Embolisation for thoracic paraspinal extramedullary haematopoiesis complicated by haemothorax: a case report
Hong Kong Med J 2026 Feb;32(1):62–5 | Epub 2 Feb 2026
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Embolisation for thoracic paraspinal extramedullary haematopoiesis complicated by haemothorax: a case report
KH Chu, MB, BS; L Xu, MB, BS, FHKAM (Radiology); HS Fung, MB, ChB, FHKAM (Radiology)
Department of Diagnostic and Interventional Radiology, Queen Elizabeth Hospital, Hong Kong SAR, China
Corresponding author: Dr L Xu (xl599@ha.org.hk)
Case presentation
A 37-year-old male with thalassaemia intermedia
(alpha and beta) had undergone cholecystectomy
and splenectomy in childhood, but had received
no regular transfusions or medications since his
haemoglobin (around 8 g/dL) and ferritin levels
(approximately 4300 pmol/L) remained stable.
He had multiple extramedullary haematopoietic
(EMH) lesions in the bilateral paraspinal regions,
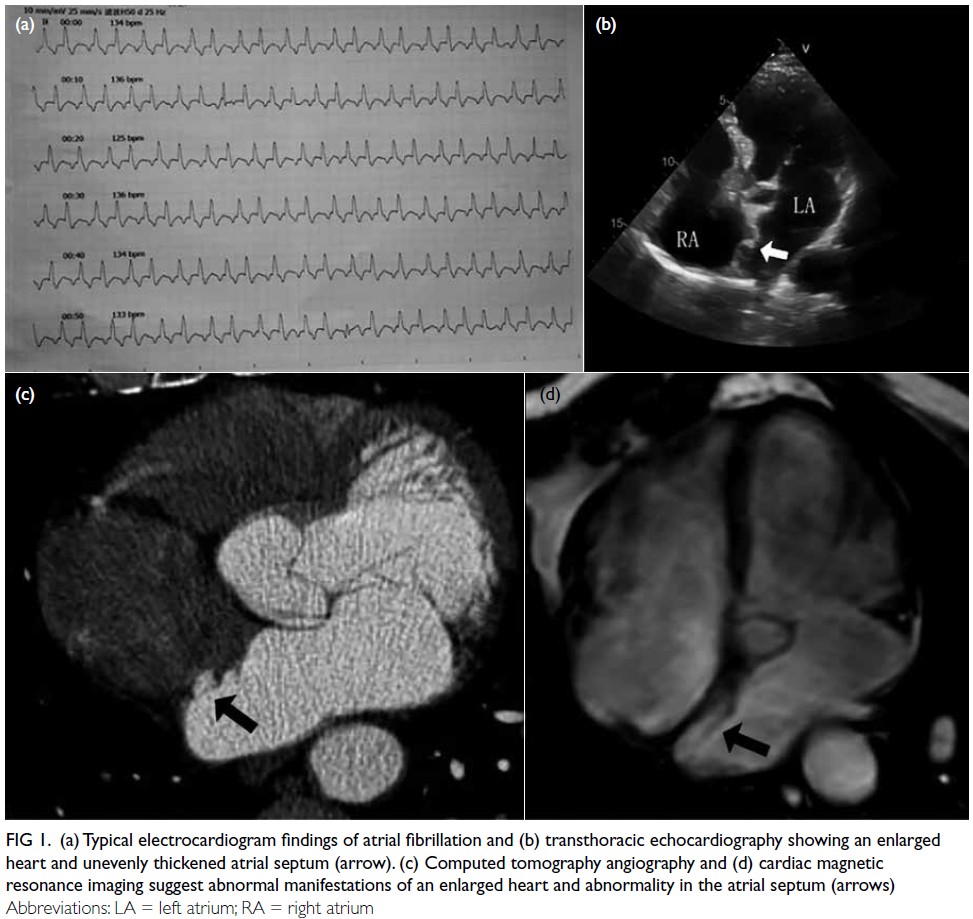
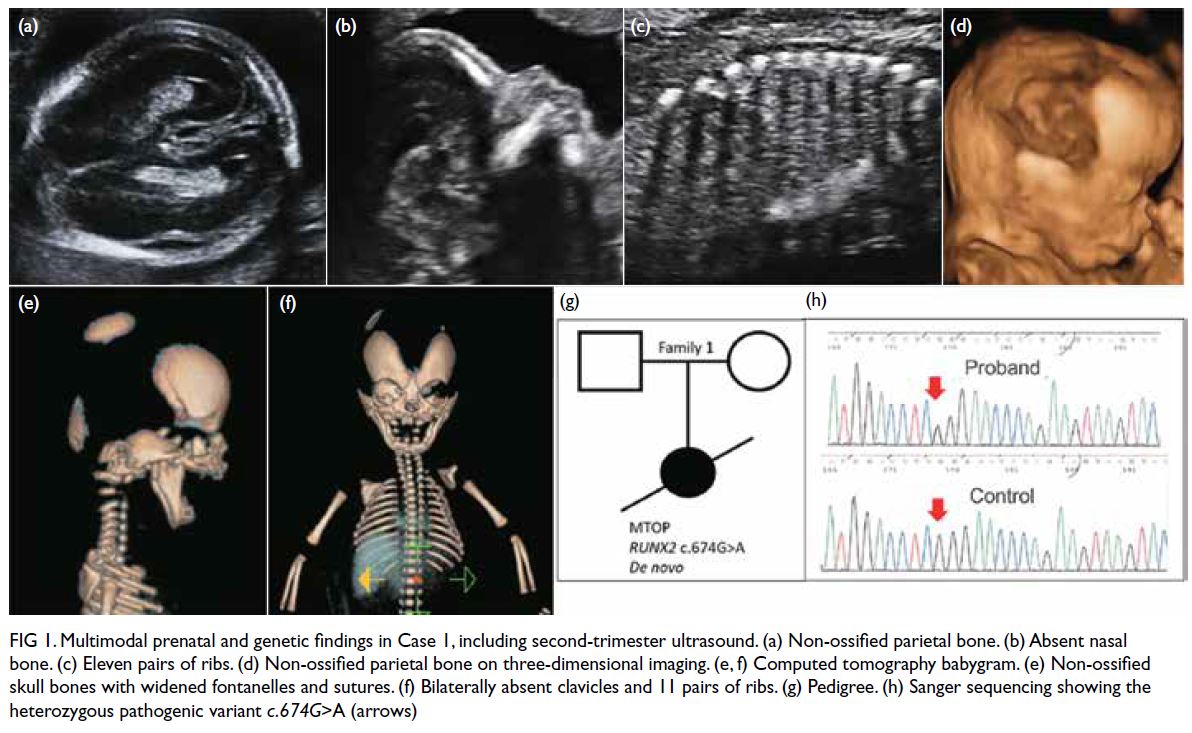
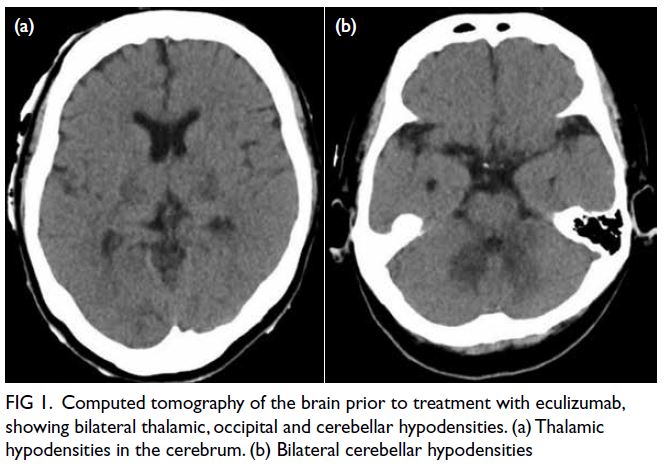
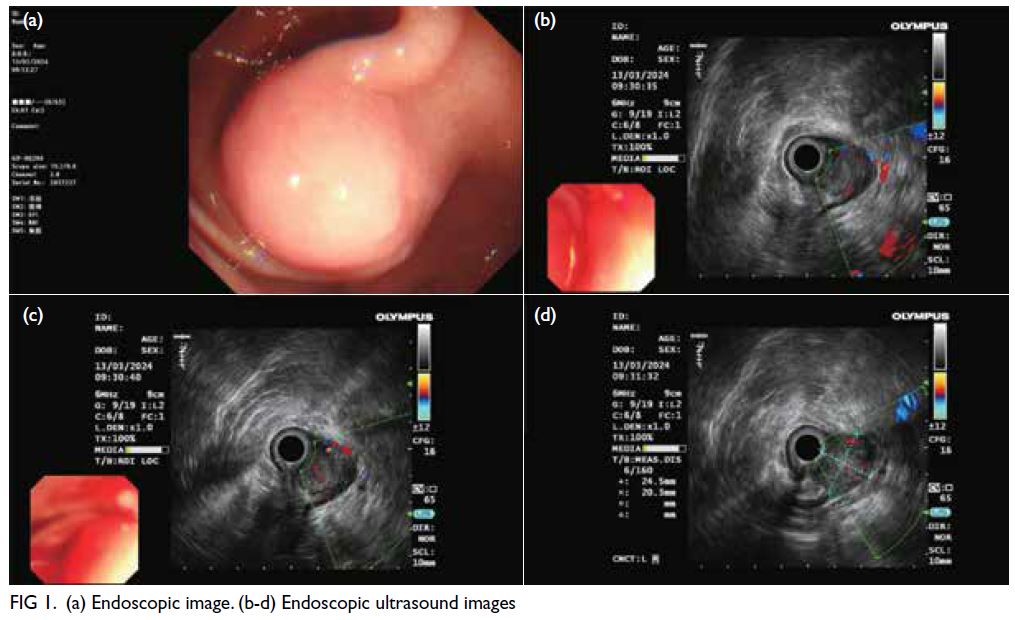
evident on previous magnetic resonance imaging (Fig 1a). Following a recent viral infection in January
2025 with nasopharyngeal swab testing positive for
influenza A and B and respiratory syncytial virus, he
reported back pain and dark-coloured urine. Blood
tests revealed a drop in haemoglobin level to 4.9 g/dL.
The working diagnosis was haemolysis precipitated
by infection. An initial computed tomography (CT)
of the thorax revealed bilateral pleural effusions and
the known EMH, but no evidence of haemorrhage
(Fig 1b).
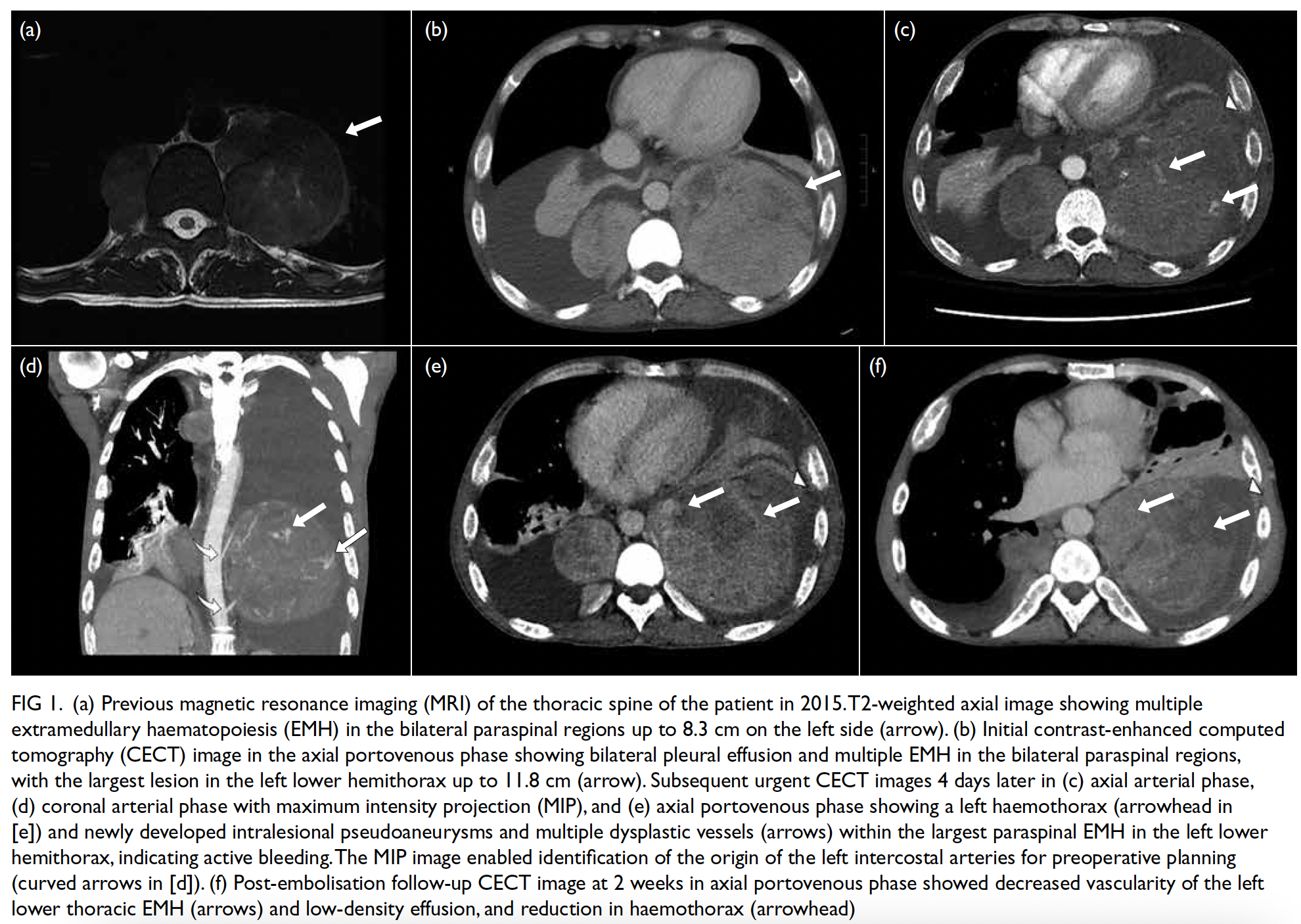
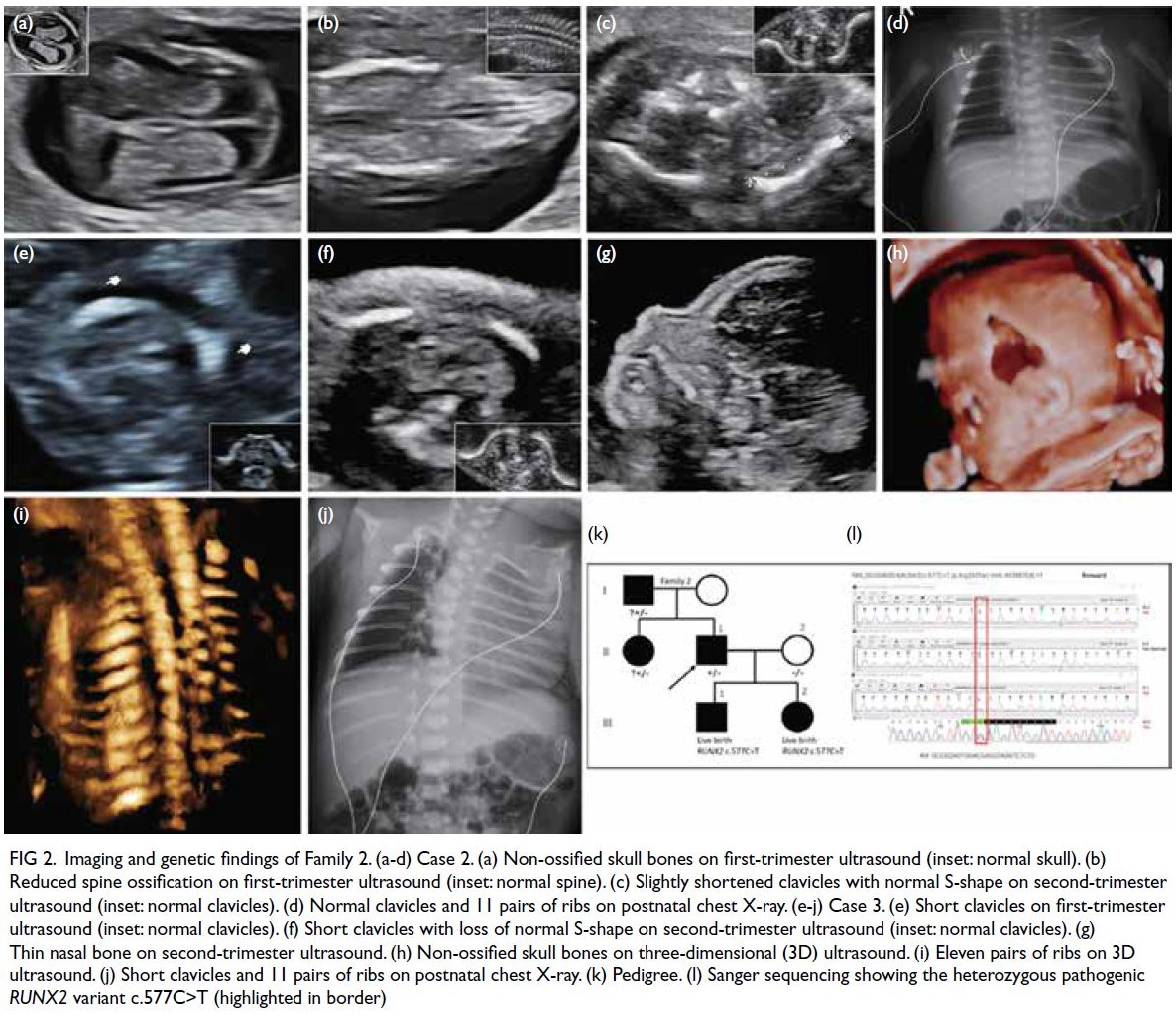
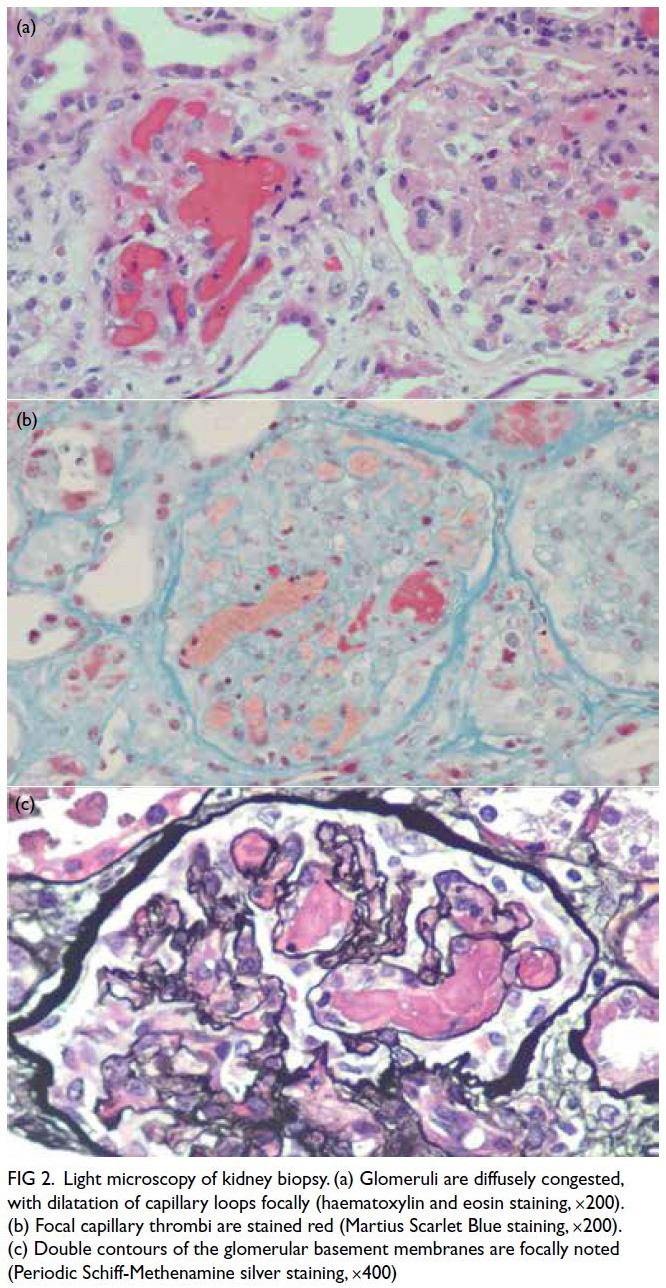
Figure 1. (a) Previous magnetic resonance imaging (MRI) of the thoracic spine of the patient in 2015. T2-weighted axial image showing multiple extramedullary haematopoiesis (EMH) in the bilateral paraspinal regions up to 8.3 cm on the left side (arrow). (b) Initial contrast-enhanced computed tomography (CECT) image in the axial portovenous phase showing bilateral pleural effusion and multiple EMH in the bilateral paraspinal regions, with the largest lesion in the left lower hemithorax up to 11.8 cm (arrow). Subsequent urgent CECT images 4 days later in (c) axial arterial phase, (d) coronal arterial phase with maximum intensity projection (MIP), and (e) axial portovenous phase showing a left haemothorax (arrowhead in [e]) and newly developed intralesional pseudoaneurysms and multiple dysplastic vessels (arrows) within the largest paraspinal EMH in the left lower hemithorax, indicating active bleeding.The MIP image enabled identification of the origin of the left intercostal arteries for preoperative planning (curved arrows in [d]). (f) Post-embolisation follow-up CECT image at 2 weeks in axial portovenous phase showed decreased vascularity of the left lower thoracic EMH (arrows) and low-density effusion, and reduction in haemothorax (arrowhead)
A few days later, the patient developed sudden
chest pain, with tachycardia and hypotension (blood
pressure: 82/43 mm Hg). Urgent CT of the thorax
revealed a left haemothorax and blood products
adjacent to the largest paraspinal EMH in the left
lower hemithorax, along with new intralesional
pseudoaneurysms and multiple dysplastic vessels,
indicative of active bleeding (Fig 1c-e). A left chest
drain was placed, yielding 1.3 L of heavily blood-stained
fluid. He was referred to interventional
radiologists for urgent embolisation to control the
bleeding.
Urgent embolisation was performed under
local anaesthesia. A 5-Fr Mikaelsson catheter (Merit
Medical, South Jordan [UT], United States) was
inserted via transfemoral access to catheterise the
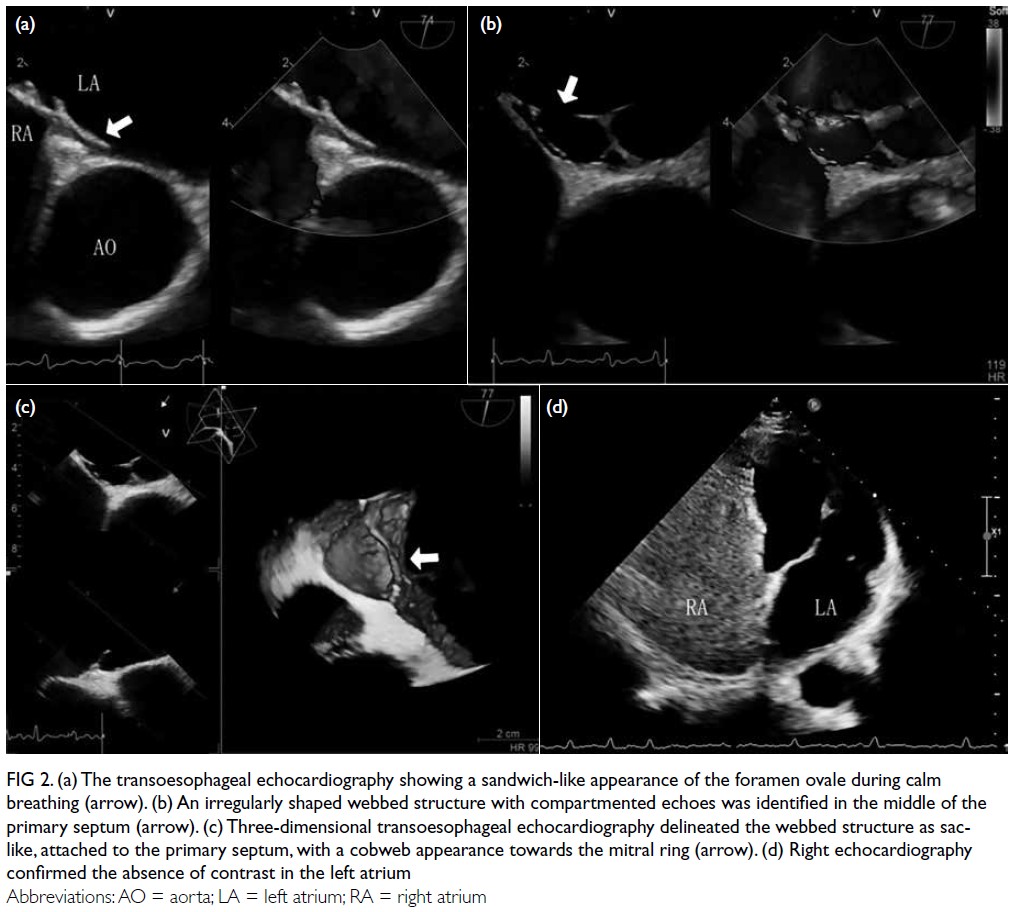
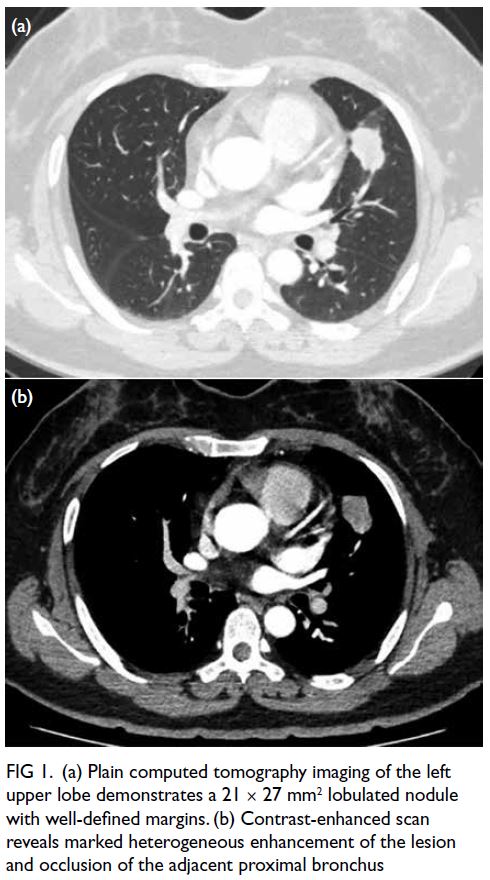
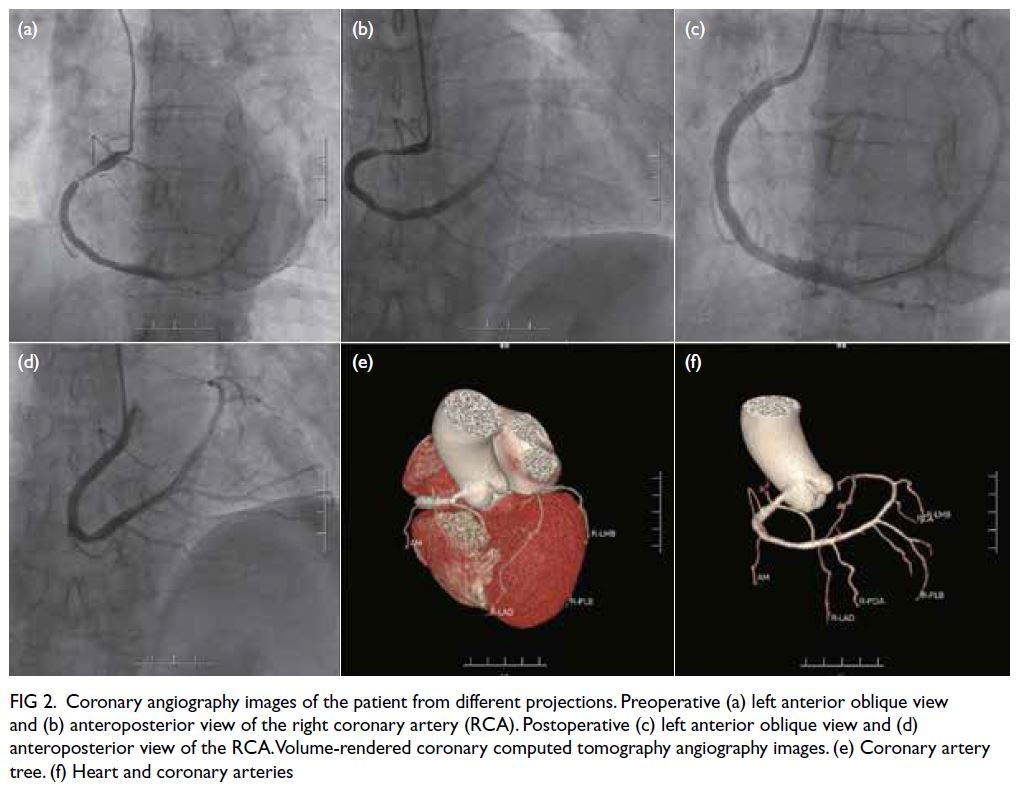
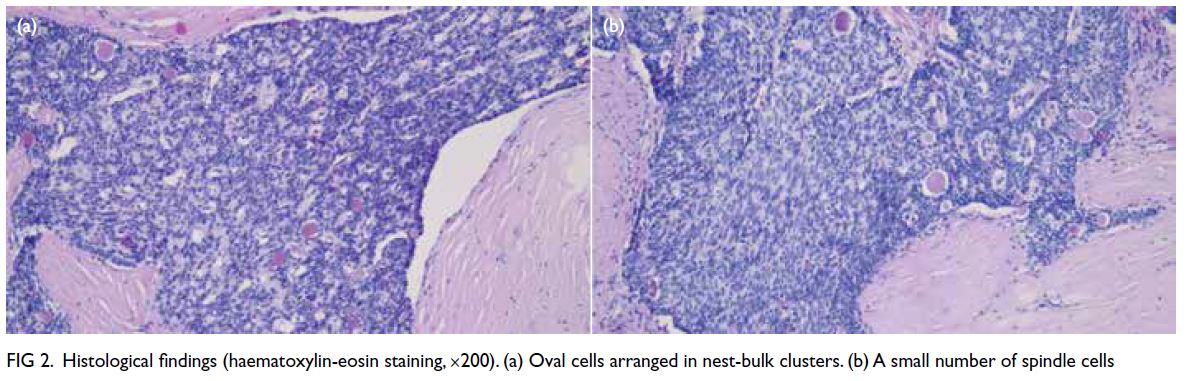
left lower intercostal arteries. Digital subtraction
angiography revealed abnormal, tortuous vessels
with small pseudoaneurysms arising from the left
10th and 11th intercostal arteries and supplying the dominant left lower thoracic EMH (Fig 2a and b). Selective cannulation of these arteries was
performed using a 2.1-Fr Maestro microcatheter
(Merit Medical). Superselective embolisation
was then performed at several branches using a
combination of 700-900 μm Embosphere (Merit
Medical) and 710-1000 μm EGgel (ENGAIN,
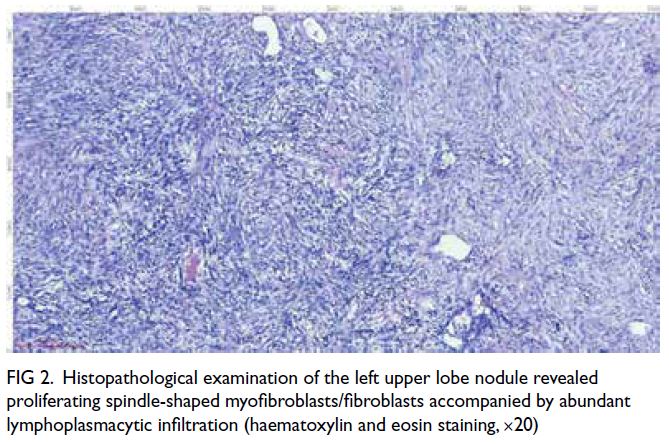
Hwaseong-si, South Korea). A postprocedural
angiogram showed successful devascularisation of
the lesion and obliteration of the pseudoaneurysms
(Fig 2c and d).
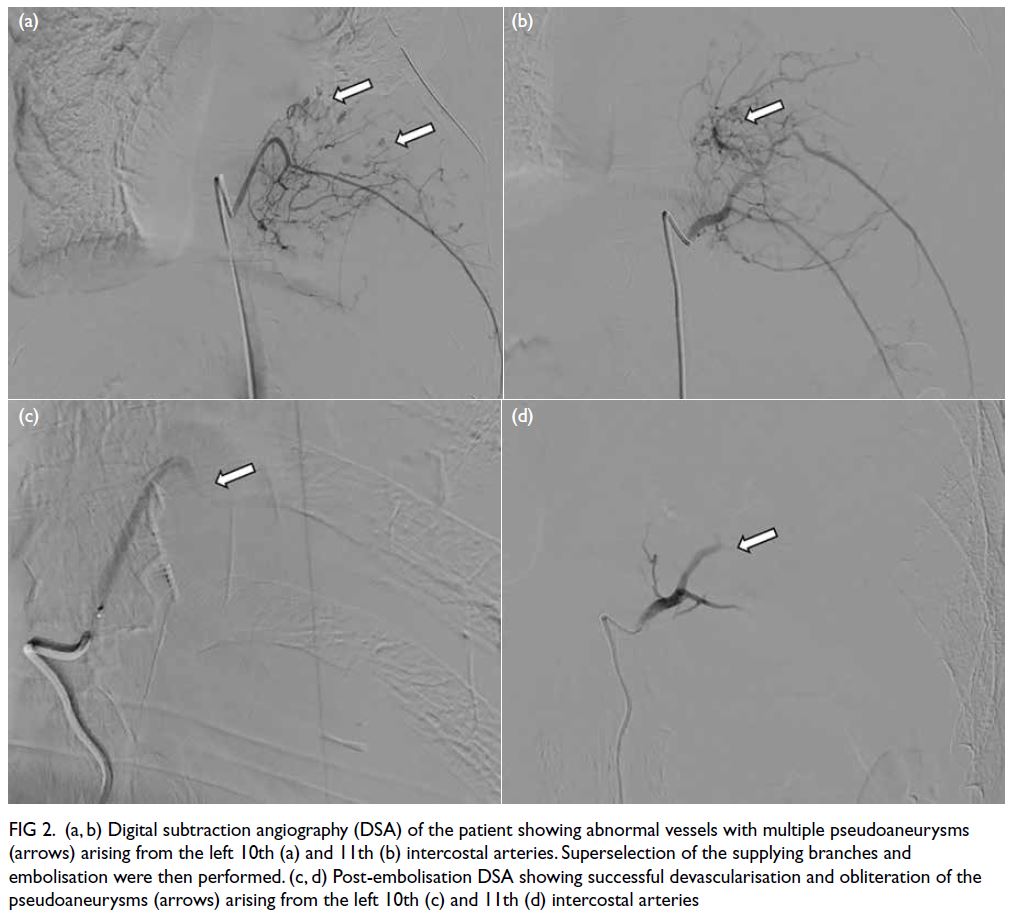
Figure 2. (a, b) Digital subtraction angiography (DSA) of the patient showing abnormal vessels with multiple pseudoaneurysms (arrows) arising from the left 10th (a) and 11th (b) intercostal arteries. Superselection of the supplying branches and embolisation were then performed. (c, d) Post-embolisation DSA showing successful devascularisation and obliteration of the pseudoaneurysms (arrows) arising from the left 10th (c) and 11th (d) intercostal arteries
Following the procedure, the patient’s vital signs
normalised and there were no neurological deficits.
His haemoglobin level stabilised at 7 to 8 g/dL and
chest drain was later removed due to minimal output.
Follow-up CT 2 weeks later showed a reduction in
the left haemothorax and decreased vascularity of
the left lower thoracic EMH (Fig 1f). The patient was
discharged and remains asymptomatic to date, with
no clinical evidence of re-bleeding.
Discussion
Extramedullary haematopoiesis refers to the
compensatory production of blood cells outside of
the bone marrow, typically occurring in patients with
insufficient bone marrow function, such as those
with thalassaemia. Diagnosis can be made clinically
and radiologically, especially when the lesions are
multifocal or bilateral, exhibiting characteristic
iron deposition or fatty replacement on imaging.1
Paraspinal EMH can lead to complications such as
spinal cord compression or, rarely, haemothorax
due to rupture and bleeding into the pleural cavity.
In our patient, it was hypothesised that haemolysis
from the recent infection increased the demand for
haematopoiesis, stimulating the existing EMH to
recruit additional blood vessels under stress. This
angiogenesis ultimately led to intralesional bleeding,
pseudoaneurysm formation and haemothorax.
There are no established evidence-based
guidelines for the treatment of EMH. Management
depends on lesion size and location, as well as the
patient’s clinical condition.2 In uncomplicated cases,
hypertransfusions aimed at correcting anaemia
and reducing haematopoietic demand can shrink
EMH lesions. Radiotherapy may also be used, as
haematopoietic tissue is radiosensitive and tends
to regress following irradiation. Nonetheless, when
complications such as haemorrhage arise, more
urgent intervention is needed. Thoracotomy with
surgical excision has traditionally been performed,
but emergency surgery carries higher risks of bleeding
and other complications.3 Embolisation has emerged
as a mainstay treatment for many haemorrhagic
conditions due to its versatility and precision. Our
case demonstrated its viability in EMH-related
haemorrhage, enabling accurate identification of
bleeding vessels and prompt haemostasis while
minimising the risks of more invasive surgery.
To ensure a safe and effective embolisation,
meticulous planning and identification of the
target vessels are essential, including superselective
cannulation to prevent non-target embolisation.
Spinal cord feeders can arise from intercostal
arteries and are identified by their characteristic
hairpin appearance as they course medially to
the vertebral pedicle.4 In particular, the artery of
Adamkiewicz, the largest anterior medullary branch
to the anterior spinal artery, commonly arises at
left-sided T9 to T12 levels. Reflux into these arteries
can lead to spinal cord ischaemia. A balance must
be struck between complete devascularisation of
the lesion and the risk of non-target embolisation.
Larger embolic agents, such as particles larger than
350 μm, are theoretically safer as they are too large
to enter the small-calibre spinal arteries. Embolic
agents should be injected slowly under fluoroscopic
guidance, with close monitoring for any interval
appearance of spinal artery supply or reflux.
The choice of embolic agents is important and
depends on factors such as the location of target
vessels, proximity to vital structures, and operator
experience. In our case, a combination of permanent
and temporary particulates was used to achieve
haemostasis. Embospheres are non-absorbable,
calibrated microspheres available in various sizes.
The 700-900 μm size was chosen to prevent entry
into spinal arteries. These provide a long-term
embolic effect with predictable delivery.5 EGgel (710-1000 μm), a porcine-derived gelatin microparticle,
was used for further proximal embolisation. It
offers temporary embolisation, complementing
Embospheres by preventing vessel recanalisation
under high intraluminal pressure.
Haemorrhage associated with EMH is a
critical condition requiring prompt and effective
intervention. Embolisation can be life-saving in
such cases. Although further studies are needed to
assess long-term outcomes, embolisation should be
considered part of the multidisciplinary management
of patients with EMH.
Author contributions
Concept or design: KH Chu, L Xu.
Acquisition of data: KH Chu, L Xu.
Analysis or interpretation of data: KH Chu, L Xu.
Drafting of the manuscript: KH Chu, L Xu.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: KH Chu, L Xu.
Analysis or interpretation of data: KH Chu, L Xu.
Drafting of the manuscript: KH Chu, L Xu.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the Central Institutional Review
Board of Hospital Authority, Hong Kong (Ref No.: CIRB-2025-064-2). The patient provided written informed consent
for all treatments and procedures, and for publication of the
case report, including the accompanying clinical images.
References
1. Hughes M. Rheumatic manifestations of haemoglobinopathies. Curr Rheumatol Rep 2018;20:61. Crossref
2. Gupta S, Krishnan AS, Singh J, Gupta A, Gupta M.
Clinicopathological characteristics and management of
extramedullary hematopoiesis: a review. Pediatr Hematol
Oncol J 2022;7:182-6. Crossref
3. Pornsuriyasak P, Suwatanapongched T, Wangsuppasawad N,
Ngodngamthaweesuk M, Angchaisuksiri P. Massive
hemothorax in a beta-thalassemic patient due to
spontaneous rupture of extramedullary hematopoietic masses: diagnosis and successful treatment. Respir Care 2006;51:272-6.
4. Papalexis N, Peta G, Gasbarrini A, Miceli M, Spinnato P,
Facchini G. Unraveling the enigma of Adamkiewicz:
exploring the prevalence, anatomical variability, and clinical impact in spinal embolization procedures for bone
metastases. Acta Radiol 2023;64:2908-14. Crossref
5. Wang CY, Hu J, Sheth RA, Oklu R. Emerging embolic
agents in endovascular embolization: an overview. Prog
Biomed Eng (Bristol) 2020;2:012003. Crossref


 Three video clips showing the webbed left atrial septal pouch and contrast flow are available at
Three video clips showing the webbed left atrial septal pouch and contrast flow are available at