Amoebic liver abscesses with an unusual source: a case report
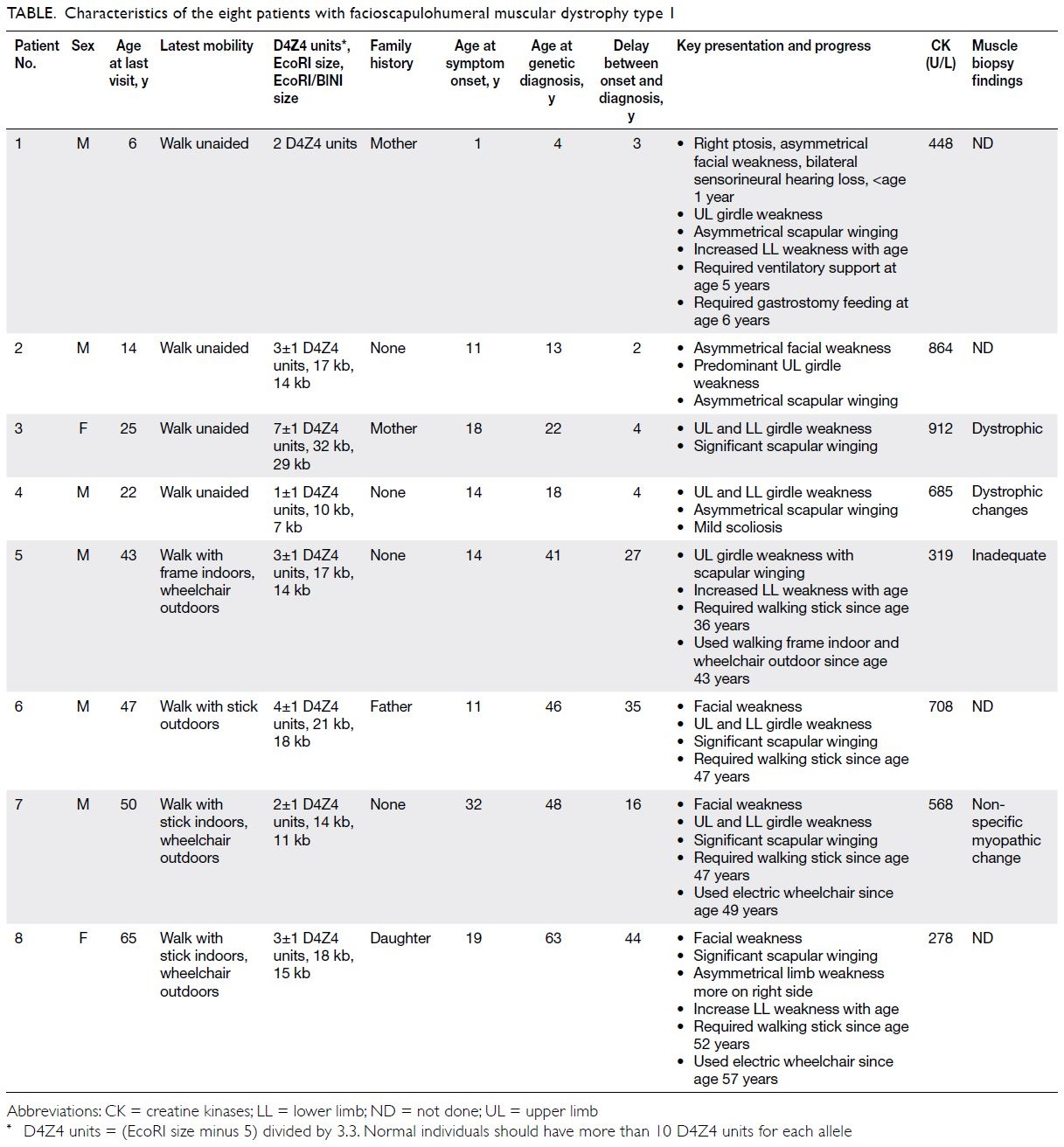
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
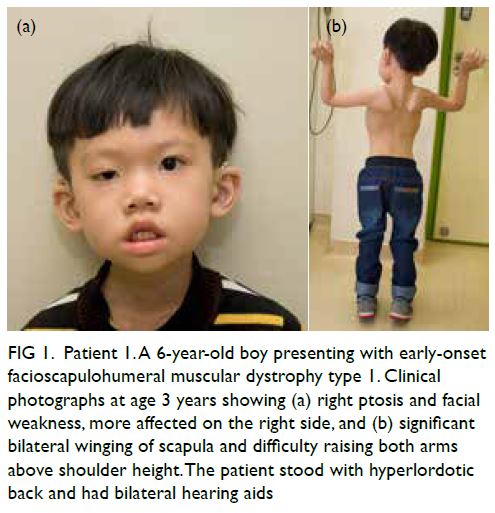
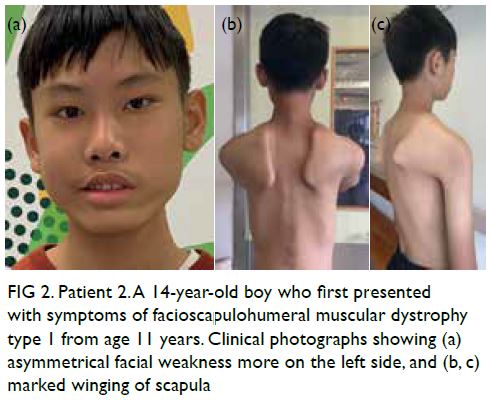
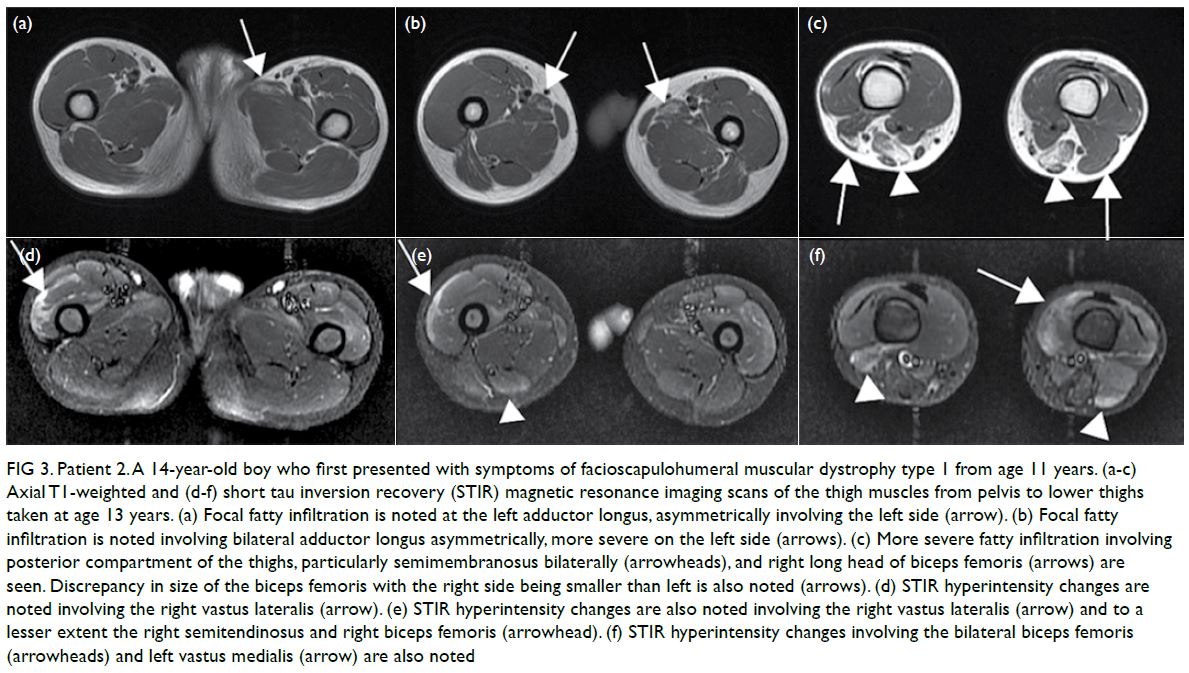
CASE REPORT
Amoebic liver abscesses with an unusual source:
a case report
Juanita N Chui, BSc (Adv), MD1; Albert KK Chui, FRACS, MD2
1 School of Medicine, University of Sydney, Sydney, Australia
2 Private Practice, 12/F, Emperor Commercial Centre, Central, Hong Kong
Corresponding author: Dr Albert KK Chui (akkchui@netvigator.com)
Case report
In July 2020, a 50-year-old man presented to a
public hospital in Hong Kong with a 3-week history
of abdominal pain localising to the right upper
quadrant, associated with low-grade fever, chills, and fatigue. He denied any diarrhoea or vomiting.
His medical history and family history were
unremarkable. He was a non-smoker and consumed
alcohol infrequently. He had recently returned from
Southern California, United States, where he was
bitten by a horse 1 month before the onset of his
symptoms. Despite sustaining deep wounds to his
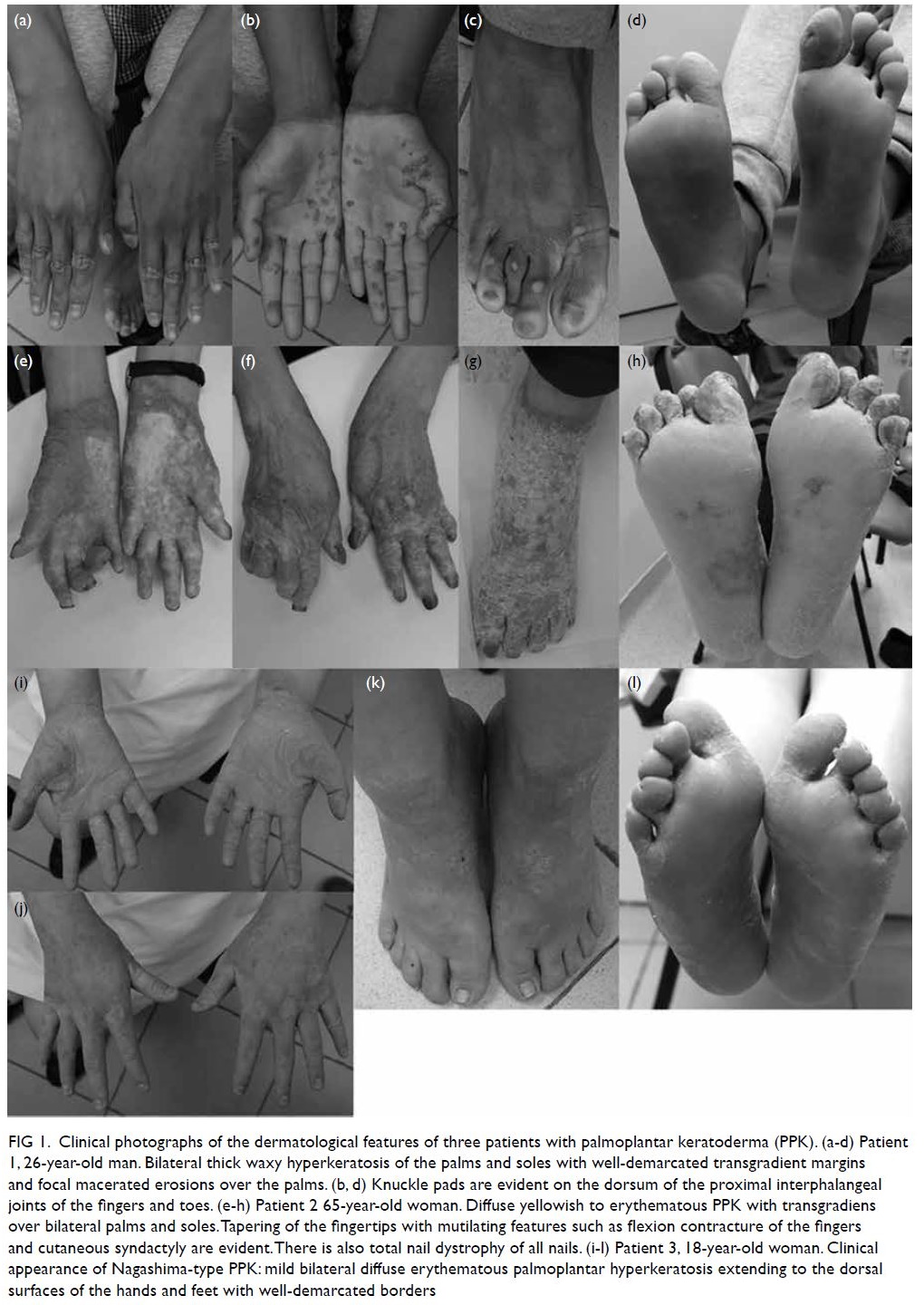
left neck (Fig 1), he did not seek medical attention
at the time.
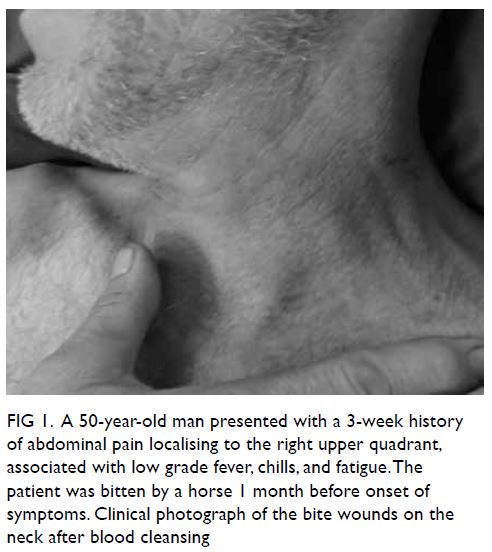
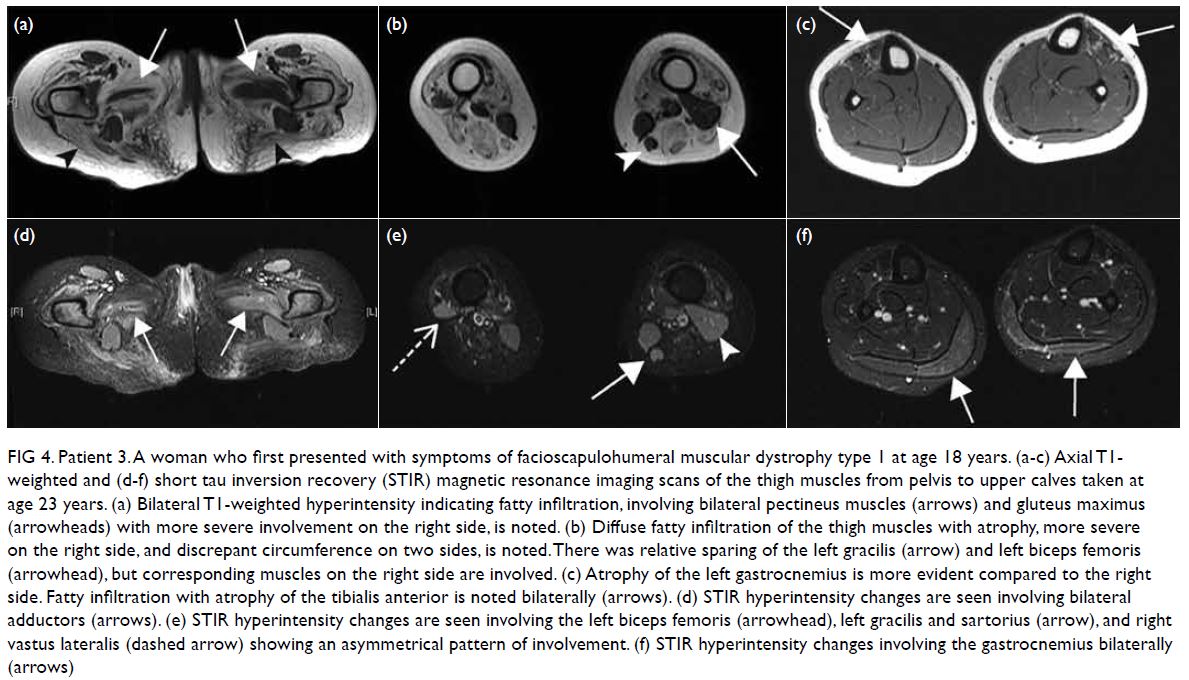
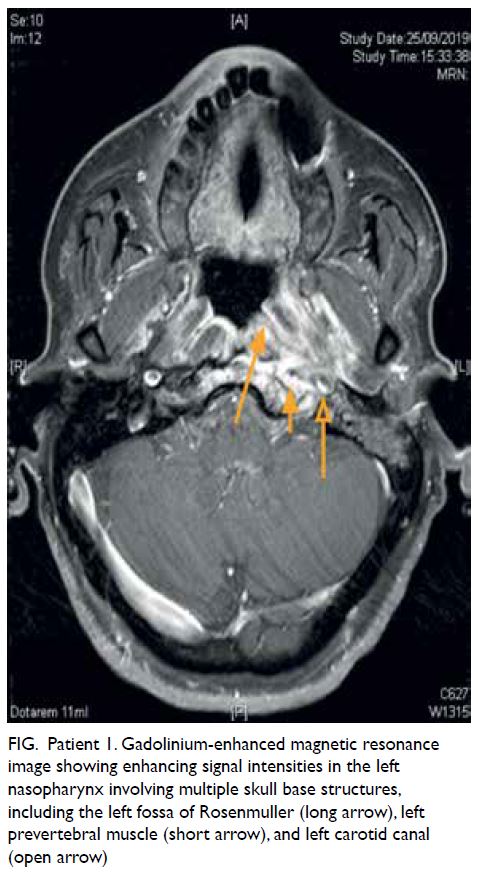
Figure 1. A 50-year-old man presented with a 3-week history of abdominal pain localising to the right upper quadrant, associated with low grade fever, chills, and fatigue. The patient was bitten by a horse 1 month before onset of symptoms. Clinical photograph of the bite wounds on the neck after blood cleansing
On admission, a contrast-enhanced computed
tomography (CT) scan of the abdomen revealed
three liver abscesses. He was treated initially with
intravenous antibiotic (amoxicillin/clavulanic
acid for 5 days before switching to piperacillin/tazobactam) but his clinical condition showed no
improvement. A new CT scan 5 days later showed
enlargement of the abscesses. Percutaneous drainage
of the abscesses was performed under ultrasound
guidance and the patient was transferred to our
private hospital for further management.
On arrival, the patient was haemodynamically
stable but clinically dehydrated, jaundiced, and
delirious. The abdomen was soft and non-tender,
with three abdominal drainage tubes in situ. A new
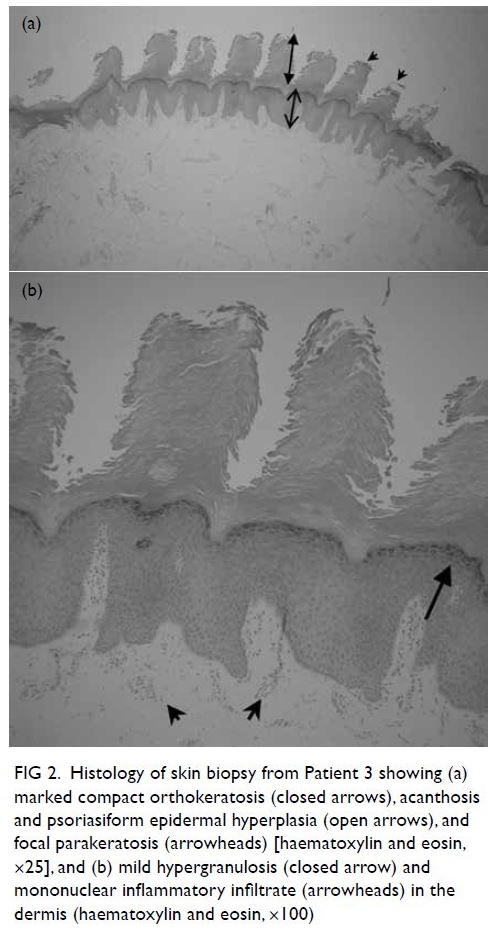
CT scan confirmed that the liver abscesses and
the drainage tubes were blocked, and they had to
be replaced (Fig 2). Drainage fluid afterwards was
noted to have the appearance of anchovy sauce and
amoebic infection was suspected.
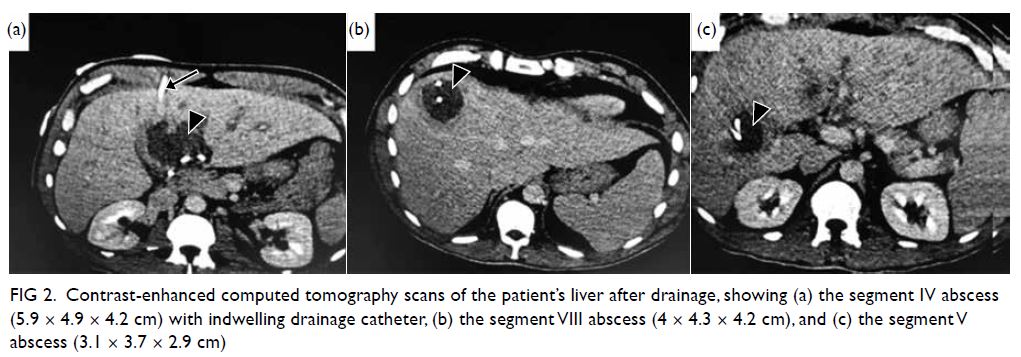
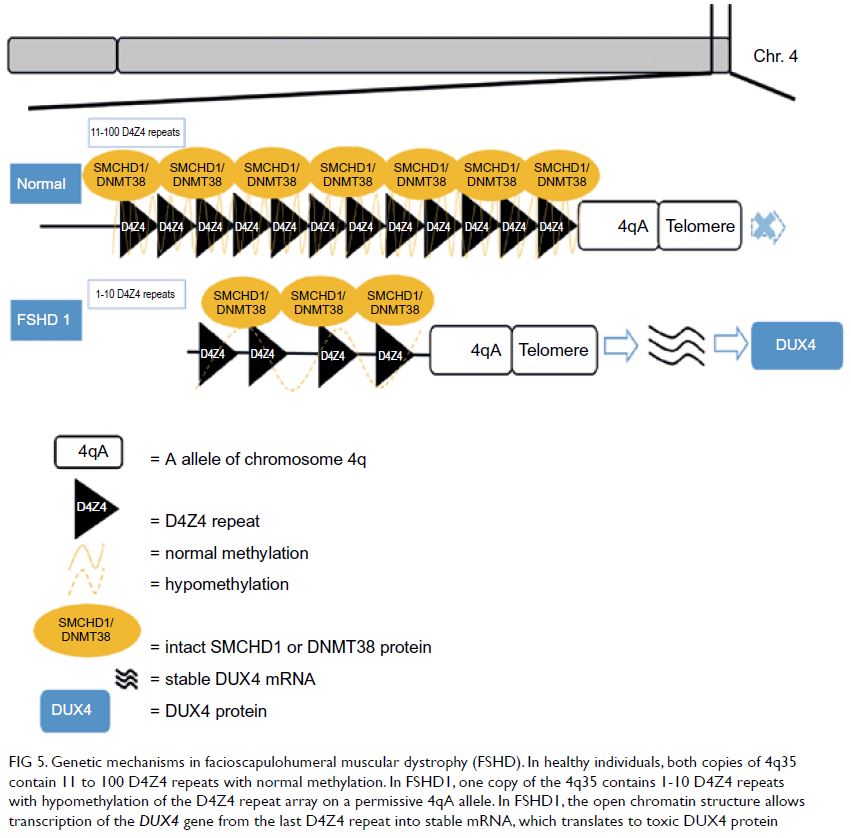
Figure 2. Contrast-enhanced computed tomography scans of the patient’s liver after drainage, showing (a) the segment IV abscess (5.9 × 4.9 × 4.2 cm) with indwelling drainage catheter, (b) the segment VIII abscess (4 × 4.3 × 4.2 cm), and (c) the segment V abscess (3.1 × 3.7 × 2.9 cm)
Blood tests on arrival showed leucocytosis
(white cell count 31 × 109/L) with markedly elevated
inflammatory markers (C-reactive protein 357 mg/L),
and deranged liver function (alkaline phosphatase
231 U/L, alanine aminotransferase 64 U/L, bilirubin
56 μmol/L and albumin 26.5 g/L). Blood cultures
were negative. No abnormalities were found on
colonoscopy and stool ova, cysts and parasite
microscopy were negative. Fluid drained from the
abscesses was negative on microscopy and culture,
but positive for Entamoeba histolytica DNA on
polymerase chain reaction. Initial amoebic serology
was negative for E histolytica immunoglobulin G
antibodies. Intravenous metronidazole was
commenced empirically in addition to piperacillin/
tazobactam. The patient subsequently made a steady
recovery. New amoebic serology demonstrated a
significant titre of E histolytica immunoglobulin G
antibodies 9 days later, indicating recent amoebic
infection. Repeated blood tests showed continued
normalisation of initial results and follow-up CT
scans showed progressive resolution of abscesses.
The patient was discharged from the hospital after
2 weeks.
Discussion
Amoebiasis is a leading parasitic infection in terms
of morbidity and mortality worldwide. It is most
prevalent in developing countries and in tropical
regions. It is caused by the protozoa, E histolytica,
transmitted via the faecal-oral route by ingestion
of contaminated food or water containing amoebic
trophozoites or cysts. Acute infection typically
presents as amoebic colitis. The most common
symptoms associated with amoebiasis are abdominal
pain (98% of cases), fever (74%), and dysentery
(30%).1 2 Although extraintestinal complications
of invasive infection are rare (<1% of cases),
liver abscesses are the most common secondary
manifestation, occurring when trophozoites invade
the colonic mucosa and penetrate mesenteric
venules to enter the portal circulation.3
This case was an unusual presentation of
amoebic liver abscess, confirmed by polymerase
chain reaction testing of aspirates and serology
results that suggested an acute infection. However,
the patient presented with no gastrointestinal
symptoms typical of amoebic colitis and stool
investigations and colonoscopy were normal. The
typical period of incubation for amoebiasis in those
with liver abscesses has been reported to be 8 to
20 weeks.4 In this case, the patient presented with
symptoms of invasive disease 4 weeks following
his horse bite. The patient denied recent travel to
an endemic area or any sick contacts. Southern
California is known to have many migrants from
South America where amoeba is prevalent. There
was little evidence from the patient’s clinical history or investigations to support a faecal-oral route of
transmission. As such, the possibility of liver abscess
from a cutaneous source was considered.
The patient was initially treated for pyogenic
liver abscess. Drainage of the abscesses and the
addition of metronidazole dramatically improved
his condition. It is conceivable that the horse bite
harboured amoebic trophozoites or otherwise
facilitated their invasion from contaminated
environmental water, soil, or vegetation. Just as
invasive trophozoites are known to reach the liver
by hematogenous dissemination to form abscesses,
in our patient they may have entered the circulation
via the bite wound to invade the liver. The short
incubation period was also consistent with direct
inoculation. Although cutaneous bacterial infection
leading to liver pyogenic abscesses is well reported
in the literature, the development of amoebic liver
abscesses from a similar source has not previously
been described. This may be the first described case
of amoebic liver abscesses of cutaneous origin and
warrants further study.
Author contributions
Concept or design: AKK Chui.
Acquisition of data: AKK Chui.
Analysis or interpretation of data: AKK Chui.
Drafting of the manuscript: Both authors.
Critical revision of the manuscript for important intellectual content: Both authors.
Acquisition of data: AKK Chui.
Analysis or interpretation of data: AKK Chui.
Drafting of the manuscript: Both authors.
Critical revision of the manuscript for important intellectual content: Both authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
As an editor of the Journal, AKK Chui was not involved in the peer review process for this article. The other author has
disclosed no conflicts of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the tenets of the Declaration of Helsinki. Patient consent was obtained.
References
1. Akgün Y, Taçyιlιdιz ÏH, Çelik Y. Amoebic liver abscess: changing trends over 20 years. World J Surg 1999;23:102-6. Crossref
2. Donovon AJ, Yellin AE, Ralls PW. Hepatic abscess. World J Surg 1991;15:162-9. Crossref
3. Swaminathan V, O’Rourke J, Gupta R, Kiire CF. An unusual presentation of an amoebic liver abscess:
the story of an unwanted souvenir. BMJ Case Rep
2013;2013:bcr2012006964. Crossref
4. Li E, Stanley SL Jr. Protozoa. Amebiasis. Gastroenterol Clin North Am 1996;25:471-92. Crossref