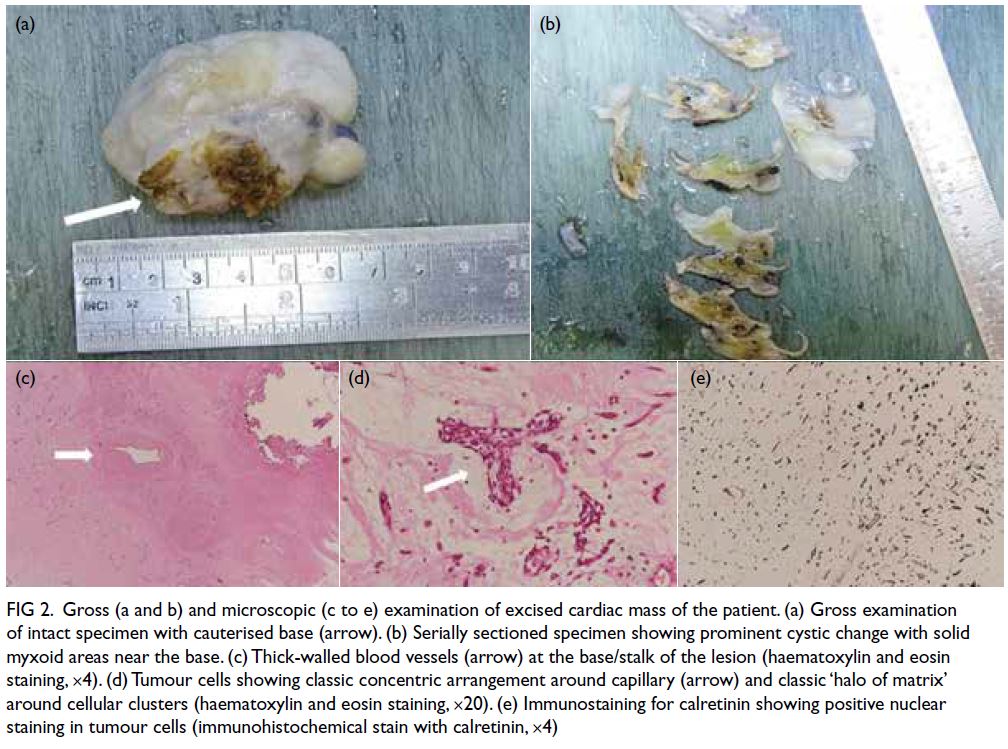
Primary penile tuberculosis masquerading as penile cancer: a case report
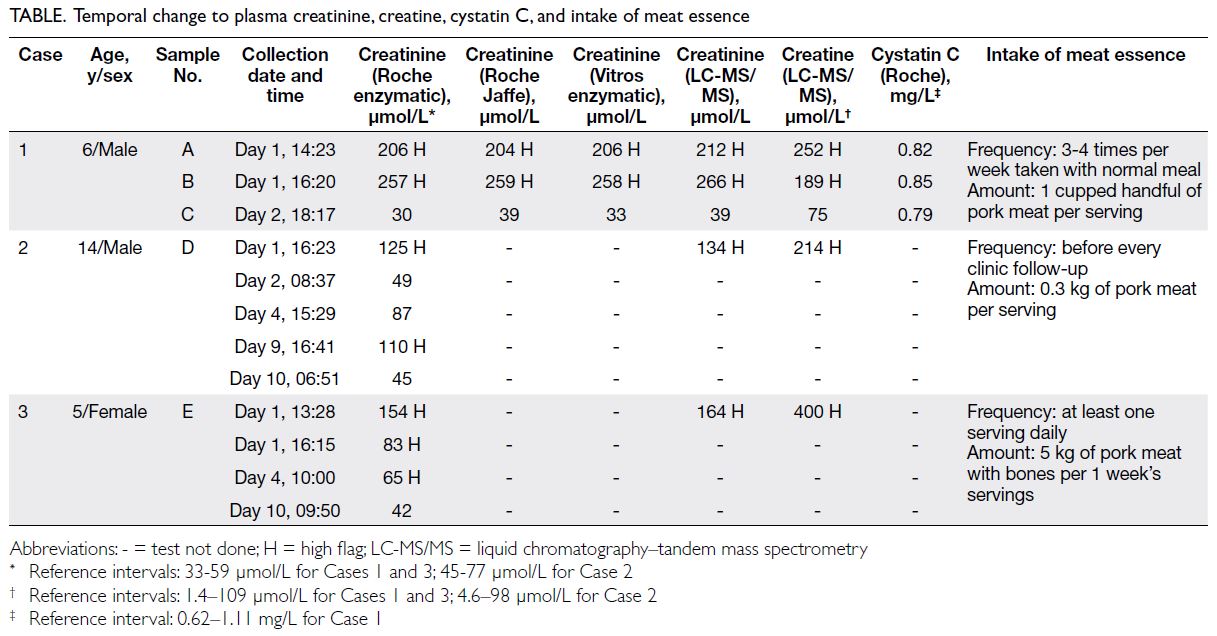
Hong Kong Med J 2023 Dec;29(6):554–5 | Epub 2 Nov 2023
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Primary penile tuberculosis masquerading as penile cancer: a case report
Ankitkumar Sharma, MS, DrNB (Urology); Deepak Velmurugan, MB, BS; Kuppurajan Narayanasamy, MS, FRCS (Urology)
Department of Urology, Kovai Medical Center and Hospital, Coimbatore, India
Corresponding author: Dr Ankitkumar Sharma (dr.ankitkumarsharma@gmail.com)
Case presentation
A 53-year-old man from northwest Tamil Nadu,
India presented to our institution in January 2021
with a 3-month history of new-onset phimosis
with swelling of the glans and an ulcer and penile
discharge. The lesion presented initially with
seropurulent discharge from the preputial sac. The
patient later noticed some induration over the dorsal
aspect that progressed further to an ulcer with
frank purulent discharge. He had no family history
of tuberculosis (TB) and no venereal disease or
significant past surgical intervention. He had type 2
diabetes mellitus controlled by regular medication.
His spouse had no significant history of pelvic
inflammatory disease or secondary infertility and
no present complaint of vaginal discharge, lower
abdominal pain or productive cough.
On examination, the patient was of average
built and nourishment. Vital signs were stable and
examination of the respiratory, cardiovascular and
gastrointestinal systems was unremarkable. Local
examination of the external genitalia revealed
staining of garments, phimosis, and an ulcer of
1×1 cm in size over the dorsal aspect of the penis
with foul smelling purulent discharge near the
corona. The glans palpable through the prepuce
was hard in consistency and non-tender. The penile
shaft was indurated up to the base and non-tender.
There was no significant inguinal lymphadenopathy.
Testes, spermatic cord and scrotum were clinically
normal.
Blood investigations revealed haemoglobin
level of 13.1 g/dL, white blood cell count of 6900/mm3,
neutrophil level of 60%, platelet count of 234 000/μL,
and glycated haemoglobin level of 7.8%; serology
showed that human immunodeficiency virus,
hepatitis B surface antigen and syphilis were non-reactive.
Ultrasound of bilateral groin revealed a
few inguinal nodes bilaterally, with the largest on
the right measuring 8.7 mm. All nodes showed
preserved fatty hilum and normal vascularity.
With the clinical diagnosis of localised
carcinoma penis in mind, the patient was counselled
about the need for intervention in the form of
penile biopsy followed by total amputation of the penis with perineal urethrostomy. Under spinal
anaesthesia, a dorsal slit was made in the penis that
revealed penile oedema with purulent discharge
from the preputial edge, multiple small granulomas
and a hard and purulent cover over the glans. The
glans penis was partially necrosed but the meatus
was preserved. On extension of the dorsal slit to
the base, the shaft was found to be covered in pus
and slough with a hard consistency of the corpora
cavernosa. Penile biopsy was taken from the glans
and shaft. The slit cut surface was approximated at
the base and haemostatic sutures placed over the
glans to let the wound drain. A 16-Fr catheter was
passed. Postoperatively, the patient was started
on 4.5-g piperacillin sodium and tazobactam
sodium via intravenous route three times a day. His
postoperative course was uneventful with the wound
showing purulent discharge, managed with twice
daily saline dressings. The possibility of infectious
origin of the disease was discussed with the patient.
After discussion with the pathologist, a
provisional report was obtained of granulomatous
inflammation with a few areas of necrosis. The need
for culture and sensitivity was discussed with the
patient, but due to financial constraints the patient
was unwilling to proceed. He was initiated on anti-tubercular
therapy as per regional antimicrobial
policy. The final histopathology confirmed
necrotising and granulomatous inflammation, with
no acid-fast bacilli noted.
The patient and his partner were screened
for pulmonary and genitourinary TB after the final
histopathology report. The patient was discharged
on postoperative day 9 with instructions to continue
ATT for 6 months. He was advised to attend a
local clinic for dressing changes every week until
the wound had healed. The wound showed signs
of healing on follow-up (Fig 1). After 6 weeks there
were signs of build-up of slough. At 8 weeks, after
completion of the intensive phase, the patient was
referred for local surgical debridement and wound
closure due to build-up of slough over the wound
surface. He completed the course of ATT and the
wound healed well (Fig 2). He has since developed
erectile dysfunction, probably owing to secondary fibrosis of the corpora cavernosa. He does not desire
treatment at this time.
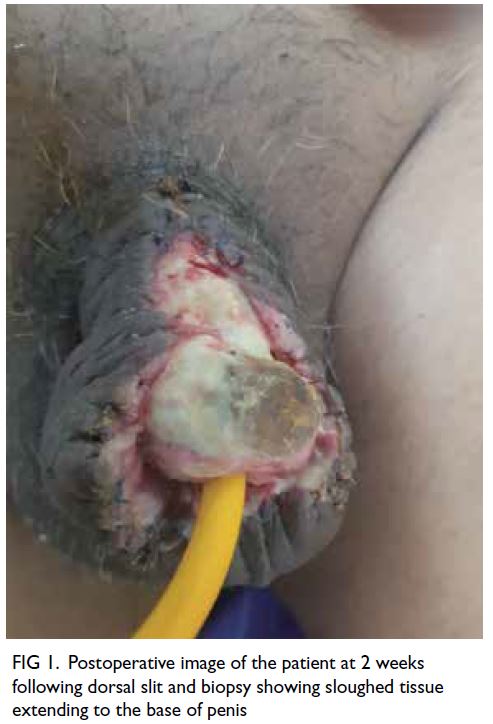
Figure 1. Postoperative image of the patient at 2 weeks following dorsal slit and biopsy showing sloughed tissue extending to the base of penis
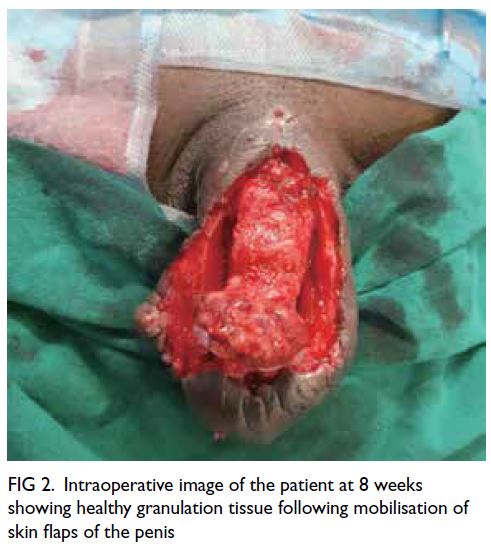
Figure 2. Intraoperative image of the patient at 8 weeks showing healthy granulation tissue following mobilisation of skin flaps of the penis
Discussion
Genitourinary TB is the most common form of
extrapulmonary TB, but penile presentation is one of
the least common forms of genitourinary TB (<1%).1
The epididymis (42%) followed by seminal vesicle
(23%), prostate (21%), testis (15%) and vas deferens
(12%) are common sites of presentation.2 In its
primary form, local spread of bacilli from clothing,
ejaculation, endometrial secretions, circumcision,
and intravesical Bacillus Calmette–Guérin has been
reported.2 The secondary form arises due to the
subsequent complication of lung TB or TB in other
parts of the urogenital tract, extending through the
urethra or via a haematogenous route.
Tuberculosis of penis may affect the skin,
glans penis or cavernous bodies. It can mimic penile
carcinoma in presentation, much like our patient.
Tuberculosis affecting the glans penis can present as
tuberculous chancre, papulo-necrotic tuberculoid,
TB cutis orificialis or tuberculous gumma. In most
cases, the lesion takes the form of an ulcer that is
difficult to differentiate from a malignant tumour.
The lesion can be extensive, with involvement of the
urethra and corpus cavernosum. Since young adults
are affected, their partner should always be evaluated
for genital TB.3 Although there are several tests
for diagnosis of TB, biopsy remains confirmatory.
Surgical management may be required in doubtful
cases in spite of successful treatment with ATT.
Organ sparing surgery coupled with ATT should be
the goal of treatment.4
Our experience highlights the similarities in
presentation of penile carcinoma and primary penile
TB, further cementing the need for a dorsal slit prior
to definitive procedure. A high index of suspicion
should be maintained considering the endemic
status of TB in South-East Asian countries. Biopsy
should always be performed in doubtful cases.
Author contributions
Concept or design: A Sharma.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: A Sharma.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: A Sharma.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki. Written informed consent was obtained from the patient for publishing of data relevant to the case report.
References
1. Nimisha E, Gupta G. Penile lupus vulgaris: a rare presentation of primary cutaneous tuberculosis. Int J STD AIDS 2014;25:969-70. Crossref
2. Amir-Zargar MA, Yavangi M, Ja’fari M, Mohseni MJ. Primary tuberculosis of glans penis: a case report. Urol J 2004;1:278-9.
3. Gangalakshmi C, Sankarmahalingam. Tuberculosis of glans penis—a rare presentation. J Clin Diagn Res 2016;10:PD05-6. Crossref
4. Rajeev TP, Pranab KR, Phukan PK, Baruna SK, Das Roop R. Tuberculosis of penis mimicking an advanced penile cancer—a case report. Int J Sci Res 2018;7:16-7.