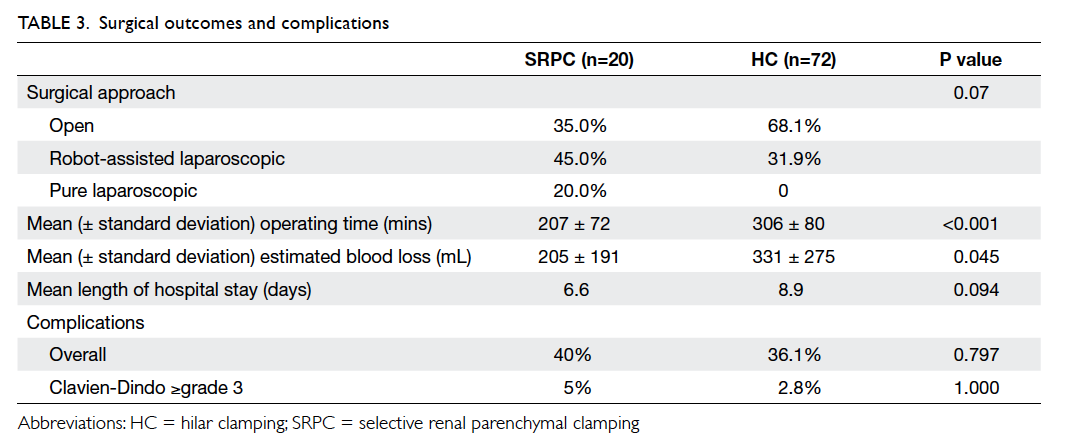
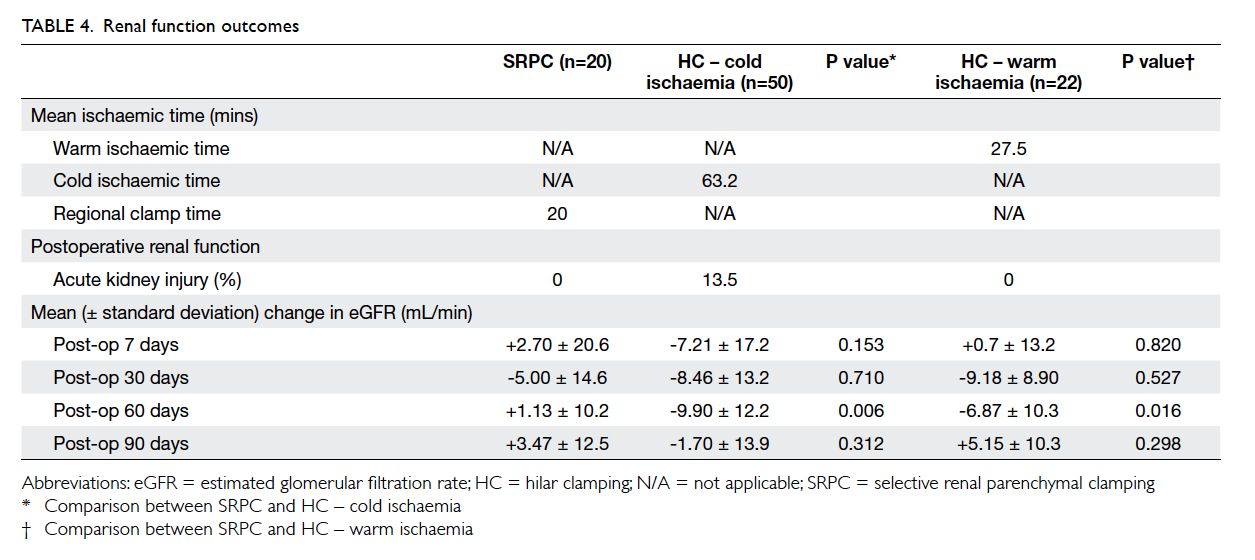
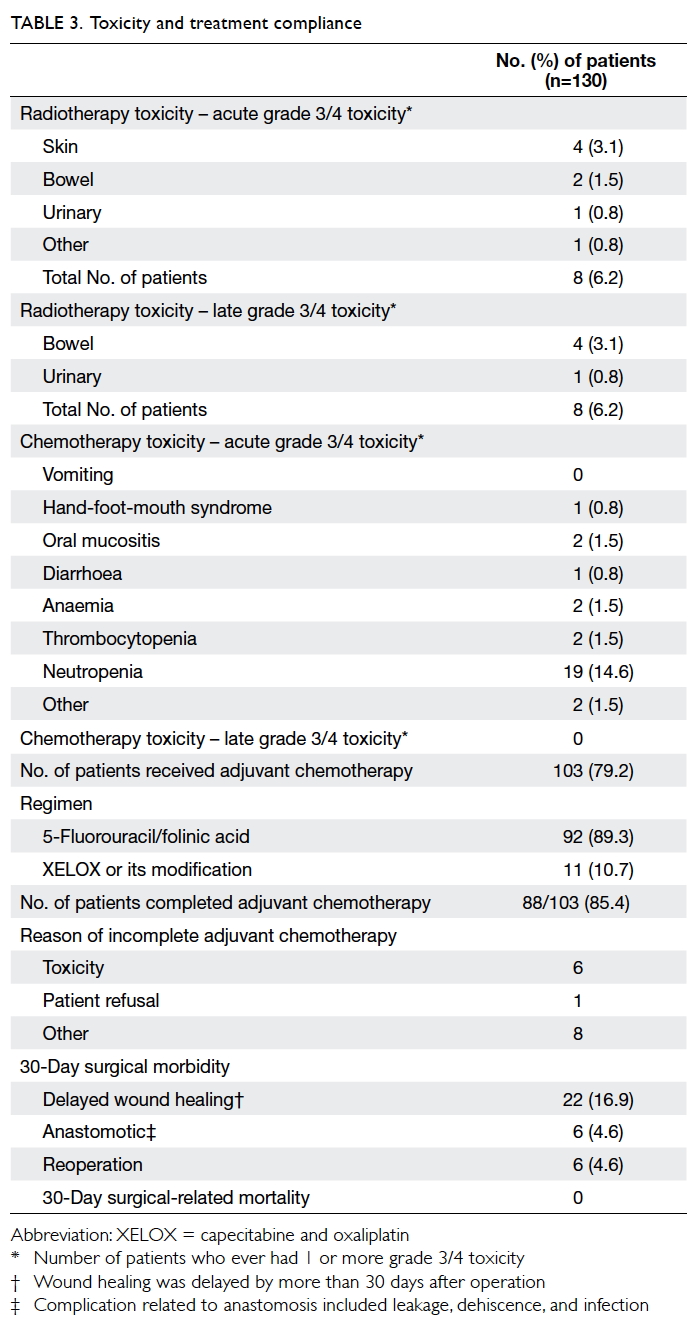
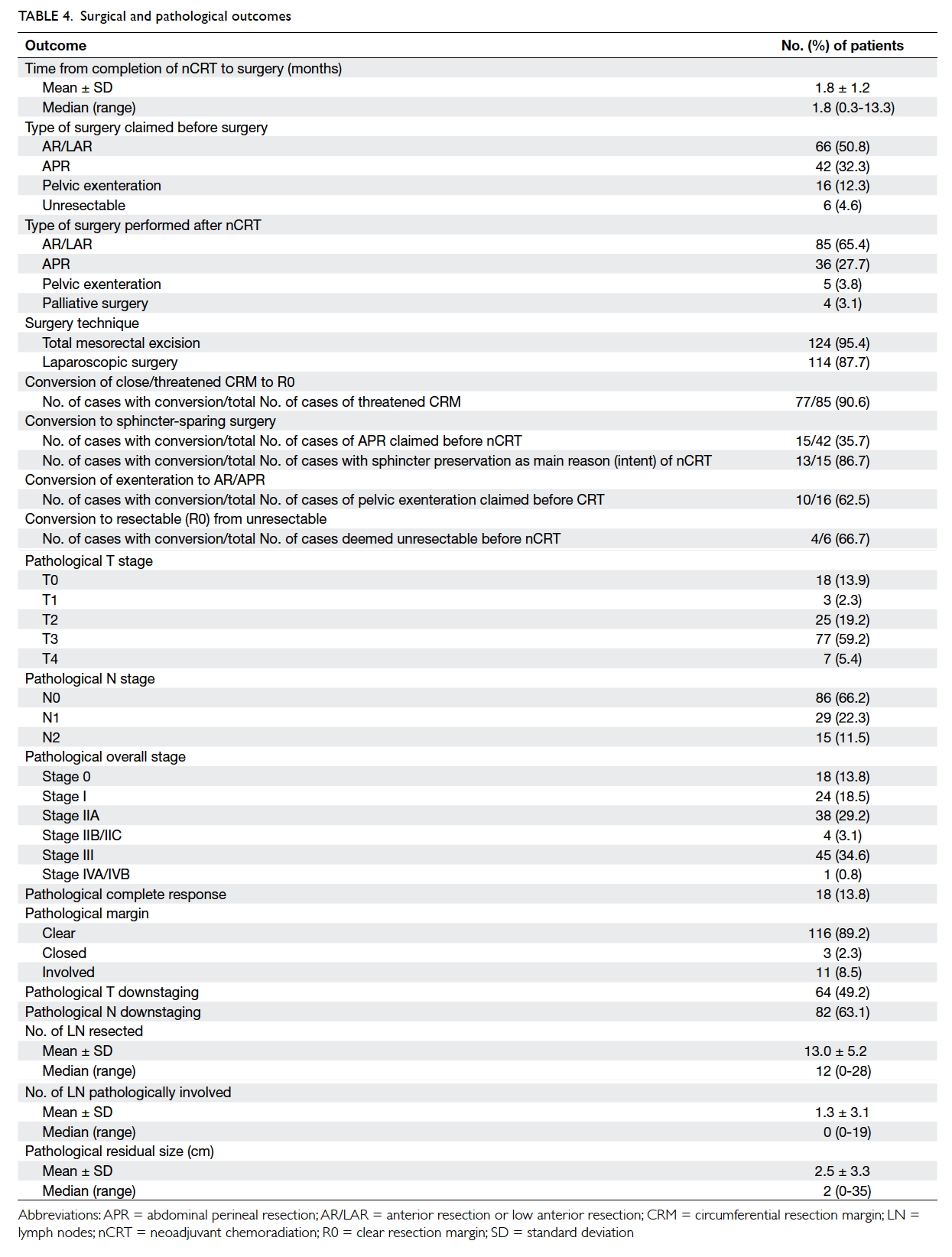
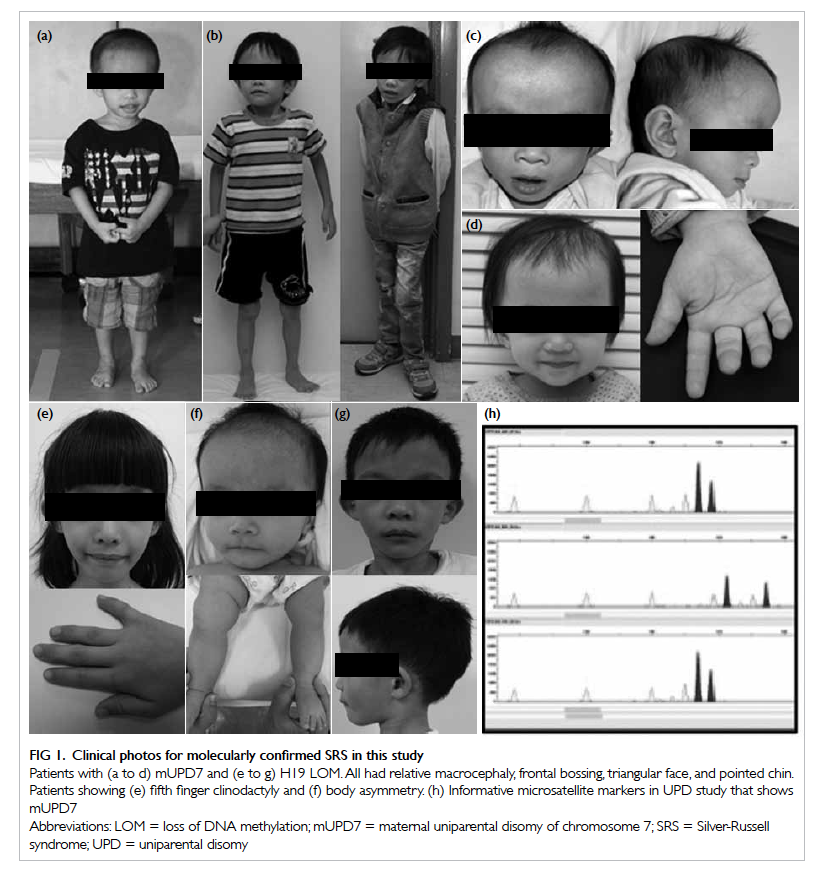
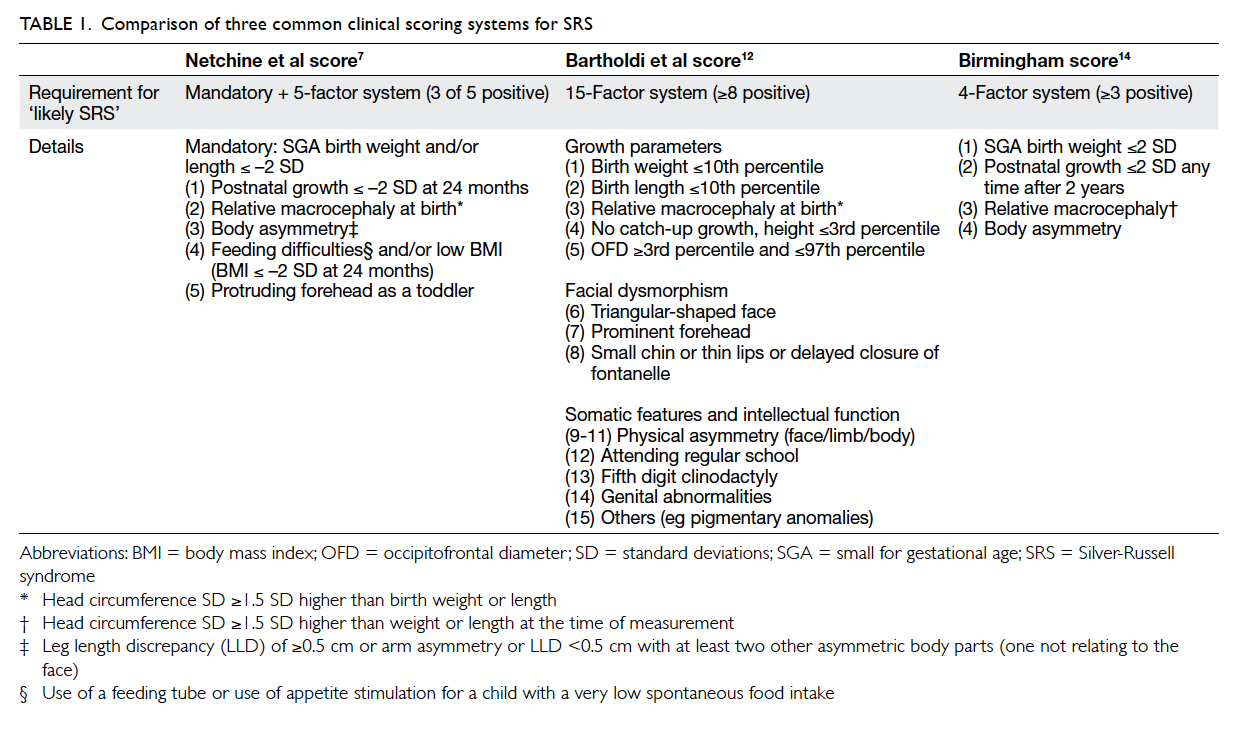
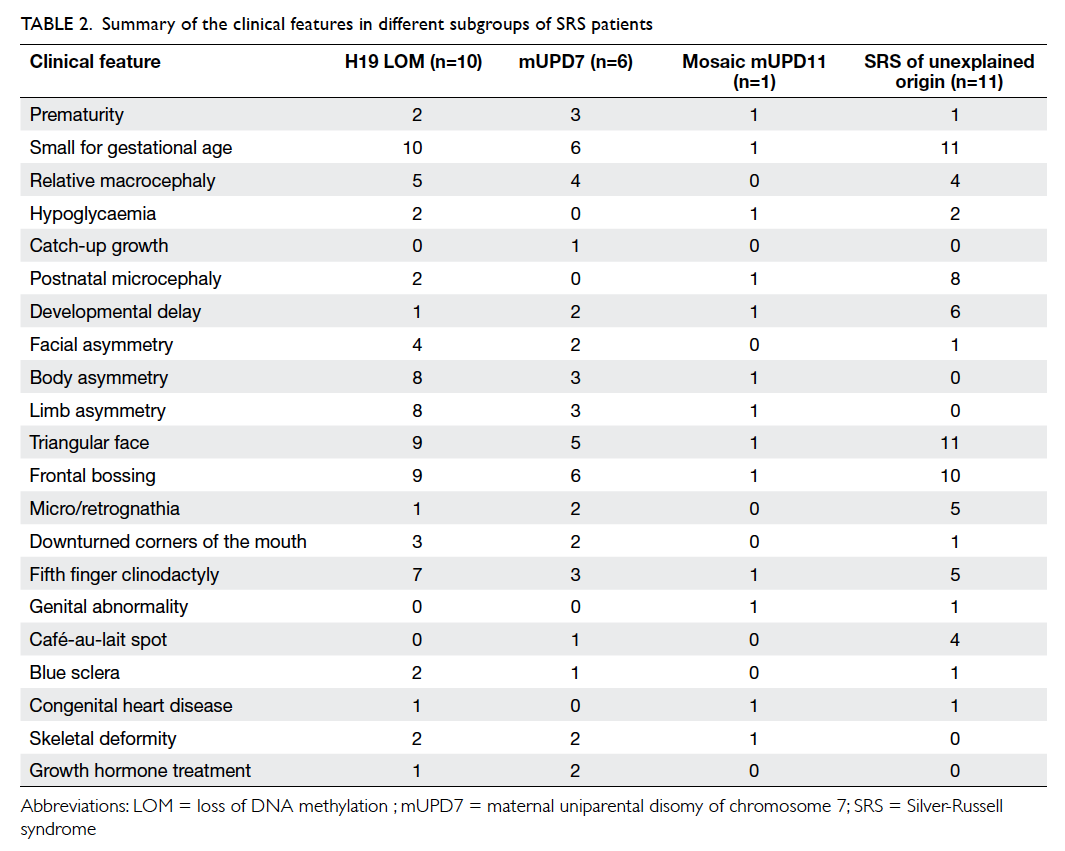
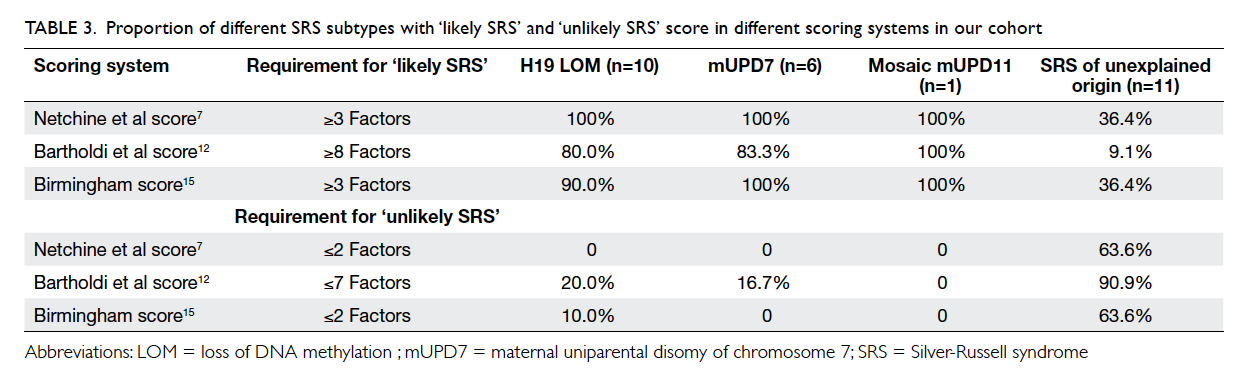
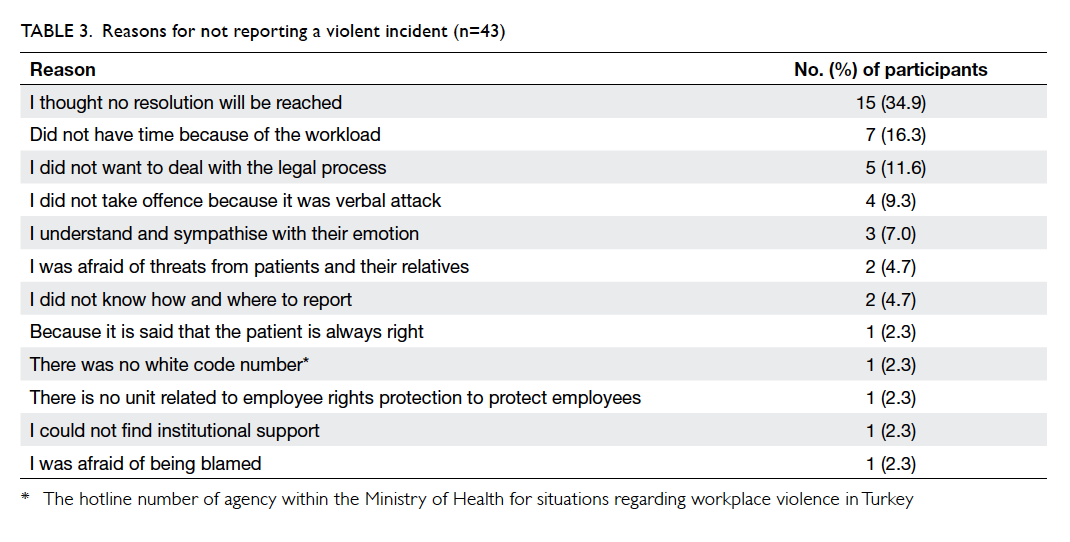
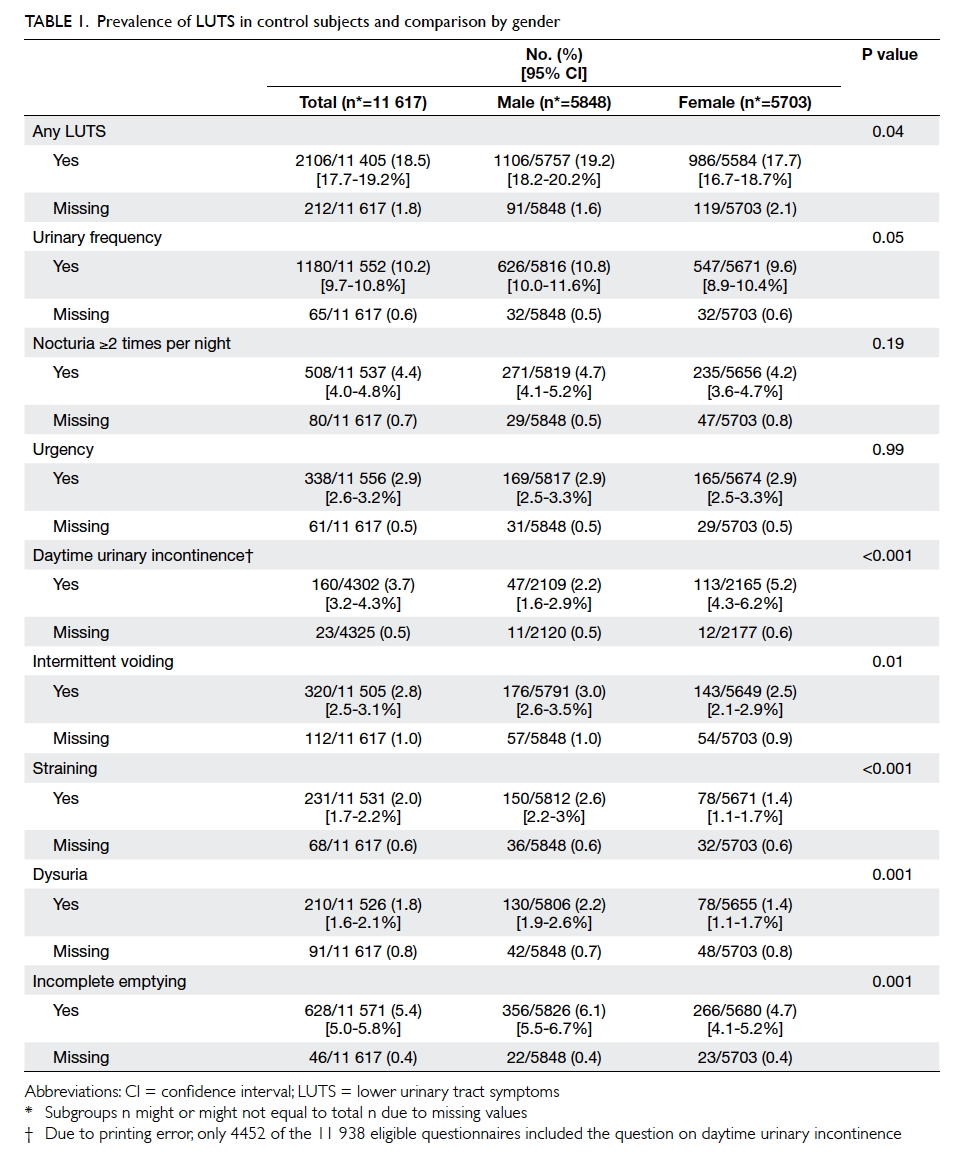
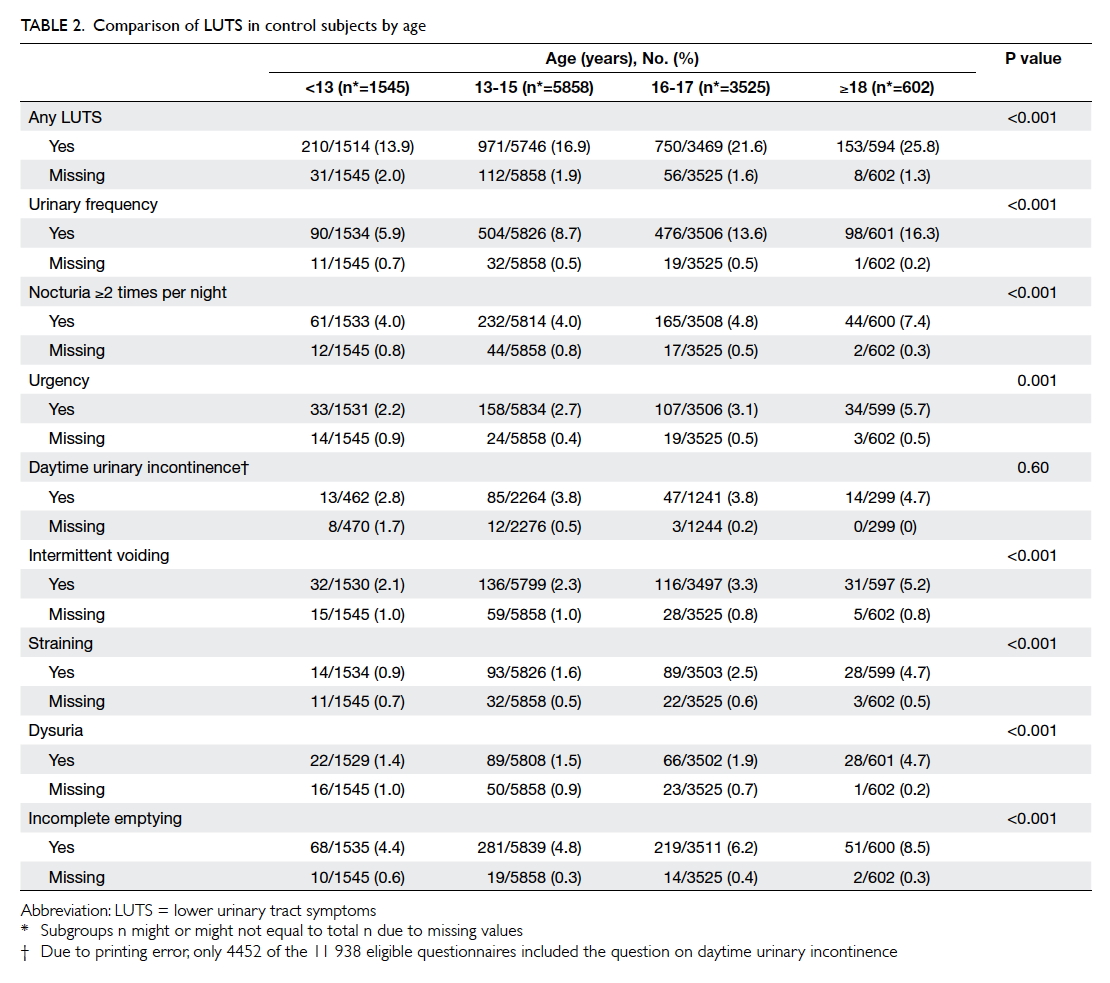
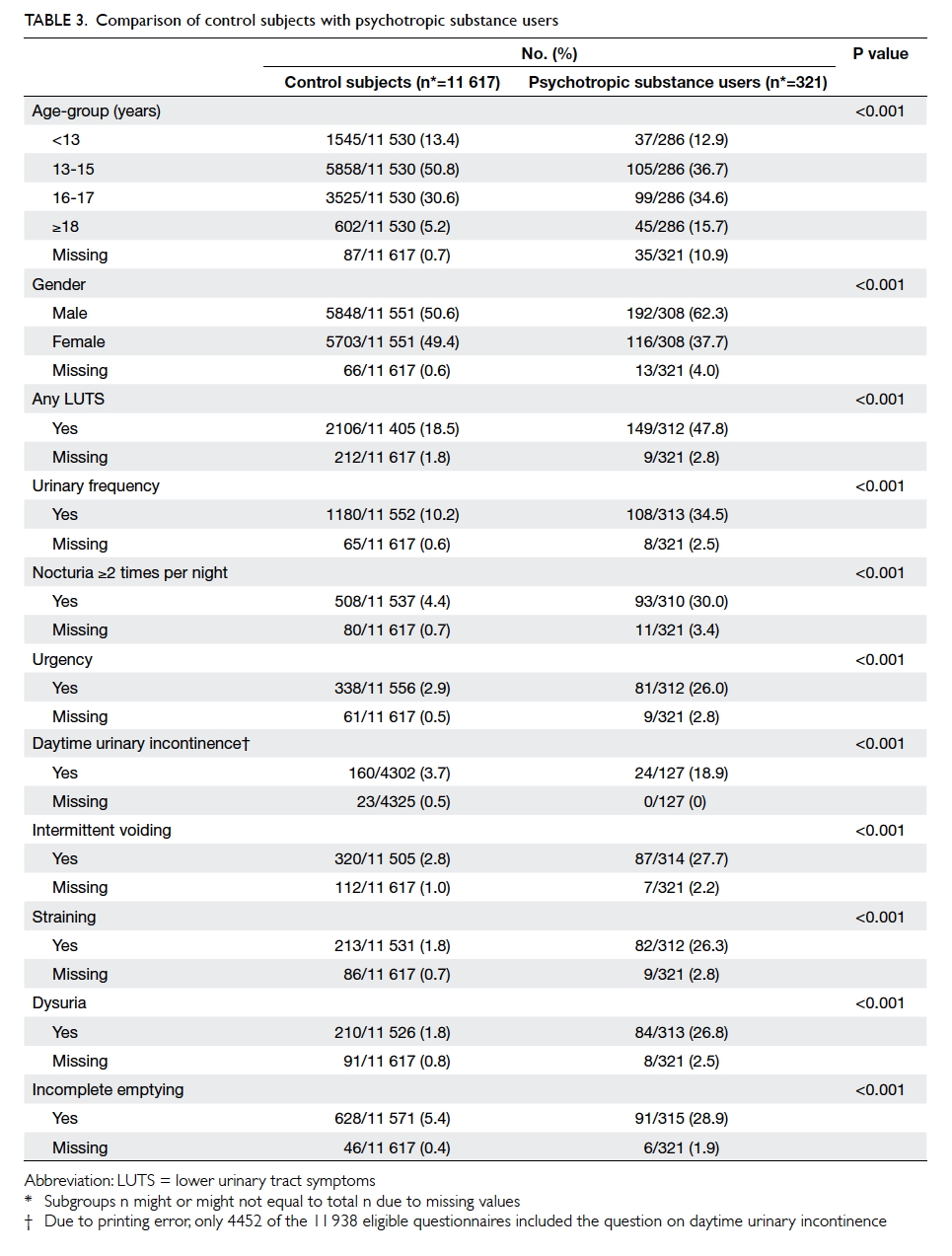
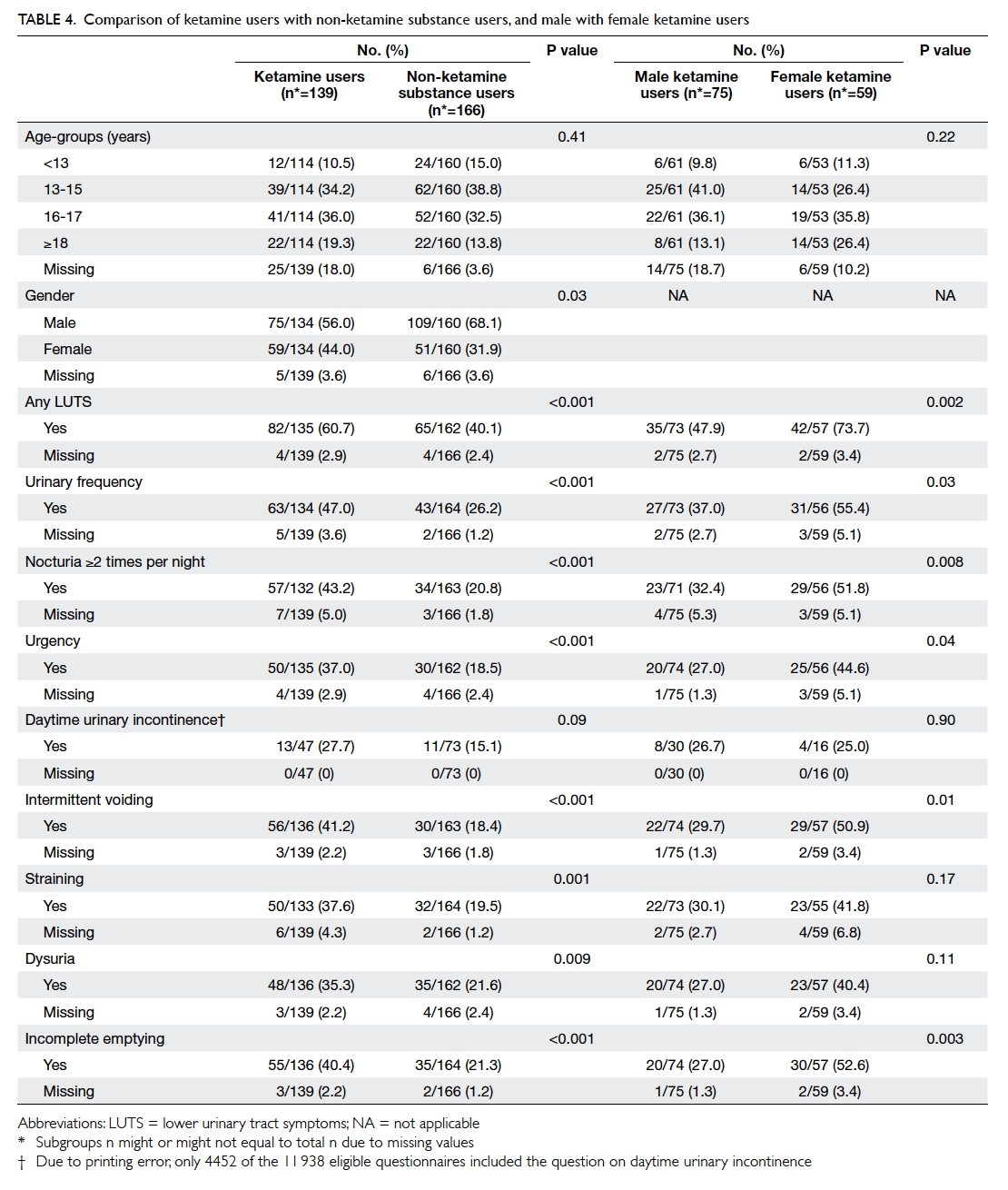
Factors affecting the deceased organ donation rate in the Chinese community: an audit of hospital medical records in Hong Kong
Hong Kong Med J 2016 Dec;22(6):570–5 | Epub 24 Oct 2016
DOI: 10.12809/hkmj164930
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Factors affecting the deceased organ donation rate in the Chinese community: an audit of
hospital medical records in Hong Kong
CY Cheung, PhD, FHKAM (Medicine)1;
ML Pong, BSc (Nursing)2;
SF Au Yeung, BSc (Nursing)2;
KF Chau, FRCP, FHKAM (Medicine)1
1 Department of Medicine, Queen Elizabeth Hospital, Jordan, Hong Kong
2 Transplant Coordinating Service, Kowloon Central Cluster, Hospital Authority, Hong Kong
2 Transplant Coordinating Service, Kowloon Central Cluster, Hospital Authority, Hong Kong
Corresponding author: Dr CY Cheung (simoncycheung@gmail.com)
Abstract
Introduction: The number of actual donors per
million population is the most commonly used
metric to measure organ donation rates worldwide.
It is deemed inadequate, however, because it does
not take into account the potential donor pool. The
aim of this study was to determine the true potential
for solid organ donation from deceased brain-dead
donors and the reasons for non-donation from
potential donors in the Chinese community.
Methods: Medical records of all hospital deaths
between 1 January and 31 December 2014 at a large
regional hospital in Hong Kong were reviewed.
Those who were on mechanical ventilation with
documented brain injury and aged ≤75 years
were classified as possible organ donors. The reasons
why some potential organ donors did not become
utilised organ donors were recorded and evaluated.
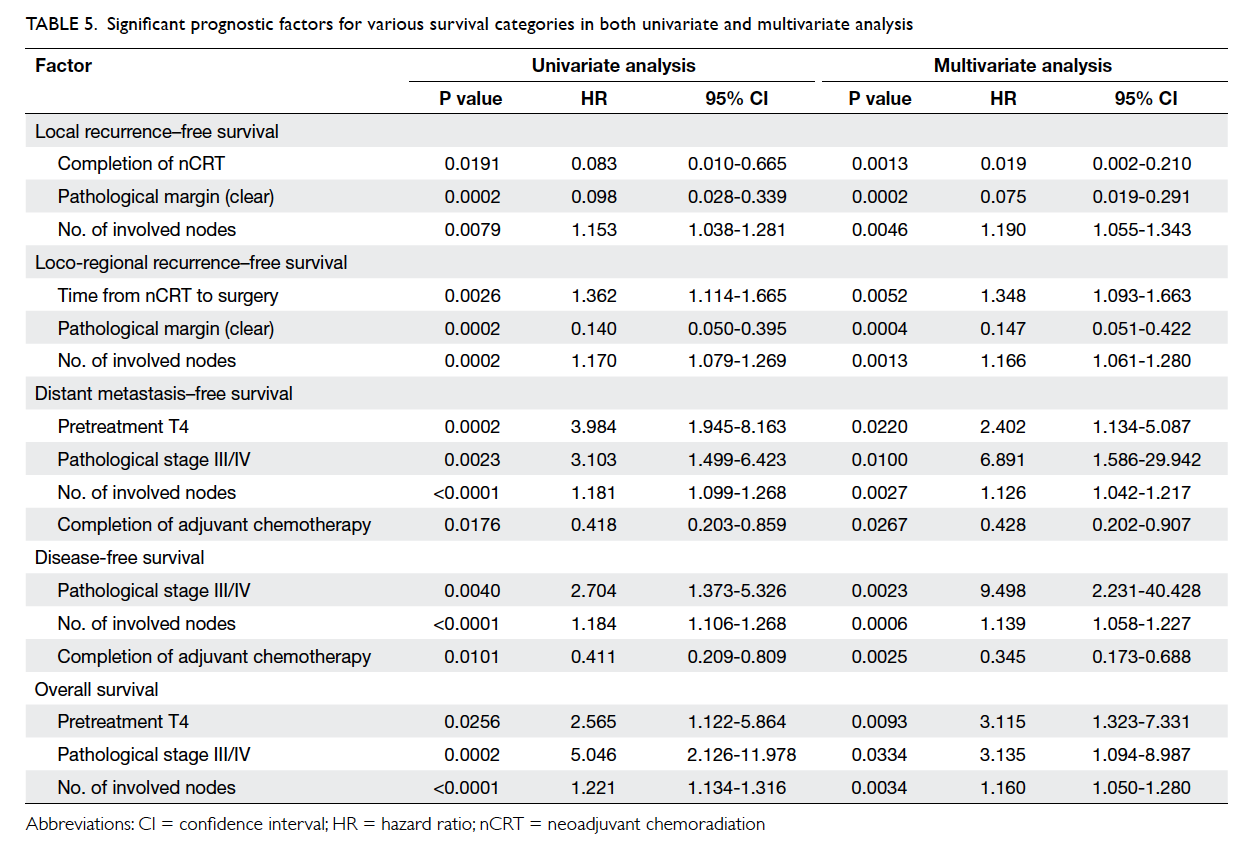
Results: Among 3659 patient deaths, 121 were
classified as possible organ donors. The mean age of the possible organ donors
was 59.4 years and 72.7% of them were male. The majority
(88%) were from non–intensive care units. Of the
121 possible organ donors, 108 were classified as
potential organ donors after excluding 13 unlikely to
fulfil brain death criteria. Finally 11 patients became
actual organ donors with an overall conversion rate
of 10%. Reasons for non-donation included medical
contra-indication (46%), failure to identify and
inform organ donation coordinators (14%), failure of
donor maintenance (11%), brain death diagnosis not
established (18%), and refusal by relatives (11%).
Conclusions: It is possible to increase the organ
donation rate considerably by action at different
stages of the donation process. Ongoing accurate
audit of current practice is necessary.
New knowledge added by this study
- There are different areas in the donation process where it may be possible to increase the organ donation rate considerably. Failure of health care professionals to identify potential donors is considered to be an important contributing factor to the shortage of cadaver organs in our community.
- All potential donors should be considered for referral to the intensive care unit for possible admission and physiological support through to brain death.
Introduction
Organ transplantation is considered to be the best
treatment for patients having end-stage organ failure.
There is a global shortage of organs, however. In the
United States, more than 100 000 potential recipients
are waiting for organs of whom only one fourth will
ultimately undergo organ transplantation.1 In a
systematic medical review, Jansen et al2 showed that
the maximum number of potential organ donors can
be approximately 3 times higher than the number of
effective organ donors. As a result, understanding
the pitfalls at each step of the process of organ
procurement, starting from donor identification to
retrieval of organs, is extremely important in the
evaluation of the size of the potential donor pool.
The number of actual donors per million
population (pmp) is the most commonly used metric
to measure organ donation rates and performances
in different countries. It has been deemed
inadequate, however, because it does not take into
account the potential donor pool that is dependent
on the rates and causes of death. Medical records
review appears to be the most accurate method
to estimate donor potential within a hospital or a
region.3 A true estimate should reflect contemporary
medical practice, donor identification, and consent
rates; thus it can provide a useful tool for measuring
organ procurement performance in a service area
and highlight areas in the procurement system that
can be improved.
Similar to other parts of the world, the shortage
of organs for transplantation remains a challenge
in Hong Kong. Organ transplants in Hong Kong,
whether cadaver or living donations, are subject
to regulation under the Human Organ Transplant
Ordinance; the main purpose of which is to ensure
that no commercial dealing is involved in organs for
transplant. Currently over 90% of organ donations in
Hong Kong are deceased donations, and the organ
procurement system is based on an opt-in policy
(voluntary decision of the patient or their family to
donate organs). No executed prisoners are involved
in the donation process. Although the deceased
organ donation rate increased from 4.3 donors pmp
in 2006 to 6.1 pmp in 2013, Hong Kong continues
to have one of the lowest donation rates among
developed countries.4 Over 2000 patients were
waiting for a solid-organ transplant in 2014 but only
112 deceased organs were utilised.5
In recent years, approximately 40% of deceased
organ donation referrals in our territory came from
intensive care units (ICUs) while the remainder
came from non-ICU areas such as medical and
neurosurgical wards. This is entirely different from
other parts of the world where more than 90% of
organ donation referrals come from the ICU.6 Most
of the current data on organ donation potential were
solely extracted from medical records in the ICU.2 7 8 9
The picture will be more complete, however, if we can
also identify and include those patients who die in
non-ICU wards but have the potential to become an
organ donor if appropriate steps are taken.10 Nearly
all studies on deceased organ donation have been
performed in western countries and data are scarce
for the Chinese population. The rates of donation
will differ from one country to another because of
differences in cultural, social, and historical factors;
the organisational characteristics of the donation
system; and various aspects of the health service.
We conducted a study to evaluate the deceased
organ donation process at our centre using the
critical pathway11 in order to identify to what extent
and why potential brain-dead donors are missed.
The main outcome measures included the potential
organ donor suitability and the various reasons for
non-donation as assessed by our organ donation
coordinator (ODC). Different ways that could help
to improve the organ donation process will also be
discussed.
Methods
This was a retrospective study conducted at Queen
Elizabeth Hospital, the largest regional acute
hospital in Hong Kong with 1833 beds, serving
approximately 7.1% of our 7.2 million population. It
is a tertiary referral centre of the major specialties
including neurology and neurosurgery. In addition,
it is one of the major organ procurement centres in
our territory and contributed approximately 30%
of all deceased organ donors in 2014. All deceased
donors in our centre are brain-dead donors as we do
not have a donation after cardiac death policy.
Hospital medical records of all those who died
at our centre (including both ICU and non-ICU
areas) between 1 January and 31 December 2014
were reviewed by the same ODC. In case of
ambiguous information, the opinion of another
ODC at our centre was sought. Both ODCs had
experience in managing patients with brain injuries
and were knowledgeable about brain death. Clinical
and demographic data including age, gender, cause
of brain injury, Glasgow Coma Scale (GCS) score,
medical co-morbidities, and likelihood of progression
to brain death were extracted from the medical
records. Only those who had been on mechanical
ventilation with documented brain injuries and
aged ≤75 years were included in our analysis. In our
hospital, patients could receive ventilator care (but
no invasive arterial pressure monitoring) in general
wards other than the 29-bed ICU because the
number of critically ill patients requiring intensive
care might exceed the number of beds available in
ICU. Patients could also be too ill and not fulfil the
ICU admission criteria. During ICU consultations,
patients were triaged by ICU specialists with
reference to a prioritisation model.12 Patients
remaining in the general ward would be cared for by
the treating teams.
The critical pathway for organ donation was
used as a tool to assess the organ donation process
after brain death.11 The various definitions of organ
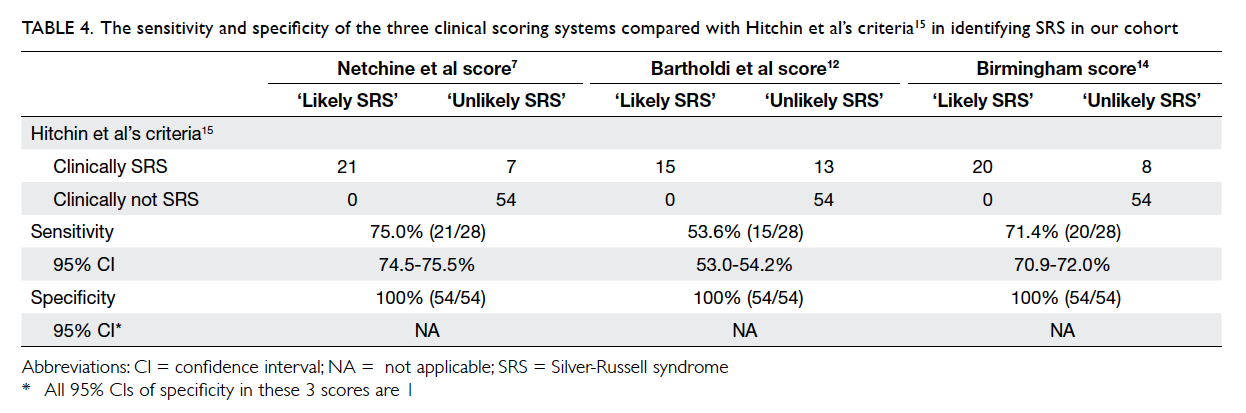
donors used in this study are shown in Figure 1. The ‘Guidelines on diagnosis of brain death’ were
first prepared in 1995 with assistance from the
Hong Kong Society of Critical Care Medicine. They
were revised later and supported by the Hospital
Authority (HA) of Hong Kong.13 Brain death is
established by the documentation of irreversible
coma and irreversible loss of brain stem reflex
responses and respiratory centre function or by the
demonstration of the cessation of intracranial blood
flow. The recommendations for the status of the two
medical practitioners certifying death are shown
in the guideline. The concept that brain death is
equivalent to death is accepted legally and within the
medical community in Hong Kong.
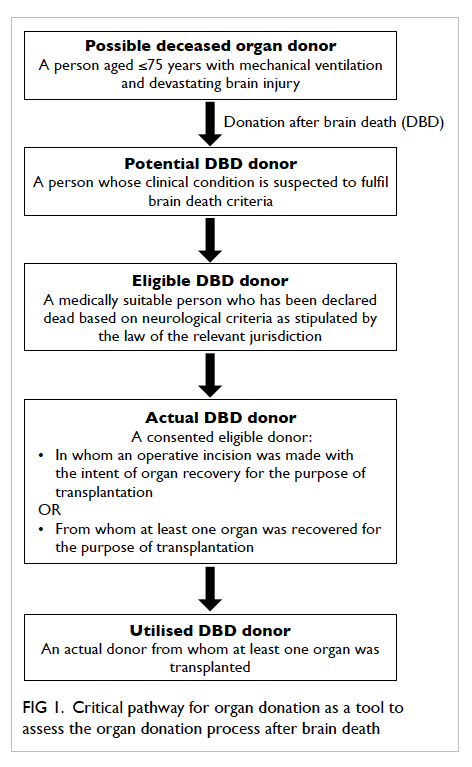
Figure 1. Critical pathway for organ donation as a tool to assess the organ donation process after brain death
Medical suitability for organ donation was
based on our ‘Guideline for evaluation and selection
of potential organ/tissue donors’ prepared by the
HA.14 The likelihood of progression to brain death
was based on the GCS score, presence or absence
of brainstem reflexes, rapidity of deterioration, and
findings of cerebral tomography. Potential brain-dead
organ donors were defined as patients with
the likelihood of progression to brain death. The
various reasons why some potential organ donors
did not become an organ donor were recorded and
evaluated. The study was approved by our hospital
ethics committee.
Statistical analyses
The Statistical Package for the Social Sciences
(Windows version 21.0; SPSS Inc, Chicago [IL],
US) was used to perform the statistical analyses.
Continuous data were expressed as means ± standard
deviations or medians (ranges), and categorical data
were expressed as percentages. Continuous data
were analysed by Mann-Whitney U tests to detect
the difference between groups while categorical data
were analysed by Chi squared test or Fisher’s exact
test. A P value of <0.05 was defined as statistically
significant.
Results
There were a total of 3659 patient deaths during the
study period. Among them, only 233 patients were
put on mechanical ventilation with documented
brain injury. On initial review, 112 patients were
excluded due to old age (>75 years). The remaining
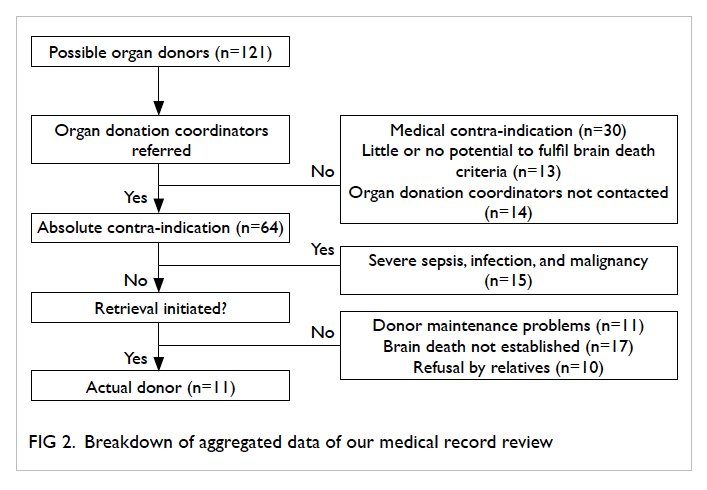
121 possible organ donors were further analysed (Fig 2).
Among the 121 possible organ donors, only 14
(12%) were from ICU. Most were from the non-ICU
areas including 64 from neurosurgical wards and 43
from general medical wards. The mean age of our
possible organ donors was 59.4 ± 13.0 years and 88
were male and 33 female.
Of the 121 possible organ donors, 64 (52.9%)
were identified and referred to ODC for consideration
of organ donation. Only eight (12.5%) patients were
from ICU and 56 (87.5%) were from non-ICU areas.
Among the 64 referred patients, 15 were medically
unsuitable and 11 had maintenance
problems related to haemodynamic instability. For
the remaining 38 patients, brain death diagnosis
could not be established or completed (did not fulfil
all the criteria) in 17. Hence only 21 patients (4 in
ICU and 17 in non-ICU) finally became eligible organ
donors after brain death. The relatives of all these
eligible donors were approached by our ODC, 11 of
them became actual organ donors (3 in ICU and 8 in
non-ICU) and 10 patients did not proceed to organ
donation because of refusal by patient relatives. The
overall consent rate at our centre was 52% and the
consent rate was higher in ICU than in the non-ICU
areas although the difference was not statistically
significant (75% vs 47%; P=0.31). All actual organ
donors finally became utilised organ donors. Of the
57 patients who had not been referred to ODC, 30
were medically unsuitable for organ donation after
careful review of hospital records, 13 had little or
no potential to progress to brain death, while the
remaining 14 could have become potential organ
donors if the ODC had been informed in a timely
manner.
Among the 108 patients who were classified as
potential brain-dead organ donors (after excluding
13 unlikely to fulfil brain death criteria), 13 patients
were from ICU and 95 were from non-ICU areas.
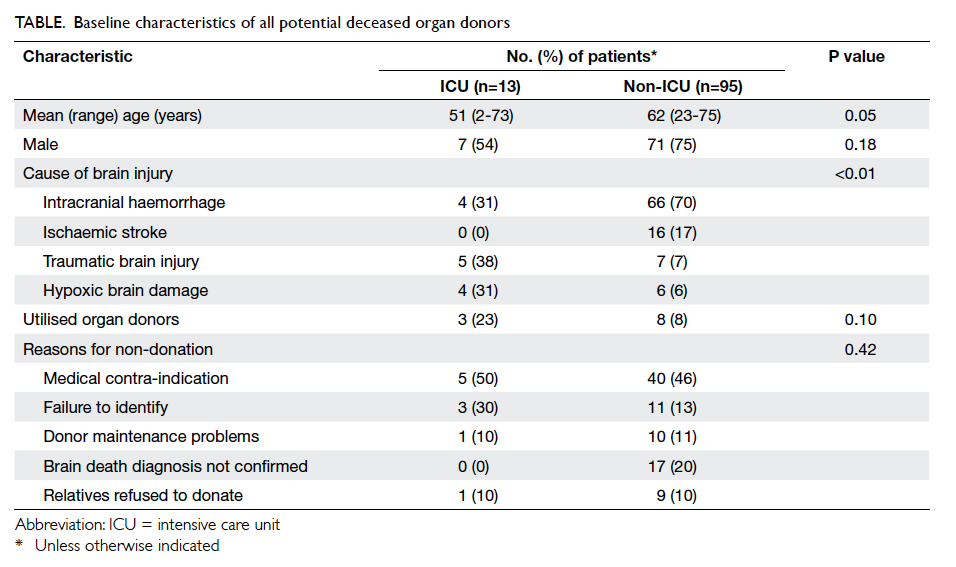
The baseline characteristics of these potential
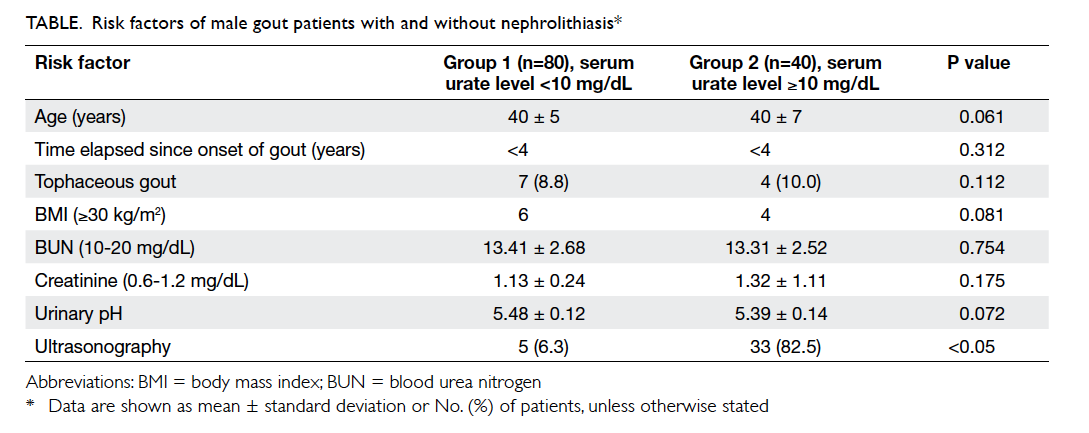
donors are shown in the Table. The potential donors in ICU were younger than those in non-ICU wards
(although only marginally significant) and there
was no significant difference in gender between
the patients from ICU and non-ICU areas. More
patients had traumatic brain injury and hypoxic
brain damage in ICU and in non-ICU wards
(neurosurgical or medical units) more patients had
intracranial haemorrhage or ischaemic stroke. Only
11 of the 108 patients finally became actual organ
donors with the overall conversion rate of 10%. The
conversion rate was higher in ICU than in non-ICU
although it was not statistically significant (23% vs
8%; P=0.10). The reasons for non-donation (n=97)
included medical contra-indication (n=45, 46%),
failure to identify and inform our ODC (n=14, 14%),
failure of donor maintenance (n=11, 11%), brain
death diagnosis not established or completed (n=17,
18%), and relative’s refusal for organ donation (n=10,
11%). There was no significant difference between
ICU and non-ICU areas concerning the reasons for
non-donation (P=0.42).
Discussion
To our knowledge, this is the first comprehensive
study to evaluate the pool of potential brain-dead
organ donors in a large regional hospital in the
Chinese community. Lack of consent to a donation
request was the primary cause of the gap between
the number of potential donors and the number
of actual donors in the United States and United
Kingdom.7 8 In order to tackle this problem, more
resources should be invested to improve the process
of obtaining consent.8 Good donor management
including identification, evaluation, and donor
maintenance are also key factors in the successful
recovery of organs in the donation process.
Failure of health care professionals to identify
potential donors is considered an important
contributing factor to the shortage of deceased
organs,11 15 and accounted for 14% of our potential
organ donor loss. Education directed at doctors and
nurses to increase their awareness of possible organ
donors is crucial to the success of an organ donation
programme. Identification of a possible deceased
organ donor should be inherently linked to the act of
referral to a key donation person/team for activation
of the deceased donation process.11 Similar to other
hospitals in Hong Kong, all possible brain-dead
donors at our centre, regardless of apparent medical
contra-indications, are referred to our ODC as
soon as they are identified. Referral usually occurs
early when the clinical condition reveals death to be
imminent or that further treatment will be futile. The
possible deceased organ donors can then be assessed
and managed by the ODC immediately as all the
ODCs in our territory have a centralised shared 24-hour
on-call system. The decision for medical suitability is
made by our ODC and transplant physicians instead
of referring teams because studies have shown that
11% of the decisions not to refer a potential donor
based on medical grounds are incorrect.16 In our
centre, only 52.9% of the possible
deceased organ donors were identified and referred
to our ODC. This figure was lower than some
European countries (approximately 80% on average)
such as 93.6% in France to 47.7% in Finland.9 One
important unique feature in our study was that most
of the potential deceased organ donors at our centre
(also in Hong Kong) were identified in the non-ICU
wards. This was in contrast to other countries where
nearly all potential donors come from ICU.2 9 As a
result, increased awareness of frontline staff and
compulsory referral of all possible deceased organ
donors may further increase the donation rate.
In our study, one third (27/87) of the non-utilised
potential organ donors in the non-ICU areas were
lost either because of a haemodynamically unstable
condition leading to cardiac death or the failure to
confirm or complete the brain death diagnosis. Brain
death is often associated with marked physiological
instability that makes it difficult to be managed in
general wards by non-ICU specialists. In addition,
due to the limited manpower and high hospital
bed occupancy rates, most potential organ donors
in general wards would no longer be supported for
organ donation if brain death could not be confirmed
within 72 hours. These patients might turn out to be
eligible organ donors if they can continue to receive
critical care management in ICU. Our study also showed that the consent rate was higher
for potential donors from ICU than from non-ICU
areas although it was not statistically significant.
Some ICU specialists believe that all potential donors
should be referred to ICU for possible admission
and physiological support through to brain death.10
Additional resources, including ICU specialists
and ICU beds, will be required however. As an
alternative, a mobile team including ICU doctors
and nurses could be set up, aiming to give advice for
potential organ donor support and optimisation of
settings towards brainstem tests in non-ICU wards.
As in many other countries, a high family
refusal rate is a significant reason for potential
donor losses in Hong Kong. In our study, the overall
refusal rate was 48%. This was higher than the family
refusal rates in Spain (24.3%), the United Kingdom
(41%), France and Belgium (10.5% in both).7 9 There
are numerous ethnic, cultural, social, and religious
factors that contribute to disparities in deceased
donation in different Asian countries. Since the
Chinese community is deeply embedded with the
traditional belief of preserving body integrity after
death, most Chinese communities such as Hong
Kong have adopted an opt-in system.16 The presumed
consent or opt-out system is a controversial topic in
our territory. It is uncertain whether the public would
support presumed consent17 because it violates our
conventional ethical and legal principle of familial
authority over a deceased body. A survey in Hong
Kong showed clear objection (66%) to presumed
consent for organ donation and only 28% agreed.18
Any health policies and educational campaigns to
increase donation rates must contend with different
cultural contexts and conceptions of autonomy to
be effective. In November 2008, the Hong Kong
SAR Government established the Centralised Organ
Donation Register to make it more convenient
for people to register their wish to donate organs
after death. Media coverage of patients’ appeals
for organs and transplant success stories can draw
public support and boost public confidence in organ
transplantation.19
One of the drawbacks of our study was the
small number of patients that might not provide
sufficient power to detect the differences between
potential donors from ICU and non-ICU areas. It
is also difficult to compare our results exactly with
other studies because of the variation in definition
of a potential donor and the difficulty in predicting
the likelihood of progression to brain death.
Furthermore, as we only analysed those patients
who were mechanically ventilated, some potential
organ donors might still be missed as it is not our
current practice to perform tracheal intubation and
mechanical ventilation solely for the purposes of
facilitating organ donation.
Conclusions
We have identified different areas in the donation
process where it may be possible to increase the organ
donation rate considerably. Among them, increasing
awareness of frontline staff in identification of
potential donors in both ICU and non-ICU areas,
and appropriate physiological support of potential
donors to accomplish the diagnosis of brain death
in general wards are key elements in our hospital.
Ongoing accurate audit of practice is a prerequisite
to improve the organ donation process.
Declaration
All authors have disclosed no conflicts of interest.
References
1. OPTN (Organ Procurement and Transplantation
Network). U. S. Department of Health and Human Services.
Available from: http://optn.transplant.hrsa.gov. Accessed 1
Jun 2015.
2. Jansen NE, van Leiden HA, Haase-Kromwijk BJ, Hoitsma
AJ. Organ donation performance in the Netherlands 2005-08; medical record review in 64 hospitals. Nephrol Dial
Transplant 2010;25:1992-7. Crossref
3. Christiansen CL, Gortmaker SL, Williams JM, et al. A
method for estimating solid organ donor potential by organ
procurement region. Am J Public Health 1998;88:1645-50. Crossref
4. IRODaT (International Registry in Organ Donation and
Transplantation). Transplant Procurement Management.
Available from: http://www.irodat.org. Accessed 1 Jun
2015.
5. Department of Health, The Hong Kong SAR Government.
Organ donation. Available from: http://www.organdonation.gov.hk/eng/statistics.html. Accessed 1 Jun
2015.
6. Mascia L, Mastromauro I, Viberti S, Vincenzi M, Zanello
M. Management to optimize organ procurement in brain
dead donors. Minerva Anestesiol 2009;75:125-33.
7. Barber K, Falvey S, Hamilton C, Collett D, Rudge C.
Potential for organ donation in the United Kingdom: audit
of intensive care records. BMJ 2006;332:1124-7. Crossref
8. Sheehy E, Conrad SL, Brigham LE, et al. Estimating the
number of potential organ donors in the United States. N
Engl J Med 2003;349:667-74. Crossref
9. Roels L, Spaight C, Smits J, Cohen B. Donation patterns
in four European countries: data from the donor action
database. Transplantation 2008;86:1738-43. Crossref
10. Opdam HI, Silvester W. Identifying the potential organ
donor: an audit of hospital deaths. Intensive Care Med
2004;30:1390-7. Crossref
11. Domínguez-Gil B, Delmonico FL, Shaheen FA, et al.
The critical pathway for deceased donation: reportable
uniformity in the approach to deceased donation. Transpl
Int 2011;24:373-8. Crossref
12. Guidelines for intensive care unit admission, discharge,
and triage. Task Force of the American College of Critical
Care Medicine, Society of Critical Care Medicine. Crit
Care Med 1999;27:633-8. Crossref
13. Hospital Authority Head Office Operations Circular No. 13 / 2010: Guidelines on diagnosis of brain death. Hong Kong: Hospital Authority; 2010.
14. Guideline for evaluation and selection of potential organ/tissue donors (revised version). Hong Kong: Hospital Authority; 2016.
15. Boey KW. A cross-validation study of nurses’ attitudes and
commitment to organ donation in Hong Kong. Int J Nur
Stud 2002;39:95-104. Crossref
16. Wu AM. Discussion of posthumous organ donation in
Chinese families. Psychol Health Med 2008;13:48-54. Crossref
17. Wu AM, Tang CS. The negative impact of death anxiety
on self-efficacy and willingness to donate organs among
Chinese adults. Death Stud 2009;33:51-72. Crossref
18. Cheng B, Ho CP, Ho S, Wong A. An overview on attitudes
towards organ donation in Hong Kong. Hong Kong J
Nephrol 2005;2:77-81. Crossref
19. Lo CM. Deceased donation in Asia: challenges and
opportunities. Liver Transpl 2012;18 Suppl 2:S5-7. Crossref