© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Posterior reversible encephalopathy syndrome
secondary to dengue fever: a case report
SW Cheo, MRCP (UK)1; HJ Wong, MRCP (UK)2; EK Ng, MRCP (UK)3; QJ Low, MRCP (UK)4; YK Chia, MRCP (UK)5
1 Department of Internal Medicine, Hospital Lahad Datu, Lahad Datu, Sabah, Malaysia
2 Department of Internal Medicine, Hospital Duchess of Kent, Sandakan, Sabah, Malaysia
3 Department of Internal Medicine, Hospital Tawau, Sabah, Malaysia
4 Department of Internal Medicine, Hospital Sultanah Nora Ismail, Batu Pahat, Johor, Malaysia
5 Department of Internal Medicine, Hospital Queen Elizabeth, Kota Kinabalu, Sabah, Malaysia
Corresponding author: Dr SW Cheo (cheosengwee@gmail.com)
Case report
A 15-year-old boy with good past health presented
with a 1-day history of fever, four episodes of vomiting,
and lethargy. He denied diarrhoea, headache, or
any neurological symptoms. On arrival, he had
blood pressure 106/36 mm Hg, heart rate 127 bpm,
temperature 39°C, and oxygen saturation 99% on air.
He had reduced pulse volume with normal capillary
refilling time. Systemic examination was otherwise
unremarkable. Full blood count analysis revealed
a haemoglobin level of 14.7 g/dL, white cell count
of 9.31 × 109/L, and platelet count of 240 × 109/L.
The patient’s blood test results were positive for
dengue non-structural protein 1, and negative for
dengue immunoglobulin M and immunoglobulin
G. His renal profile was normal, with mild raised
liver enzymes: alanine aminotransferase 82 U/L and
aspartate aminotransferase 310 U/L.
In view of the tachycardia and reduced pulse
volume, he was treated as a case of severe dengue
fever with compensated shock. He was admitted to
the intensive care unit and treated with fluid boluses
according to standard protocol. On day 3 of illness he
developed severe plasma leakage and was intubated
for respiratory distress. His condition soon stabilised
and he remained normotensive throughout his stay
in intensive care unit. He was extubated on day 7
when he entered a recovery phase. At that time, he
had a normal level of consciousness.
Three days later (day 10 of illness), he became
encephalopathic, disorientated and able to obey
only single step commands. Examination revealed
a Glasgow Coma Scale score of E4V4M6 with
normal motor power over both upper and lower
limbs. A plain computed tomography (CT) brain
scan showed hypodensities at bilateral occipital
regions and semiovale, predominantly involving the
white matter, and suggestive of posterior reversible
encephalopathy syndrome (PRES) [Fig 1]. A diagnostic lumbar puncture was offered but refused
by his parents. Blood and urine cultures were
negative and he was prescribed a 1-week course of intravenous meropenem. He subsequently improved
and was discharged well on day 16. Serum dengue
polymerase chain reaction was later confirmed as
DEN-2. At clinic follow-up, the patient was well and
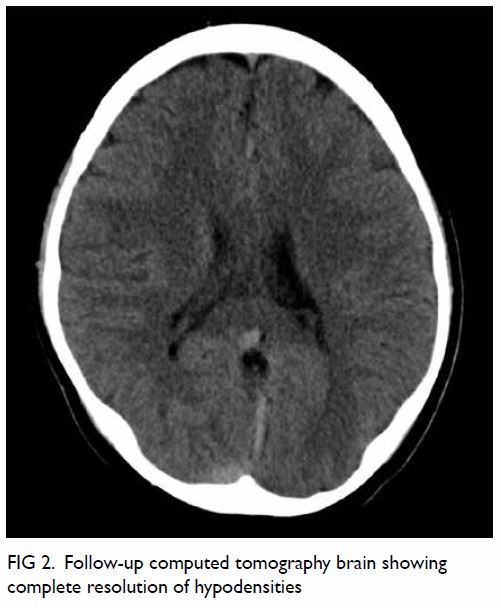
asymptomatic. Repeat CT brain at 3 months showed
complete resolution of white matter oedema (Fig 2).
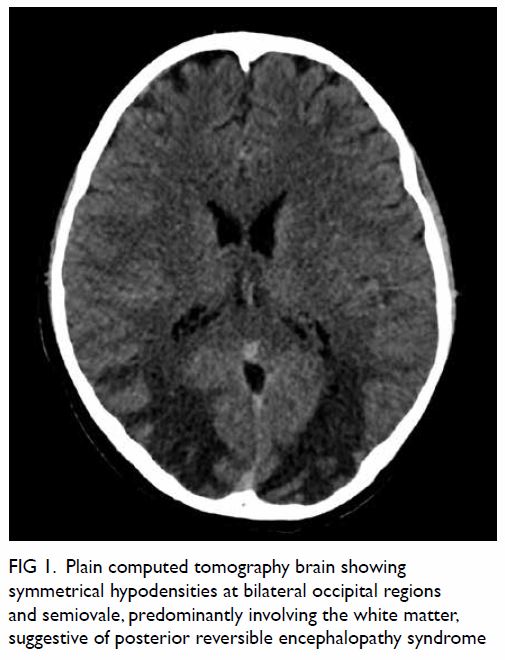
Figure 1. Plain computed tomography brain showing symmetrical hypodensities at bilateral occipital regions and semiovale, predominantly involving the white matter, suggestive of posterior reversible encephalopathy syndrome
Discussion and conclusion
Dengue fever is an important public health problem
in the tropics. At present, it is endemic in more
than 100 countries. The World Health Organization
estimates there to be around 390 million infections
per year with 96 million manifesting clinically.1 The
clinical features vary from those of a mild febrile illness to severe dengue with systemic complications.
Systemic complications include shock, bleeding,
plasma leakage, myocarditis and, in some patients,
neurological complications. The latter include
dengue encephalitis, dengue encephalopathy,
meningitis, acute disseminated encephalomyelitis,
and Guillain-Barré syndrome.2 Posterior reversible
encephalopathy syndrome can occur but is rare.
Posterior reversible encephalopathy
syndrome is a distinct clinicoradiological syndrome
characterised by headache, seizures, altered
consciousness and visual disturbances with the
presence of reversible brain vasogenic oedema.3 It can be caused by acute hypertension, hypertensive
disease in pregnancy, autoimmune diseases
(systemic lupus erythematosus, systemic sclerosis),
immunosuppressants, cytotoxic agents, renal failure,
and infection. An infectious aetiology such as virus
and gram-positive or gram-negative sepsis have all
been associated with PRES as has dengue virus.4 5
The pathophysiology of PRES in dengue is still
not fully understood. It is postulated to be related
to endothelial dysfunction4 that subsequently leads
to vasogenic oedema and decreased cerebral blood
flow. In addition, inflammatory cytokines can
increase vascular permeability with consequent
interstitial brain oedema.3 Neuroimaging is essential
for diagnosis with characteristics that include
vasogenic oedema in the parieto-occipital region of
both cerebral hemispheres. The subcortical white
matter is always affected. Three primary patterns
of magnetic resonance imaging (MRI) brain have been described, namely dominant parieto-occipital
pattern, holohemispheric watershed pattern, and
superior frontal sulcus pattern. Treatment is mainly
supportive with removal of causative factors.
In our patient, diagnosis of PRES was
based on clinical and radiological features. He
developed acute onset encephalopathy coupled with
typical neuroimaging features. Brain CT showed
symmetrical hypodensities at bilateral occipital
lobes and predominantly involving white matter,
suggestive of PRES. He was treated conservatively,
and he improved. Follow-up CT brain revealed
complete resolution of vasogenic oedema. In terms of
aetiology, our patient had no history of hypertension
or autoimmune disease or recent cytotoxic agent. In
addition, blood pressure remained stable throughout
his hospital stay with no decompensated shock. The
limitation of our case is the lack of MRI facilities.
Compared with CT, MRI better delineates lesions
and is preferable if available.
In terms of timing of PRES in relation to dengue
fever, PRES has been reported to have occurred
on day 5 to 6 of illness in one case,4 and within the
non-structural protein 1 antigen positive period
(first 7 days of illness) in another.5 In our case, it
occurred on day 10 of illness and was considered
compatible with the cases reported previously. A
diagnosis of PRES secondary to dengue is one of
exclusion, supported by typical imaging features and
within a reasonable time frame. Alternative causes of
PRES should be actively searched for and excluded
in every case.
In conclusion, this case highlights PRES as
an important differential diagnosis in a dengue
patient with neurological deficits. It is important to
differentiate PRES from other clinical syndromes
since management is mainly supportive and by
controlling the underlying conditions. Neuroimaging
plays an important role in establishing the diagnosis
in the compatible clinical context. The prognosis
of PRES is usually good. However, more studies
are needed to further elucidate the underlying
pathophysiology of PRES in dengue.
Author contributions
Concept or design: SW Cheo and QJ Low.
Acquisition of data: SW Cheo and HJ Wong.
Analysis or interpretation of data: HJ Wong and EK Ng.
Drafting of the manuscript: SW Cheo and QJ Low.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: SW Cheo and HJ Wong.
Analysis or interpretation of data: HJ Wong and EK Ng.
Drafting of the manuscript: SW Cheo and QJ Low.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
The authors have disclosed no conflicts of interest.
Funding/support
This case report received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki. The patient provided written informed consent
for all treatments and procedures, and written consent for
publication.
References
1. World Health Organization. Dengue and severe dengue: Fact sheet. Available from: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.Accessed 4 Nov 2019.
2. Murthy JM. Neurological complications of dengue infection. Neurol India 2010;58:581-4. Crossref
3. Fugate JE, Rabinstein AA. Posterior reversible
encephalopathy syndrome: clinical and radiological
manifestations, pathophysiology, and outstanding
questions. Lancet Neurol 2015;14:914-25. Crossref
4. Mai NT, Phu NH, Nghia HD, et al. Dengue-associated posterior reversible encephalopathy syndrome, Vietnam.
Emerg Infect Dis 2018;24:402-4. Crossref
5. Sohoni CA. Bilateral symmetrical parieto occipital involvement in dengue infection. Ann Indian Acad Neurol
2015;18:358-9. Crossref