Hong Kong Med J 2016 Aug;22(4):327–33 | Epub 17 Jun 2016
DOI: 10.12809/hkmj154707
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Impact of 18FDG PET and 11C-PIB PET brain imaging on the diagnosis of Alzheimer’s disease and other dementias in a regional memory clinic in Hong Kong
YF Shea, MRCP, FHKAM (Medicine)1;
Joyce Ha, BSc1;
SC Lee, BHS (Nursing)1;
LW Chu, MD, FRCP1,2
1 Division of Geriatrics, Department of Medicine, LKS Faculty of Medicine,
The University of Hong Kong, Queen Mary Hospital, Pokfulam, Hong Kong
2 The Alzheimer’s Disease Research Network, SRT Ageing, The University
of Hong Kong, Pokfulam, Hong Kong
Corresponding author: Dr YF Shea (elphashea@gmail.com)
Abstract
Objective: This study investigated the improvement
in the accuracy of diagnosis of dementia subtypes
among Chinese dementia patients who underwent
[18F]-2-fluoro-2-deoxy-D-glucose positron emission
tomography (18FDG PET) with or without carbon 11–labelled Pittsburgh
compound B (11C-PIB).
Methods: This case series was performed in the
Memory Clinic at Queen Mary Hospital, Hong
Kong. We reviewed 109 subjects (56.9% were female) who
received PET with or without 11C-PIB between January
2007 and December 2014. Data including age, sex,
education level, Mini-Mental State Examination
score, Clinical Dementia Rating scale score,
neuroimaging report, and pre-/post-imaging clinical
diagnoses were collected from medical records. The
agreement between the initial and post-PET with or
without 11C-PIB dementia diagnosis was analysed by the
Cohen’s kappa statistics.
Results: The overall accuracy of initial clinical
diagnosis of dementia subtype was 63.7%, and
diagnosis was subsequently changed in 36.3% of
subjects following PET with or without 11C-PIB. The
rate of accurate initial clinical diagnosis (compared
with the final post-imaging diagnosis) was 81.5%,
44.4%, 14.3%, 28.6%, 55.6% and 0% for Alzheimer’s
disease, dementia with Lewy bodies, frontotemporal
dementia, vascular dementia, other dementia, and mixed dementia, respectively. The agreement
between the initial and final post-imaging dementia
subtype diagnosis was only fair, with a Cohen’s kappa
of 0.25 (95% confidence interval, 0.05-0.45). For the
21 subjects who underwent 11C-PIB PET imaging, 19%
(n=4) of those with Alzheimer’s disease (PIB positive)
were initially diagnosed with non–Alzheimer’s
disease dementia.
Conclusions: In this study, PET with or without
11C-PIB brain imaging helped improve the accuracy of
diagnosis of dementia subtype in 36% of our patients
with underlying Alzheimer’s disease, dementia with
Lewy bodies, vascular dementia, and frontotemporal
dementia.
New knowledge added by this study
- Positron emission tomography (PET) with or without Pittsburgh compound B (PIB) brain imaging helps improve the accuracy of dementia subtype diagnosis in Chinese patients.
- PET with or without PIB brain imaging should be considered in patients with dementia who attend the memory clinic, especially if there is diagnostic difficulty.
Introduction
With ageing of the world’s population, the prevalence
of dementia increases: 46.8 million people worldwide
were living with dementia in 2015. This is projected to
reach 74.7 million in 2030 and 131.5 million in 2050,
with 60% suffering from Alzheimer’s disease (AD).1
In Hong Kong, the prevalence of mild dementia has
been reported to be 8.9% for adults aged 70 years or
over, with 64.6% suffering from AD.2 Appropriate
management of demented patients begins with
correct diagnosis of dementia subtype that allows
earlier implementation of disease-specific treatment.
In particular, cholinesterase inhibitors (ChEIs) or N-methyl-D-aspartate receptor antagonists are mostly
suitable for the treatment of AD. The current clinical
diagnostic guidelines for various types of dementia
have limited sensitivities and specificities, however.
The sensitivity and specificity of clinical diagnostic
criteria for AD, dementia with Lewy bodies (DLB),
and frontotemporal dementia (FTD) have been
reported as 81% and 70%, 50% and 80%, 85% and
95%, respectively.3 4 5 6 In the most recent diagnostic
criteria for AD, additional use of biomarkers of AD
has been recommended by the National Institute
on Aging and Alzheimer’s Association to improve
the accuracy of AD diagnosis.3 Biomarkers for the
diagnosis of AD include cerebrospinal fluid (CSF),
amyloid pathological imaging (eg carbon 11–labelled
Pittsburgh compound B [11C-PIB] positron emission
tomography [PET]), and functional imaging (eg [18F]-2-fluoro-2-deoxy-D-glucose [18F-FDG] PET) that
yield sensitivities and specificities of at least 90% and
85%, respectively in the diagnosis of AD, DLB, and
FTD.3 7 8 9 10 11 Because of the invasive nature of lumbar puncture in the collection of CSF, neuroimaging
modalities such as 18F-FDG PET and 11C-PIB PET
are more accepted in routine clinical practice to
improve the diagnosis of dementia subtype.
The most common functional neuroimaging is
with 18F-FDG12 and the most common pathological
neuroimaging is with 11C-PIB.13 These molecular
imaging markers are imaged using PET. The 18F-FDG
measures metabolic activity of the brain; 18F-FDG
PET distinguishes well between AD and non-AD
dementia.11 In a systematic review, the sensitivity
and specificity for 18F-FDG PET in distinguishing
between AD and DLB was 83%-99% and 71%-93%,
respectively; and the sensitivity and specificity for
18F-FDG PET in distinguishing between AD and
FTD was 97.6%-99% and 65%-86%, respectively.11 In
the same systematic review, 18F-FDG PET predicted
patients with mild cognitive impairment (MCI)
deteriorating into dementia with sensitivity and
specificity of 81%-82% and 86%-90%, respectively.11
Besides, 11C-PIB can detect the presence of fibrillar
amyloid plaques that are a neuropathological marker
of AD.13 Correlation studies with neuropathology
have shown a sensitivity of 90% and specificity
of 100%; 11C-PIB can reasonably distinguish AD
from other types of dementia, eg FTD.13 Using
neuropathology as the gold standard, the sensitivity
and specificity was 89% and 83%, respectively.13
The presence of 11C-PIB retention also predicts the
progression of patients with MCI: 50% progress
to AD in 1 year and 80% progress to AD within 3
years.14
Previous studies with 18F-FDG and 11C-PIB
PET have focused on highly selected diagnostic
groups, and only a few studies have studied their
impact in the routine clinical setting of a memory
clinic at a tertiary university hospital. The latter are
referral centres, and often encounter patients with
complicated diagnostic issues. Ossenkoppele et al15
reported a cohort of 145 patients who underwent
18F-FDG and 11C-PIB PET after clinical assessment.
Change in clinical diagnosis was required in 23% with
the diagnostic confidence increased from a mean of
71% to 87%. Diagnosis remained unchanged in 96%
after PET over the next 2 years.15 In seven patients
with MCI and positive amyloid deposition on 11C-PIB
PET, six progressed to AD during follow-up (5 had
AD pattern of hypometabolism on 18F-FDG PET).15
In a retrospective study of 94 patients with MCI or
dementia, Laforce et al16 showed that 18F-FDG PET
brain scan led to a change in diagnosis in 29% of
patients, and reduced the frequency of atypical or
unclear diagnoses from 39.4% to 16%.
To the best of our knowledge, there are
no published data on the impact of molecular
neuroimaging on accuracy of diagnosis of AD or
other dementias in the Chinese population. We
hypothesised that brain 18F-FDG with or without
11C-PIB PET imaging can improve the accuracy
of diagnosis of common dementia subtypes in a
memory clinic. The objective of this study was to
investigate the impact of brain 18F-FDG with or
without 11C-PIB imaging in improving the accuracy
of diagnosis of dementia subtype in a local memory
clinic in Hong Kong.
Methods
This was a retrospective study conducted at the
Memory Clinic of Queen Mary Hospital, the
University of Hong Kong. Patients were referred by
general practitioners, neurologists, geriatricians,
surgeons, or psychiatrists. All patient records
between January 2007 and December 2014 were
reviewed. Inclusion criteria were a clinical diagnosis
of MCI, dementia of any type, or unclassifiable
dementia; and 18F-FDG with or without 11C-PIB
PET performed within 3 months after the initial
clinical diagnosis. The initial clinical assessment was
performed by a geriatrician experienced in dementia
care and included detailed history taking from
primary carers of the patient, physical examination,
cognitive assessment, and laboratory studies
(including thyroid function test, vitamin B12 level,
folate level, and syphilis serology [Venereal Disease
Research Laboratory]). Clinical criteria for AD, FTD,
DLB, and vascular dementia (VaD) were employed to
establish the clinical diagnosis initially, without using
any biomarker. The diagnosis of different dementia
subtype before neuroimaging was based on
the respective diagnostic guidelines. Patients with
AD were diagnosed according to the NINCDS-ADRDA
(National Institute of Neurological
and Communicative Disorders and Stroke and
Alzheimer’s Disease and Related Disorders
Association) diagnostic criteria.17 Patients with DLB
were diagnosed by the McKeith criteria.4 Behavioural
variant (bv) of FTD was diagnosed by revised
diagnostic criteria reported by the International
bvFTD Criteria Consortium5 and language variant
of FTD was diagnosed by latest published criteria.6
Patients with VaD were diagnosed according to the
criteria of the NINDS-AIREN (National Institute
of Neurological Disorders and Stroke/Association
Internationale pour la Recherche et l’Enseignement
en Neurosciences).18 In this study, we reviewed the
medical records of eligible subjects and collected
data including age, sex, education level, Mini-Mental
State Examination score, Clinical Dementia Rating scale score,
molecular imaging report including the standardised
uptake value ratio (SUVR) of 11C-PIB PET, and the
pre- and post-imaging diagnoses. For patients who
were diagnosed with MCI, their progression during
subsequent follow-up visits was also reviewed.
The need for 18F-FDG with or without 11C-PIB
PET was determined by the geriatrician who
performed the initial clinical assessment. The images
were evaluated by a radiologist with more than 10
years of experience in reading PET scans. Dementias
were classified using the generally accepted criteria.
Patients were fasted for at least 4 hours before the
PET. The serum glucose level was measured in all
patients. For 18F-FDG PET, the patient was rested
in a dimly lit room with eyes closed for 30 minutes
prior to injection of 18F-FDG via a venous catheter.
Another 30 minutes of rest was observed before
starting the acquisition. The acquired data were
semi-quantitatively compared with age-stratified
normal controls using three-dimensional stereotactic
surface projections. For PIB imaging, acquisition was
performed at 5 minutes and 35 minutes after 11C-PIB
injection via a venous catheter, and SUVR images of
11C-PIB between 5 and 35 minutes were generated.
Cerebellar grey matter was chosen as reference
tissue. In this study, 11C-PIB PET scans were rated as
positive (PIB+; if binding occurred in more than one
cortical brain region; ie frontal, parietal, temporal, or
occipital) or negative (PIB–; if predominantly white
matter binding).
The pattern of 18F-FDG PET hypometabolism
that is suggestive of each subtype of dementia is as
follows6 12 19:
(1) AD—uni- or bi-lateral parietotemporal
hypometabolism with posterior cingulate
gyrus involvement or bilateral parietal and
precuneal hypometabolism.
(2) DLB—same as AD with added hypometabolism
in occipital lobes.
(3) bvFTD—uni- or bi-lateral frontotemporal
hypometabolism with or without less-severe
parietal hypometabolism.
(4) Semantic dementia—anterior temporal lobe
hypometabolism.
(5) Progressive non-fluent aphasia—left posterior
frontoinsular hypometabolism.
(6) VaD—well-defined focal defects not fitting the
above described patterns.
Statistical analyses
Descriptive statistics were used for data analyses.
Continuous variables were expressed as mean ±
standard deviation or median (interquartile range)
as appropriate. Categorical data were expressed as
number and percentages. The agreement between
pre- or post-imaging diagnoses of dementia subtype
was analysed by the Cohen’s kappa (κ) statistic. The
Cohen’s κ reflected the degree of agreement: <0 = no
agreement, 0-0.20 = slight agreement, 0.21-0.40 = fair
agreement, 0.41-0.60 = moderate agreement, 0.61-0.80 = substantial agreement, and 0.81-1.00 = almost
perfect agreement. All analyses were performed
with the Statistical Package for the Social Sciences
(Windows version 18.0; SPSS Inc, Chicago [IL], US).
Results
A total of 109 patients (56.9% were female) were recruited of whom 102
had dementia and seven had MCI. Both 18F-FDG
and 11C-PIB PET data were available for 45 (41.3%)
patients, and 64 patients underwent 18F-FDG only.
The final diagnosis of the 102 demented patients
after neuroimaging is shown in Table 1.
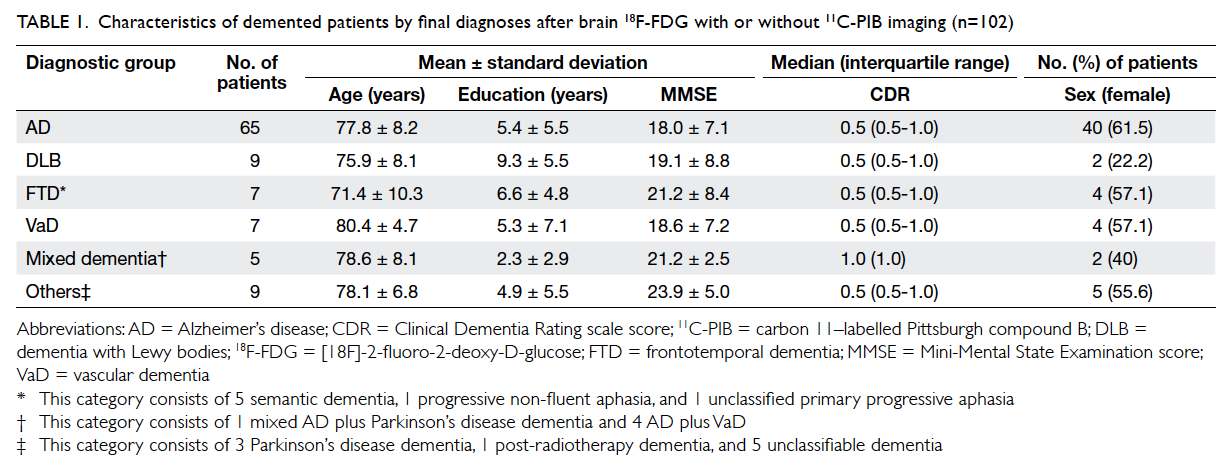
Table 1. Characteristics of demented patients by final diagnoses after brain 18F-FDG with or without 11C-PIB imaging (n=102)
The accuracy of clinical diagnoses is
summarised in Table 2. Overall, PET scans confirmed
the clinical impression in 63.7% of patients, and
corrected the diagnosis in 36.3%. Using the result
of PET scan as the gold standard, the frequency of
accurate initial clinical diagnosis was low for FTD,
VaD, and mixed dementia (14.3%, 28.6%, and 0%,
respectively). The accuracy of clinical diagnosis for
AD and DLB was 81.5% and 44.4%, respectively. After
excluding subjects with an initial MCI diagnosis, the
agreement between the initial and final post-imaging
dementia diagnosis was only fair, with a Cohen’s κ of
0.25 (95% confidence interval, 0.05-0.45).
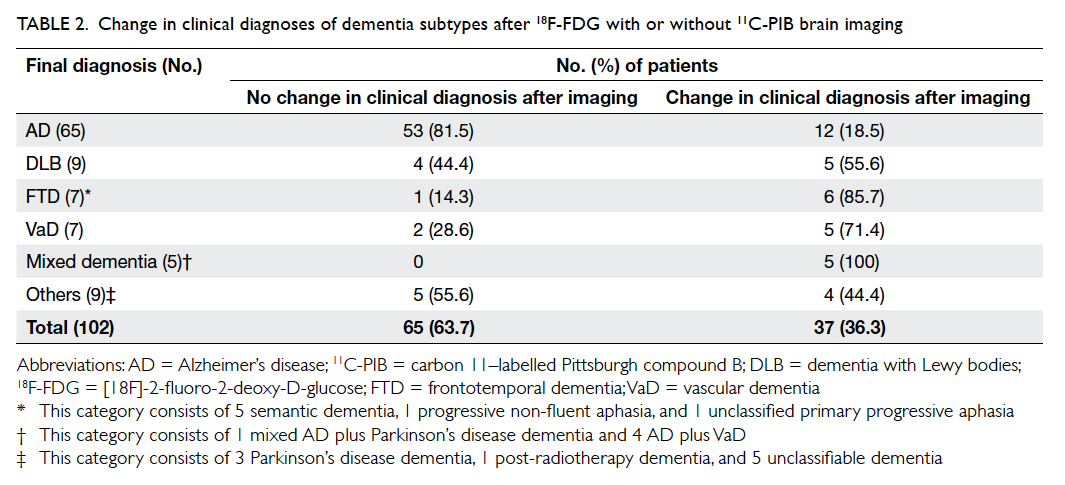
Table 2. Change in clinical diagnoses of dementia subtypes after 18F-FDG with or without 11C-PIB brain imaging
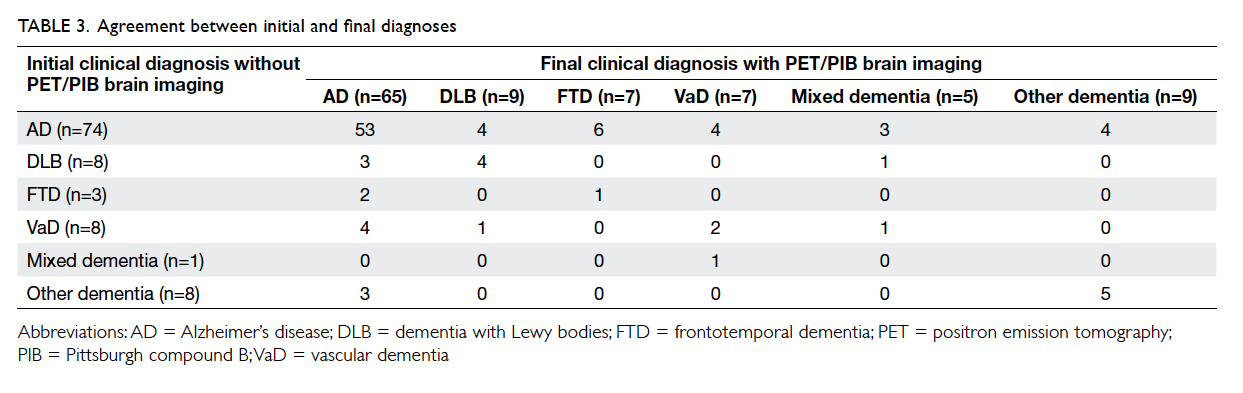
Table 3 lists the diagnosis of subjects before
and after the availability of 18F-FDG with or without
11C-PIB PET neuroimaging. For subjects with a final
diagnosis of AD (n=65), 18.5% (n=12) were initially
diagnosed with non-AD dementia (including 3 with
DLB, 2 with FTD, 4 with VaD, and 3 with other
dementia) and subsequently received symptomatic
AD therapy (ie ChEIs and/or memantine). For
the 21 subjects who underwent PIB PET imaging,
19% (n=4) of those with AD (PIB+) were initially
diagnosed with non-AD dementia. For subjects
with an initial diagnosis of AD (n=74), 28.4% (n=21)
had a change in diagnosis (including 4 DLB, 6 FTD,
4 VaD, 3 mixed AD plus VaD, and 4 with other
dementia). Excluding subjects with DLB and mixed
AD plus VaD, 13.7% of all subjects (14 out of 102)
had discontinued their previous symptomatic AD
therapy. For subjects with a final diagnosis of FTD
(n=7), 85.7% (n=6) were initially misdiagnosed as
AD. For subjects with a final diagnosis of DLB (n=9),
44.4% (n=4) were misdiagnosed as AD.
Five patients were diagnosed with unclassifiable
dementia following neuroimaging, which comprised
four females and one male with a mean age of
78 ± 9.4 years. All presented with amnesia. In
addition, one patient presented with apraxia and
dysexecutive syndrome and another presented with
hyperorality. All of them were PIB-. An AD pattern
of hypometabolism was present in four patients (2
with hypometabolism in posterior cingulate gyrus
and 2 with hypometabolism in temporoparietal
lobes). Isolated hypometabolism in the temporal
lobes was present in one patient.
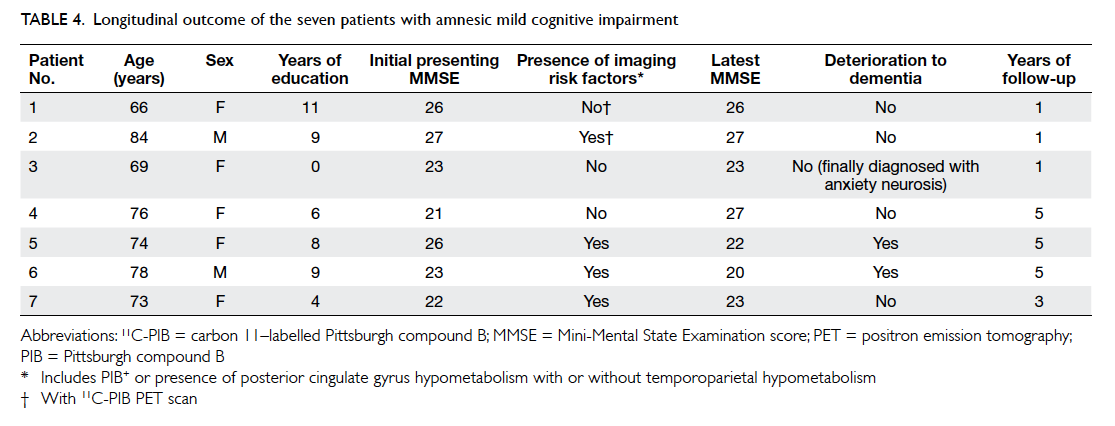
The clinical information of the seven amnesic
MCI subjects are summarised in Table 4. None of the
three subjects without imaging risk factors for AD
deteriorated over a follow-up period of 1 to 5 years.
Of the four amnesic MCI subjects with imaging risk
factors, two deteriorated into AD over a follow-up
period of 5 years.
Discussion
In this study, we showed that 18F-FDG with or
without 11C-PIB PET clarified and improved the
accuracy of dementia diagnosis in 36.3% of patients,
and confirmed the initial diagnosis in 63.7%. Using
the results of PET scan as the gold standard, the
accuracy of clinical diagnosis was low for FTD, VaD,
and mixed dementia collectively. On the one hand,
11.7% of patients (ie 12 out of 102) were started on
symptomatic AD therapy after the 18F-FDG with or
without 11C-PIB PET neuroimaging investigations.
On the other hand, 13.7% of patients (ie 14 out of
102) discontinued symptomatic AD therapy after
18F-FDG with or without 11C-PIB PET because they
did not have AD.
We also showed that the accuracy of clinical
diagnosis of DLB and FTD was low (44.4% and 14.3%,
respectively). This finding was in agreement with a
previous study.20 Both DLB and FTD are commonly
misdiagnosed clinically as AD (50% for DLB and
85.7% for FTD).20 We have previously reported that
100% of our patients with biomarkers that confirmed
DLB and FTD presented with memory impairment
in our memory clinic.20 A previous study also
reported that 26% of DLB patients were initially
misdiagnosed with AD, and 57% of these DLB
patients presented with memory impairment.21 We
understand that an accurate diagnosis of DLB is very
important for subsequent management. Patients
with DLB are particularly sensitive to neuroleptics.21
Neuroleptic sensitivity can present as drowsiness,
confusion, abrupt worsening of parkinsonism,
postural hypotension, or neuroleptic malignant
syndrome.21 Other clinical features of DLB that need
to be observed and tackled include well-formed
visual hallucinations, rapid eye movement sleep
behavioural disorder, and autonomic symptoms
(including postural hypotension, sialorrhoea, and
urinary and bowel symptoms).21 By accurately
establishing the diagnosis of DLB, careful
observation of classic DLB symptoms may reduce
unnecessary investigations. Regarding therapeutic
implications, DLB is characterised by far greater
cholinergic deficits than AD. Hence, most DLB
patients will benefit from ChEIs, and the extent of
symptomatic improvement should be monitored
after such therapy.22
Similarly, FTD may be misdiagnosed as AD.
The former can also present initially with memory
impairment, as illustrated by our FTD patients.
There is increasing evidence that elderly patients
with FTD often present with memory impairment.5 23 24
In one autopsy study, 64% (n=7) of 11 elderly
patients with FTD had anterograde memory loss.23
Current treatment guidelines do not advise giving
ChEIs or memantine treatments to FTD patients.
Thus, such medications should be stopped to prevent
unnecessary adverse effects.25
In the past few years, disease-modifying
treatments (eg bapineuzumab) have failed to
demonstrate their efficacy in clinical trials with
AD patients.26 Detailed post-hoc analyses with AD
biomarkers have shown the problem of diagnosing
AD in subjects recruited in these studies. Only
approximately 80% of these subjects had AD amyloid
pathology, according to the presence of amyloid
PET scan.26 Thus, including 11C-PIB PET to confirm
brain amyloid in study inclusion criteria can help
ensure recruitment of genuine AD patients to future
clinical trials of disease-modifying treatments for
AD.27 Given the minimally invasive nature of 11C-PIB
PET compared with CSF amyloid-beta (Aβ) 42 measurements,7 it is likely to be a more acceptable choice for patients in clinical trials. At present,
there are ongoing clinical trials of AD treatments
including secretase inhibitors, Aβ aggregation
inhibitors, Aβ and tau immunotherapy.27 We believe
that 11C-PIB PET will play an important role in these
clinical trials.
It is considered that 18F-FDG and 11C-PIB PET
may detect underlying AD in patients with MCI.28 In
the present study, 50% of MCI patients (ie 2 out of
4) with 18F-FDG and 11C-PIB PET imaging findings
positive for AD showed deterioration over a follow-up period of 5
years. Although recommending PET brain imaging
in MCI patients is still debatable, we believe that this
investigation can help clinicians to better plan future
and long-term treatments. In particular, disease-modifying
drugs for AD or MCI due to AD may
prove to be effective in the coming decade. Finally,
in the present study, five patients were diagnosed
with unclassifiable dementia. In the four patients
with an AD pattern of hypometabolism, AD may
still be present as they may have diffuse plaques
or amorphous plaques that do not bind well to
PIB. Alternatively they may have another type of
dementia that requires pathological confirmation,
eg argyrophilic grain disease or neurofibrillary
tangle–only dementia.29 We will follow up the
remaining patient with isolated hypometabolism in
the temporal lobes to see whether additional FTD
features develop.
There were several limitations to the present
study. This was a retrospective case series and as
such we were unable to collect further information
such as the pre-imaging or post-imaging confidence
of diagnosis. The diagnosis of dementia relied on
the clinical diagnostic criteria without pathological
confirmation. Therefore, we were also unable to
compare the relative accuracy of clinical diagnosis
and PET diagnosis with pathological diagnosis. For
patients with MCI, some were not followed up for
sufficiently long to ascertain whether or not they had
deteriorated and developed dementia. Structural
imaging (including computed tomography or
magnetic resonance imaging) of the brain was not
analysed as a separate variable but integrated into the
pre-functional imaging clinical diagnoses of dementia
subtypes. Our case series is likely to have selection
bias as PET imaging is mostly a self-paid service in
Hong Kong. The exception is for patients who are
retired civil servants or recipients of Comprehensive
Social Security Assistance. Demented patients who
could not afford PET may differ to the patients
selected. Although the PET images were analysed
and read by radiologists experienced in PET, the
interpretations depended heavily on individual
experience and training; also, radiologists were not
blinded to clinical information written on the request
form. Despite these limitations, our study should be
more reflective of day-to-day practice in a memory
clinic and how 18F-FDG with or without11C-PIB PET
imaging may assist clinical diagnosis.
Conclusions
In this study, 18F-FDG with or without 11C-PIB brain
imaging improved the accuracy of diagnosis of
dementia subtype in 36% of patients with underlying
AD, DLB, VaD, and FTD who presented to our
memory clinic.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Alzheimer’s Disease International World Alzheimer Report 2015: executive summary. Available from: http://www.alz.co.uk/research/WorldAlzheimerReport2015-sheet.pdf. Accessed Sep 2015.
2. Lam LC, Tam CW, Lui VW, et al. Prevalence of very mild
and mild dementia in community-dwelling older Chinese
people in Hong Kong. Int Psychogeriatr 2008;20:135-48. Crossref
3. McKhann GM, Knopman DS, Chertkow H, et al. The
diagnosis of dementia due to Alzheimer’s disease:
recommendations from the National Institute on Aging and Alzheimer’s Association workgroups on diagnostic
guidelines for Alzheimer’s disease. Alzheimers Dement
2011;7:263-9. Crossref
4. McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and
management of dementia with Lewy bodies: third report
of the DLB Consortium. Neurology 2005;65:1863-72. Crossref
5. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of
revised diagnostic criteria for the behavioural variant of
frontotemporal dementia. Brain 2011;134:2456-77. Crossref
6. Harris JM, Gall C, Thompson JC, et al. Classification and
pathology of primary progressive aphasia. Neurology
2013;81:1832-9. Crossref
7. Shea YF, Chu LW, Zhou L, et al. Cerebrospinal fluid
biomarkers of Alzheimer’s disease in Chinese patients:
a pilot study. Am J Alzheimers Dis Other Demen
2013;28:769-75. Crossref
8. Duits FH, Teunissen CE, Bouwman FH, et al. The
cerebrospinal fluid “Alzheimer profile”: easily said, but
what does it mean? Alzheimers Dement 2014;10:713-723.e2. Crossref
9. Sinha N, Firbank M, O’Brien JT. Biomarkers in dementia
with Lewy bodies: a review. Int J Geriatr Psychiatry
2012;27:443-53. Crossref
10. Harris JM, Gall C, Thompson JC, et al. Sensitivity and
specificity of FTDC criteria for behavioral variant
frontotemporal dementia. Neurology 2013;80:1881-7. Crossref
11. Davison CM, O’Brien JT. A comparison of FDG-PET and
blood flow SPECT in the diagnosis of neurodegenerative
dementias: a systematic review. Int J Geriatr Psychiatry
2014;29:551-61. Crossref
12. Schöll M, Damián A, Engler H. Fluorodeoxyglucose PET in
neurology and psychiatry. PET Clin 2014;9:371-90. Crossref
13. Vandenberghe R, Adamczuk K, Dupont P, Laere KV,
Chételat G. Amyloid PET in clinical practice: Its place
in the multidimensional space of Alzheimer’s disease.
Neuroimage Clin 2013;2:497-511. Crossref
14. Cummings JL. Biomarkers in Alzheimer’s disease drug
development. Alzheimers Dement 2011;7:e13-44. Crossref
15. Ossenkoppele R, Prins ND, Pijnenburg YA, et al. Impact of
molecular imaging on the diagnostic process in a memory
clinic. Alzheimers Dement 2013;9:414-21. Crossref
16. Laforce R Jr, Buteau JP, Paquet N, Verret L, Houde
M, Bouchard RW. The value of PET in mild cognitive
impairment, typical and atypical/unclear dementias: A
retrospective memory clinic study. Am J Alzheimers Dis
Other Demen 2010;25:324-32. Crossref
17. McKhann G, Drachman D, Folstein M, Katzman R, Price
D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease:
report of the NINCDS-ADRDA Work Group under the
auspices of Department of Health and Human Services Task
Force on Alzheimer’s Disease. Neurology 1984;34:939-44. Crossref
18. Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular
dementia: diagnostic criteria for research studies. Report
of the NINDS-AIREN International Workshop. Neurology
1993;43:250-60. Crossref
19. Waldö ML. The frontotemporal dementias. Psychiatr Clin
North Am 2015;38:193-209. Crossref
20. Shea YF, Ha J, Chu LW. Comparisons of clinical symptoms
in biomarker-confirmed Alzheimer’s disease, dementia
with Lewy bodies, and frontotemporal dementia patients
in a local memory clinic. Psychogeriatrics 2014;15:235-41. Crossref
21. Zweig YR, Galvin JE. Lewy body dementia: the impact on
patients and caregivers. Alzheimers Res Ther 2014;6:21. Crossref
22. Gauthier S. Pharmacotherapy of Parkinson disease
dementia and Lewy body dementia. Front Neurol Neurosci
2009;24:135-9. Crossref
23. Baborie A, Griffiths TD, Jaros E, et al. Frontotemporal
dementia in elderly individuals. Arch Neurol 2012;69:1052-60. Crossref
24. Hornberger M, Piguet O. Episodic memory in
frontotemporal dementia: a critical review. Brain
2012;135:678-92. Crossref
25. Portugal Mda G, Marinho V, Laks J. Pharmacological
treatment of frontotemporal lobar degeneration: systematic
review. Rev Bras Psiquiatr 2011;33:81-90. Crossref
26. Blennow K, Mattsson N, Schöll M, Hansson O, Zetterberg
H. Amyloid biomarkers in Alzheimer’s disease. Trends
Pharmacol Sci 2015;36:297-309. Crossref
27. Wisniewski T, Goñi F. Immunotherapeutic approaches for
Alzheimer’s disease. Neuron 2015;85:1162-76. Crossref
28. Langa KM, Levine DA. The diagnosis and management
of mild cognitive impairment: a clinical review. JAMA
2014;312:2551-61. Crossref
29. Kovacs GG. Tauopathies. In: Kovacs GG, editor.
Neuropathology of neurodegenerative diseases: a practical
guide. Cambridge: Cambridge University Press;
2015: 125-8.