Hong Kong Med J 2016 Aug;22(4):347–55 | Epub 6 Jul 2016
DOI: 10.12809/hkmj164865
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Prevalence of infections among residents of Residential Care Homes for the Elderly in Hong Kong
Carmen SM Choy, MB, BS1;
H Chen, MB, BS, FHKAM (Community Medicine)2;
Carol SW Yau, MB, BS, FHKAM (Community Medicine)3;
Enoch K Hsu, BSc, MSc2;
NY Chik, BNurs2;
Andrew TY Wong, MB, BS, FHKAM (Medicine)2
1 Accident and Emergency Department, Queen Elizabeth Hospital, Jordan,
Hong Kong
2 Infection Control Branch, Centre for Health Protection, Hong Kong
3 Surveillance and Epidemiology Branch, Centre for Health Protection,
Hong Kong
Corresponding author: Dr Carmen SM Choy (carmencly@yahoo.com.hk)
Abstract
Introduction: A point prevalence study was
conducted to study the epidemiology of common
infections among residents in Residential Care
Homes for the Elderly in Hong Kong and their
associated factors.
Methods: Residential Care Homes for the Elderly in
Hong Kong were selected by stratified single-stage
cluster random sampling. All residents aged 65 years
or above from the recruited homes were surveyed.
Infections were identified using standardised
definitions. Demographic and health information—including medical history, immunisation record,
antibiotic use, and activities of daily living (as
measured by Barthel Index)—was collected by a
survey team to determine any associated factors.
Results: Data were collected from 3857 residents
in 46 Residential Care Homes for the Elderly from
February to May 2014. A total of 105 residents had
at least one type of infection based on the survey
definition. The overall prevalence of all infections was
2.7% (95% confidence interval, 2.2%-3.4%). The three
most common infections were of the respiratory
tract (1.3%; 95% confidence interval, 0.9%-1.9%),
skin and soft tissue (0.7%; 95% confidence interval,
0.5%-1.0%), and urinary tract (0.5%; 95% confidence
interval, 0.3%-0.9%). Total dependence in activities
of daily living, as indicated by low Barthel Index score
of 0 to 20 (odds ratio=3.0; 95% confidence interval,
1.4-6.2), and presence of a wound or stoma (odds
ratio=2.7; 95% confidence interval, 1.4-4.9) were
significantly associated with presence of infection.
Conclusions: This survey provides information
about infections among residents in Residential Care
Homes for the Elderly in the territory. Local data
enable us to understand the burden of infections and
formulate targeted measures for prevention.
New knowledge added by this study
- Characteristics of local Residential Care Homes for the Elderly (RCHE) residents were explored. Most individuals had medical co-morbidities and required assistance with activities of daily living (ADL); use of an indwelling medical device was also common.
- Local prevalence of infections among residents in RCHE was 2.7% and the most common infection was of the respiratory tract.
- Total dependence in ADL and presence of a wound or stoma were associated with presence of infections among residents in RCHE in Hong Kong.
- Measures that focus on prevention of respiratory tract infection among the elderly should be emphasised and an infection control programme should be designed to enhance such practice in RCHE.
- Infection control protocols can be developed according to specific areas of nursing care, for example, wound care or catheter care.
Introduction
Ageing is a worldwide phenomenon, and Hong
Kong, without exception, is encountering the same
population change. In 2014, the proportion of our
elderly population aged 65 years or above was 15%.
This proportion is expected to double over the next
20 years, to 28% in 2034.1
In Hong Kong, Residential Care Homes for
the Elderly (RCHEs) provide different levels of care
for the elderly who—for personal, social, health, or
other reasons—can no longer live alone or with their
family. These RCHEs can be broadly categorised as
private homes (PH) and non-private homes (NPH);
the former are run by private entrepreneurs and
vary in size and capacity, while the latter are run by
non-governmental organisations and include care
and attention homes for the elderly and subvented,
and self-financing and contract homes that provide
subsidised care for the elderly. In 2015, there were
around 740 RCHEs providing over 73 000 residential
placements over the territories and residential care
for approximately 7% of the elderly in Hong Kong.1 2
These numbers are expected to increase further.
Generally speaking, RCHEs are very
heterogeneous in terms of size, facilities, manpower,
and level of care. Some residents are encouraged
to participate in various types of group activities
while some residents may require assistance in daily
activities. These activities, together with the confined
and shared living environment, may promote the
transmission of infectious diseases.
Infections are an important cause of morbidity
and mortality among the elderly, and place a
significant burden on our health care system.
Residents of RCHEs are usually frail; compared
with their community-dwelling counterparts,
they are more susceptible to infections and related
complications. Overseas studies conducted in a
hospital setting have shown that the mortality rate
of community-acquired pneumonia is 30%, while
that in nursing homes is substantially higher, with a
reported rate of up to 57%.3 Infections may be a cause
as well as the consequence of functional impairment
among RCHE residents, leading to a reduction in
their quality of life.4
Local studies of infectious diseases in the RCHE
setting are scarce. The last such survey was conducted
in 2006.5 Continuous or regular surveillance serves
to reveal the disease burden to increase awareness of
infections and to identify critical areas for infection
control. It is important that we understand the
local epidemiology and burden of infections among
RCHE residents and apply measures to control these
infections and safeguard the health of this vulnerable
group.
Methods
We conducted a point prevalence survey of common
infections among residents of RCHEs in Hong Kong.
Population and setting
All RCHEs in Hong Kong were included. All residents
aged 65 years or above who were present at 9 am (the
reference time) on the survey day were included.
Residents were excluded from the survey if: (1) he/she was not present at the reference time (owing
to medical appointment, admission to hospital, or
home leave from the RCHE); or (2) he/she attended
the RCHE as a day patient/resident.
Sampling strategy
A list of all RCHEs in Hong Kong was retrieved from
the Social Welfare Department website.6 All RCHEs
on the list were stratified according to the main
geographical region of Hong Kong (Kowloon, Hong
Kong Island, and New Territories) and type of RCHE
(PH and NPH) into six strata. Stratified single-stage
cluster random sampling was performed using the
captioned list as the sampling frame. All residents
were surveyed in each recruited home.
Data collection
The survey was conducted from February to May
2014. A survey team comprising doctors, nurses,
and research staff visited the RCHEs for data
collection on any one day during the survey period.
A standardised survey form was developed based on
a previous similar prevalence survey.5 This survey
form comprised four parts: (1) socio-demographic
information about the resident; (2) health information
including medical history, vaccination history, and
antibiotic use; (3) measurement of activities of daily
living (ADL) using the Barthel Index (BI)7; and (4) a
checklist of acute symptoms of common infections.
Symptoms of acute infections were obtained by
doctors by interviewing the residents or their major
carers with the help of RCHE staff. All health-related
information was verified by doctors or nurses;
functional status of residents was assessed by trained
nurses using the BI.
A pilot study was conducted in two RCHEs in
February 2014 to field test the data collection tools.
Inter-rater reliability on BI was assessed during the
period for the six trained nurses. Cohen’s kappa of
BI estimated ranged from 0.8 to 1, suggesting good
inter-rater reliability among them.
The survey was conducted in an anonymous
manner. Written consent was obtained from the
RCHEs. Verbal consent was obtained from residents
and/or their relatives. If any residents (or their
relatives) refused to participate in the survey, their
information was not retrieved. If relevant data
were not available on the survey day, data would be
retrieved within 1 week. The study was approved by
the Ethics Committee of the Department of Health.
Outcome measures
Infection was defined using any one of the following
criteria: (1) presence of symptoms and/or signs of
infection that developed in the 24 hours preceding
the survey day, that fulfilled the surveillance criteria
of the Canadian Consensus Conference8; (2)
infection diagnosed by a locally registered physician
(eg visiting medical officers, general practitioners);
or (3) consumption of antimicrobial agents on the
survey day for a specific infection.
Sample size and power estimation
Sample size was estimated to determine the
prevalence of infections among residents in RCHEs
in Hong Kong. Based on a previous prevalence
survey in Hong Kong,5 the prevalence of infections
was 5.7%, the design effect (DEFF) was 1.765 with an
intraclass correlation coefficient (ICC) of 0.025 and
average cluster size of 31.61. Assuming the margin of
error to be 0.2, given a conservative DEFF of 2, the
sample size calculated was 3178.
Statistical analyses
R (version 3.0.2) was used for statistical analysis.
“Survey” package (version 3.29-5) in R was used
to calculate the prevalence of infections adjusted
for cluster sampling. Prevalence rates of infections
and other study variables were calculated using
“svyciprop” function from the “Survey” package.
Logistic regression with adjustment on cluster
sampling was performed using “svyglm” function
from the “Survey” package to identify risk factors for
infection. Variables were included for multivariate
analysis if: (1) the P value was <0.25 in univariate
analysis or (2) the variables had been considered
as risk factors of infection in previous studies,
such as mobility status, use of medical devices,
presence of wound, home size, gender, and recipient
of the Comprehensive Social Security Assistance
(as a surrogate measurement of social economic
status).9 10 11 12 In addition, subgroup analyses were
performed to explore the association of specific risk
factors with different types of infection, such as the
presence of chronic obstructive pulmonary disease
(COPD) with respiratory tract infection (RTI) and
diabetes mellitus (DM) with skin and soft tissue
infection (SSTI). In order to adjust for multiple
comparisons, P values calculated for exploration
of association between risk factors and different
types of infection were adjusted using Bonferroni
correction. A P value of <0.05 was considered to be
statistically significant.
Results
A total of 100 RCHEs were invited. The overall
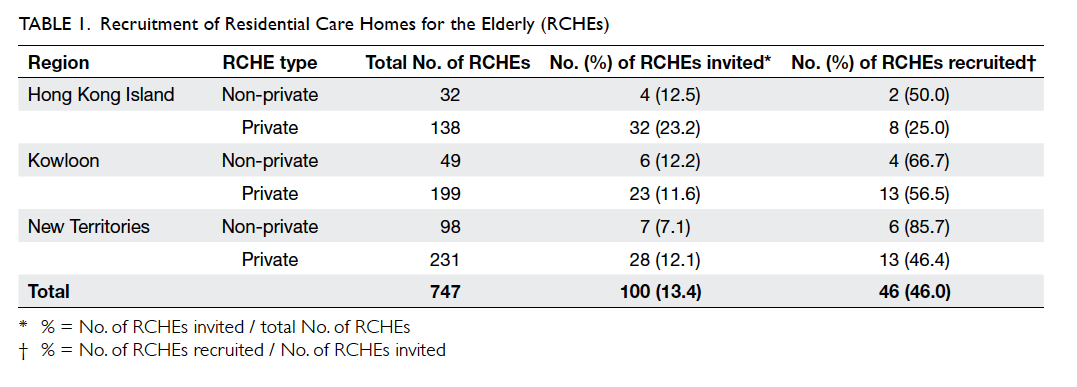
response rate was 46% (n=46). Table 1 illustrates the number of recruited RCHEs stratified by region
and home type. A higher response rate was noted in
NPHs (70.6%, n=12) than PHs (42.2%, n=35). The
ICC and DEFF calculated from the sample collected
were 0.0035 and 1.45, respectively. The mean
home capacity of the participating RCHEs was 111
residents per home (95% confidence interval [CI],
88-133 residents per home) with a median occupancy of 89%. Among the
staff in participating RCHEs, 45.9% were personal
care workers, 14.7% were health care workers, and
11% were nurses (including registered nurses and
enrolled nurses). There was no significant difference
in terms of home capacity, occupancy, or staffing
level between participating and non-participating
RCHEs based on data from the annual assessment
of all RCHEs conducted by Elderly Health Service,
Department of Health.
Demographics and underlying co-morbidity of residents
Among the 4127 residents in the participating
RCHEs, 261 (6.3%) were excluded from the survey as
they were not available due to hospitalisation, medical
appointment, home leave, or other personal reasons.
All 3866 residents who were at the participating
RCHEs at 9 am on the survey day were invited and
joined the survey. Nine (0.2%) residents were not
included in the analysis as their RCHEs failed to
provide relevant information subsequently. Among
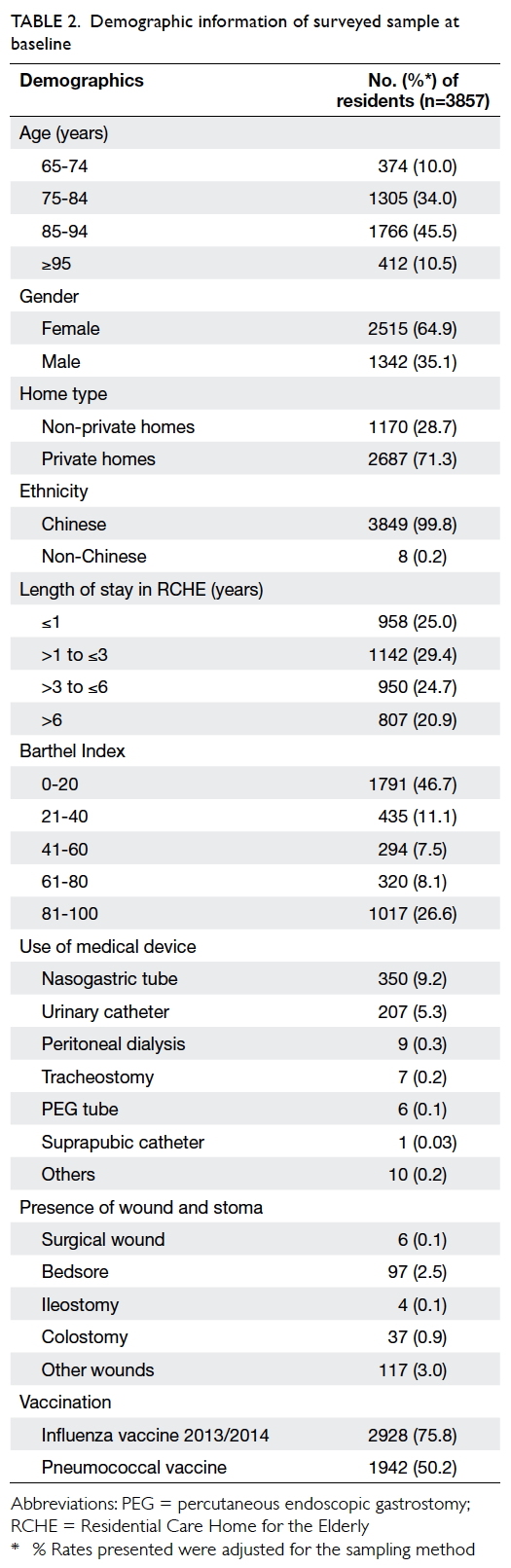
the 3857 residents surveyed, the mean age was 85.2
years, and the female-to-male ratio was 1.9:1. Most
residents were Chinese (99.8%, n=3849), and 56.5%
(n=2178) of those surveyed were above 84 years
old. The mean age of male and female residents was
82.4 and 86.8 years, respectively. Table 2 shows the
demographic information of the surveyed sample.
Duration of their stay in RCHEs varied; a quarter had
resided in a RCHE for less than 1 year, 29.4% for 1 to 3
years, 24.7% for 3 to 6 years, and 20.9% for more than
6 years. For ADL of residents, the median BI score
was 30, and 46.7% of residents scored 0-20 indicating
they were totally dependent in ADL.13 Regarding use
of an indwelling medical device, 14.4% of residents
required at least one device, mostly a nasogastric
tube (9.2%) or urinary catheter (5.3%). Up to 75.8% of
residents received the 2013/2014 seasonal influenza
vaccine and 50.2% had received the pneumococcal
vaccine. Most residents (87.1%) had more than one
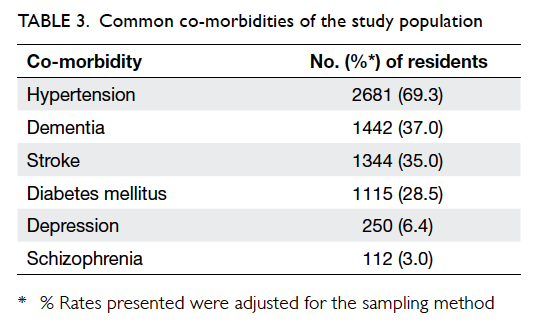
underlying co-morbidity with the most common
diagnosis being hypertension (69.3%), followed by
dementia (37.0%) and stroke (35.0%) [Table 3].
Prevalence of infections
A total of 105 residents were diagnosed with at least
one infection based on the survey definition. Among
these residents, 102 had one type of infection
and three had two types of infection. The overall
prevalence of infections was 2.7% (95% CI, 2.2%-3.4%). Table 4 shows the prevalence of different infections.
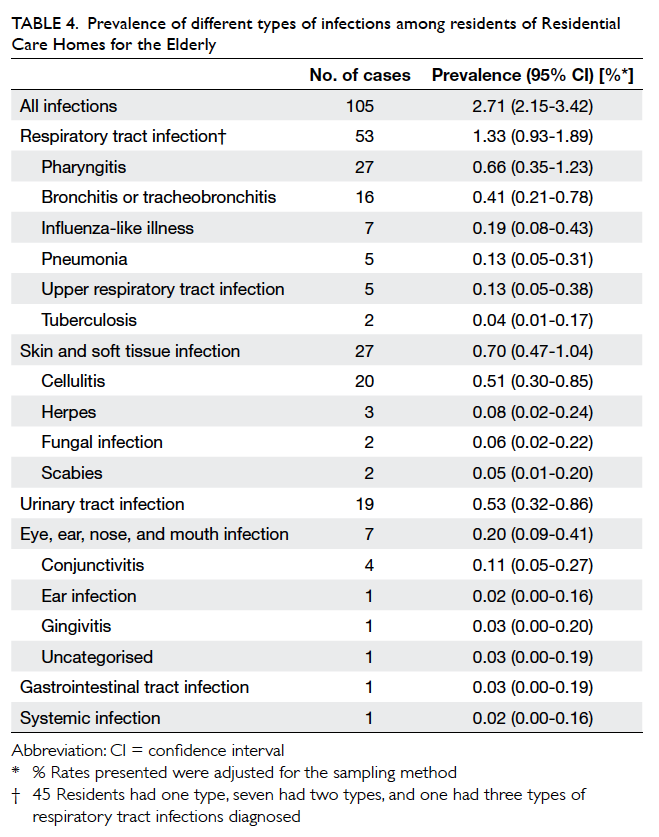
Table 4. Prevalence of different types of infections among residents of Residential Care Homes for the Elderly
Of all the infections, RTI was the most common
type, comprising 49.1% (n=53) of all infections,
followed by SSTI (25.0%, n=27) and urinary tract
infection (UTI) [17.6%, n=19; Fig].
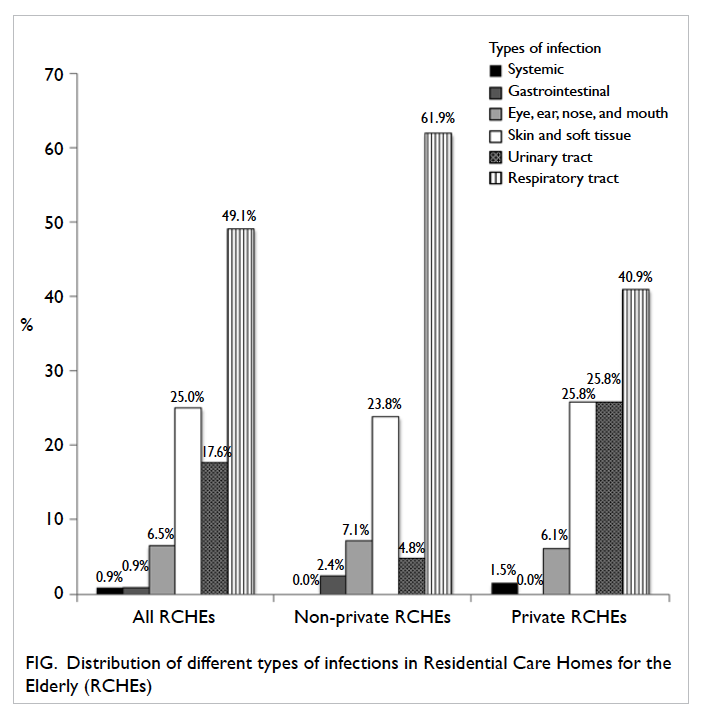
Figure. Distribution of different types of infections in Residential Care Homes for the Elderly (RCHEs)
Factors associated with infectious diseases
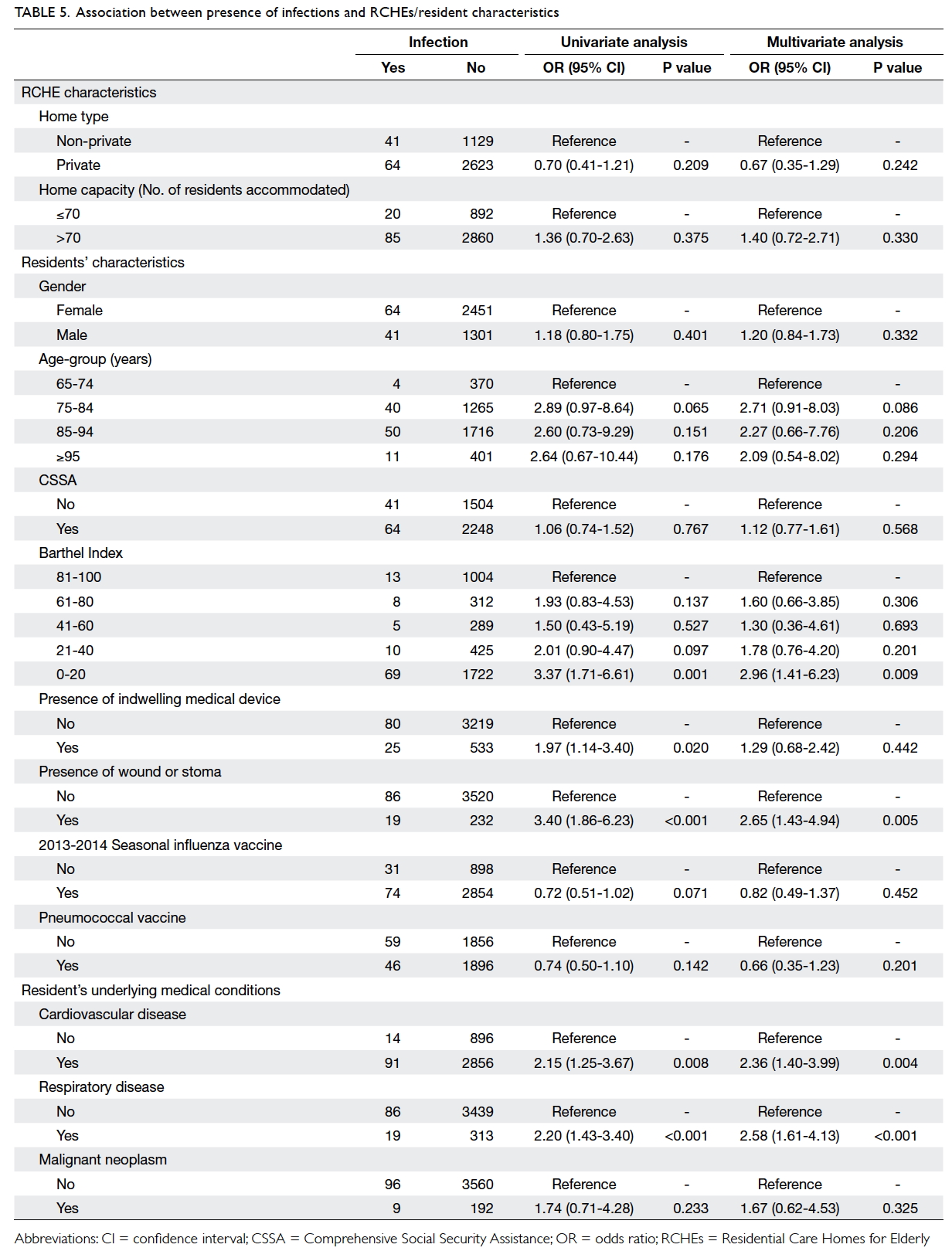
Table 5 illustrates factors associated with presence of
any infection and specific infections. Residents with
ADL dependency, as reflected by low BI score of
0-20 (odds ratio [OR]=3.0; 95% CI, 1.4-6.2), presence
of a wound or stoma (OR=2.7; 95% CI, 1.4-4.9), or
co-morbidities including cardiovascular diseases
(CVD) [OR=2.4; 95% CI, 1.4-4.0] and respiratory
diseases (OR=2.6; 95% CI, 1.6-4.1) were significantly
likely to have an infection. Seasonal influenza
vaccination (OR=0.82; P=0.452) and pneumococcal
vaccination (OR=0.66; P=0.201) were associated
with a lower risk of infection but neither reached
statistical significance.
Subgroup analysis by site of infection showed
that low BI score (OR=2.6; 95% CI, 1.3-5.2) and COPD (OR=3.7; 95%
CI, 1.5-9.1) were significantly associated with RTI. Factors significantly associated with
SSTI included low BI score (OR=5.5; 95% CI, 1.7-17.5), presence of wound(s) and stoma (OR=9.0;
95% CI, 4.7-17.1), having DM (OR=1.9; 95% CI,
1.0-3.6), mental illness (OR=3.7; 95% CI, 1.2-11.8),
and CVD (OR=4.6; 95% CI, 1.3-16.3). Presence of
a urinary catheter was significantly associated with
UTI (OR=5.6; 95% CI, 1.9-16.2).
Discussion
This point prevalence survey aimed to investigate
the prevalence of infections among residents
living in RCHEs in Hong Kong. It is essential to
understand that this specific group of elderly
differs significantly from their community-dwelling
counterparts in terms of health condition, level of
mobility, daily routine behaviour, and level of care
received. The confined living environment, shared
bathing equipment, group dining facilities, and close
human-to-human contact potentially foster the
transmission of infection. A local study has shown
that nursing home residency is an independent
predictor of infection-related mortality, pneumonia-related
mortality, and all-cause mortality.14
In this study, the overall prevalence of infections
was 2.7%. Among all infections, RTI, SSTI, and UTI
ranked top with a prevalence of 1.3%, 0.7%, and
0.5%, respectively. Low BI score of 0-20, presence
of a wound or stoma, and co-morbidities including
CVD and respiratory diseases were significantly
associated with presence of infection.
Compared with the previous prevalence
survey of infections among residents of RCHEs in
Hong Kong conducted in November 2006,5 a lower
overall prevalence was noticed in this survey. In
2006 the prevalence was 5.7%.5 A similar pattern of
prevalence regarding type of infection was observed
for common cold or pharyngitis (included under RTI
in our study) that was the most common type of
infection, followed by SSTI and UTI. This reduction
in overall prevalence over a 6-year period may be due
to a better awareness of infection control among the
general public and health care workers, particularly
after the severe acute respiratory syndrome endemic
in 2003 and H1N1 swine influenza endemic in 2009.
Another encouraging finding in this study may also
account for this improved trend: an increased uptake
of seasonal influenza vaccine was noted, from 60.3%
in year 2012-2013 to 75.8% in year 2013-2014 among
surveyed residents.
Prevalence surveys conducted in long-term
care facilities (LTCF) overseas have generally
reported an overall higher prevalence of infection,
from 3.4% to 11.8%.15 16 17 18 19 20 21 22 23 24 Most reported UTI as the most common type of infection.15 16 19 20 21 22 23 Despite a lower prevalence in our survey compared with
overseas surveys, we must interpret the results with
caution for a few reasons. First, the difference in
survey method, study population, and case definition
among these studies may render direct comparison
of prevalence inappropriate. Second, it is important
to understand the differences between settings and
the elderly population in LTCF in Hong Kong and
those overseas. In the US, LTCF can further be
categorised into veteran care centres that provide
care for elderly military officers, and nursing homes
and residential care communities that offer different
levels of assistance in ADL depending on the elderly
individual’s capacity for self-care.25 On the contrary,
in Europe, more than two thirds of those receiving
institutional care are above 80 years of age.26 Third,
staff levels, occupancy,25 local infection practice and
guidelines, and accessibility to health care facilities,
such as emergency room or secondary health care
facilities in overseas LTCF differ significantly from
our local setting. These factors may explain the
difference in prevalence between local RCHEs and
overseas LTCF.
This study also investigated the risk factors
associated with the presence of infection among
residents. Low BI score of 0-20 representing total
dependency in ADL, and presence of a wound
or stoma were associated with presence of any
type of infection. The findings are consistent with
past studies that suggest limitations in ADL or
functional impairment, and presence of skin ulcers
are risk factors for infection.15 17 21 Nevertheless, the
protective effect of immunisation with seasonal
influenza vaccine and pneumococcal vaccine was
not clearly demonstrated in this study.
Regarding RTI, which essentially includes upper tract infections (eg common cold or influenza-like illness) and lower tract infections (eg pneumonia), COPD and lower BI score of 0-20
were two associated factors. A few previous studies
that focused on risk factors for pneumonia (or
specifically nursing home–associated pneumonia)
also suggested that a low BI score,27 low ADL score,27
profound debility (measured by Karnofsky score of
≤40),28 and COPD5 28 are associated factors, and is
compatible with our findings.
Although multiple factors were significantly
associated with SSTI in our study, including low BI
score, presence of wounds and stoma, co-morbidities
like DM, mental illnesses and CVD, limited studies
have determined risk factors for SSTI in LTCF. In
Cotter et al’s study,16 presence of a urinary catheter,
vascular catheter, pressure sores, or other wounds
was significantly associated with SSTI. It is possible
that individuals with DM or CVD are more prone to
development of an ulcer or poor wound healing, and
thus have a higher risk of SSTI. Further studies may
be necessary to delineate the association between
SSTI and other co-morbidities.
Presence of an indwelling urinary catheter
is not surprisingly associated with UTI, and is
compatible with the previous local study5 and most
overseas studies.16 29 30 This reflects the importance
of proper care for indwelling urinary catheters in
RCHEs.
Our study provides more information
regarding prevalence and risk factors associated
with infectious diseases in RCHEs in Hong Kong.
Readers, however, must take note of a few limitations
of this study.
First, a point prevalence study offers only a
snapshot of events and thus a causal relationship
between risk factors and infections cannot be
established. Our study was conducted during
February to May, which was late winter to early
spring time in Hong Kong, and the prevalence of
different infections may have a seasonal variation,
for example, influenza.31 32 Comparison needs to take account of the season during which the study was
conducted.
Second, only 46 of the 100 invited RCHEs
participated in the survey. This response rate may
affect the generalisability of results. It is possible that
the RCHEs with stronger compliance with infection
control measures volunteered to participate whilst
those homes that refused were less compliant and
had a higher infection prevalence.
Third, the exclusion of residents who were not
present at the RCHEs at the reference time may have
led to underestimation of the prevalence of infection.
We reviewed the list of residents excluded from the
survey and found 18 of them had been admitted to
hospital in the 2 days preceding the survey, of whom
10 were admitted because of symptoms or signs
suggestive of infection. Assuming they all fulfilled
the criteria for infection in this survey, the effect
was likely minimal, with an adjusted prevalence of
infections of 2.9% (95% CI, 2.3%-3.7%).
Fourth, demographic data, medical history,
and vaccination history were retrieved from records
maintained by RCHEs, but different RCHEs had
different practices of record keeping. Data may have
been incomplete or inadequate in certain RCHEs
while others may have provided more detailed data.
These differences were minimised by a standard
protocol and training of the survey team and
verification of data with RCHE staff on site.
Finally, we did not include any infection control
practice measures in our study, such as hand hygiene
compliance of staff and environmental hygiene
measures. While the aim of the study was not to
assess the infection control practices of RCHEs,
these factors could potentially affect the results in
the risk factor analysis, and hence, readers should
interpret the regression result in the context that
confounding may present.
Conclusions
The overall prevalence of infections among RCHE
residents was estimated to be 2.7%. Associated
factors were identified. It is recommended that
infection control measures be targeted towards
these factors. Training for RCHE staff and a policy
to execute infection control guidelines in RCHEs
should be planned early in view of an increasing
demand for services provided by RCHEs. Further
study can be carried out at different times of the
year to identify any seasonal changes and pattern
of infections, or targeted at residents admitted to
public hospitals with acute infections to estimate the
overall burden on our health care sector.
Acknowledgements
The authors would like to thank the survey team
for their hard work in study design and fieldwork.
Furthermore, we extend our heartfelt gratitude to
all the participating RCHEs and their staff for their
assistance throughout the study. Without their
support, this survey would not have been possible.
The authors would like to thank the Elderly Health
Service, Department of Health for sharing their data
on annual assessment of RCHEs.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Census and Statistics Department, Hong Kong SAR
Government. Hong Kong population projections 2015-2064. September 2015. Available from: http://www.statistics.gov.hk/pub/B1120015062015XXXXB0100.pdf.
Accessed 22 Dec 2015.
2. Social Welfare Department, Hong Kong SAR Government.
Provision of residential care services for elders (non-governmental organisations versus private sector). March 2015. Available from:
http://www.swd.gov.hk/doc/elderly/ERCS/Overview%20item(b)English(31-3-2015).pdf. Accessed 22 Dec 2015.
3. Gavazzi G, Krause KH. Ageing and infection. Lancet Infect
Dis 2002;2:659-66. Crossref
4. Büla CJ, Ghilardi G, Wietlisbach V, Petignat C, Francioli
P. Infections and functional impairment in nursing home
residents: a reciprocal relationship. J Am Geriatr Soc
2004;52:700-6. Crossref
5. Chen H, Chiu AP, Lam PS, et al. Prevalence of infections in
residential care homes for the elderly in Hong Kong. Hong
Kong Med J 2008;14:444-50.
6. Social Welfare Department, Hong Kong SAR Government.
List, licences and briefs of residential care homes.
Available from: http://www.swd.gov.hk/en/index/site_pubsvc/page_elderly/sub_residentia/id_listofresi/.
Accessed 31 Dec 2013.
7. Mahoney FI, Barthel DW. Functional evaluation: The
Barthel Index. Md State Med J 1965;14:61-5.
8. McGeer A, Campbell B, Emori TG, et al. Definitions of
infection for surveillance in long-term care facilities. Am
J Infect Control 1991;19:1-7. Crossref
9. Magaziner J, Tenney JH, DeForge B, Hebel JR, Muncie HL
Jr, Warren JW. Prevalence and characteristics of nursing
home–acquired infections in the aged. J Am Geriatr Soc
1991;39:1071-8. Crossref
10. Li J, Birkhead GS, Strogatz DS, Coles FB. Impact of
institution size, staffing patterns, and infection control
practices on communicable disease outbreaks in New York
State nursing homes. Am J Epidemiol 1996;143:1042-9. Crossref
11. Cohen S. Social status and susceptibility to respiratory
infections. Ann N Y Acad Sci 1999;896:246-53. Crossref
12. Harrington RD, Hooton TM. Urinary tract infection risk
factors and gender. J Gend Specif Med 2000;3:27-34.
13. McDowell I. Measuring health: a guide to rating scales and
questionnaires. Oxford: Oxford University Press; 2006. Crossref
14. Chan TC, Hung IF, Cheng VC, et al. Is nursing home
residence an independent risk factor of mortality in
Chinese older adults? J Am Geriatr Soc 2013;61:1430-2. Crossref
15. Chami K, Gavazzi G, Carrat F, et al. Burden of infections
among 44,869 elderly in nursing homes: a cross-sectional
cluster nationwide survey. J Hosp Infect 2011;79:254-9. Crossref
16. Cotter M, Donlon S, Roche F, Byrne H, Fitzpatrick F.
Healthcare-associated infection in Irish long-term care
facilities: results from the First National Prevalence Study.
J Hosp Infect 2012;80:212-6. Crossref
17. Eriksen HM, Iversen BG, Aavitsland P. Prevalence of
nosocomial infections and use of antibiotics in long-term
care facilities in Norway, 2002 and 2003. J Hosp Infect
2004;57:316-20. Crossref
18. Moro ML, Mongardi M, Marchi M, Taroni F. Prevalence
of long-term care acquired infections in nursing and
residential homes in the Emilia-Romagna Region. Infection
2007;35:250-5. Crossref
19. Lim CJ, McLellan SC, Cheng AC, et al. Surveillance of
infection burden in residential aged care facilities. Med J
Aust 2012;196:327-31. Crossref
20. Tsan L, Langberg R, Davis C, et al. Nursing home–associated
infections in Department of Veterans Affairs community
living centers. Am J Infect Control 2010;38:461-6. Crossref
21. Tsan L, Davis C, Langberg R, et al. Prevalence of nursing
home–associated infections in the Department of Veterans
Affairs nursing home care units. Am J Infect Control
2008;36:173-9. Crossref
22. Marchi M, Grilli E, Mongardi M, Bedosti C, Nobilio L,
Moro ML. Prevalence of infections in long-term care
facilities: how to read it? Infection 2012;40:493-500. Crossref
23. Dwyer LL, Harris-Kojetin LD, Valverde RH, et al. Infections
in long-term care populations in the United States. J Am
Geriatr Soc 2013;61:342-9. Crossref
24. European Centre for Disease Prevention and Control.
Point prevalence survey of healthcare-associated
infections and antimicrobial use in European long-term
care facilities. May-September 2010. Available from:
http://www.ecdc.europa.eu/en/publications/_layouts/forms/Publication_DispForm.aspx?List=4f55ad51-4aed-4d32-b960-af70113dbb90&ID=1086. Accessed 31 Dec
2013.
25. Harris-Kojetin L, Sengupta M, Park-Lee E, Valverde
R. Long-term care services in the United States: 2013
overview. Vital Health Stat 3 2013;(37):1-107.
26. Rodrigues R, Huber M, Lamura G, editors. Facts and figures
on healthy ageing and long-term care. European Centre for
Social Welfare Policy and Research; 2012. Available from:
http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.303.1022&rep=rep1&type=pdf. Accessed 22 Jan 2016.
27. Wójkowska-Mach J, Gryglewska B, Romaniszyn D, et
al. Age and other risk factors of pneumonia among
residents of Polish long-term care facilities. Int J Infect Dis
2013;17:e37-43. Crossref
28. Muder RR. Pneumonia in residents of long-term care
facilities: epidemiology, etiology, management, and prevention. Am J Med 1998;105:319-30. Crossref
29. Michel JP, Lesourd B, Conne P, Richard D, Rapin CH.
Prevalence of infections and their risk factors in geriatric
institutions: a one-day multicentre survey. Bull World
Health Organ 1991;69:35-41.
30. Eriksen HM, Koch AM, Elstrøm P, Nilsen RM, Harthug
S, Aavitsland P. Healthcare-associated infection among
residents of long-term care facilities: a cohort and nested
case-control study. J Hosp Infect 2007;65:334-40. Crossref
31. Saha S, Chadha M, Al Mamun A, et al. Influenza seasonality
and vaccination timing in tropical and subtropical areas of
southern and south-eastern Asia. Bull World Health Organ
2014;92:318-30. Crossref
32. Yang L, Wong CM, Lau EH, Chan KP, Ou CQ, Peiris JS.
Synchrony of clinical and laboratory surveillance for
influenza in Hong Kong. PLoS One 2008;3:e1399. Crossref