Hong Kong Med J 2025;31:Epub 6 Aug 2025
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Corneal perforation in breast cancer patients
receiving combination chemotherapy with pertuzumab/trastuzumab targeted therapy: a case report
Anita LW Li *, FRCOphth, FCOphthHK, Vivian WM Ho, FRCOphth, PG DIP CRS, Daniel HT Wong, FRCOphth, FCOphthHK,
Kenneth KW Li, FRCOphth, FCOphthHK
Department of Ophthalmology, Kowloon East Cluster, Hospital Authority, The University of Hong Kong, Hong Kong SAR, China
Corresponding author: Dr Anita LW Li (lal505@ha.org.hk)
Case presentation
A 67-year-old woman with an unremarkable
ophthalmic history presented to the ophthalmology
department with a ‘gush of fluid’ and reduced vision
in her left eye, from 6/20 in the Snellen test (recorded
in 2023 at a private hospital), to hand movements,
after her sixth cycle of combination chemotherapy
(paclitaxel and carboplatin) and targeted therapy
(combined pertuzumab/trastuzumab in a
subcutaneous injection) for metastatic breast
cancer. She had experienced bilateral eye discomfort
and intermittent epiphora since the start of her
combined chemotherapy/targeted therapy. She
denied any topical medication use such as steroid or
nonsteroidal anti-inflammatory drugs.
Clinical findings revealed left upper lid
swelling and conjunctival injection, a central
corneal perforation with iris plugging and a flat
anterior chamber in the patient (Fig 1a). Her right
eye showed mild punctate epithelial erosions in
keeping with mild dry eye disease; there was no sign
of corneal melting. Corneal sensation in both eyes was intact. Schirmer’s test was not performed at
first presentation due to the emergent nature of the
condition.
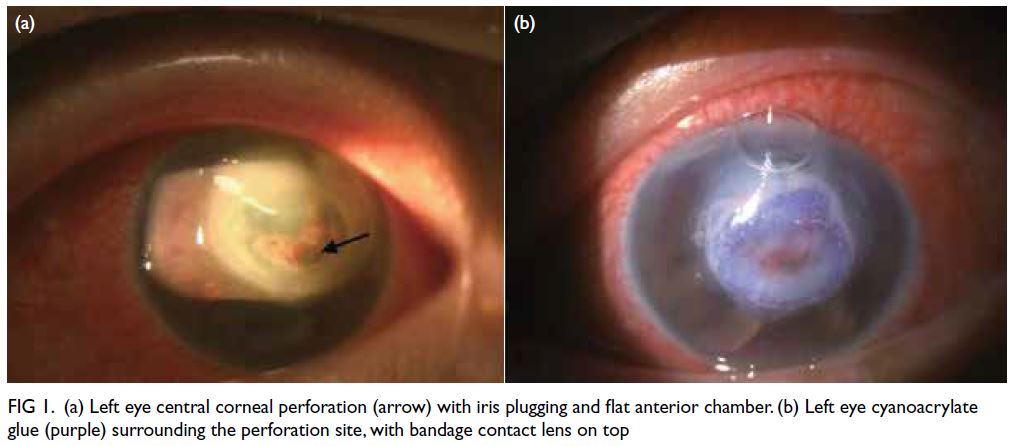
Figure 1. (a) Left eye central corneal perforation (arrow) with iris plugging and flat anterior chamber. (b) Left eye cyanoacrylate glue (purple) surrounding the perforation site, with bandage contact lens on top
Viral and bacterial conjunctival swabs were
taken and cyanoacrylate glue and a bandage contact
lens were applied immediately to the left eye corneal
perforation (Fig 1b). Viral and bacterial cultures were
negative. Blood tests for rheumatoid factor, anti–extractable nuclear antigen screen and anti–nuclear
antigen to look for an autoimmune cause were also
negative.
The combined therapy was discontinued
immediately and the patient underwent a multilayer
amniotic membrane transplant along with anterior
chamber reformation to restore globe integrity while
awaiting a subsequent corneal graft transplant (Fig 2a). One month later, with her ocular condition
temporarily stabilised, she underwent radical
mastectomy for her breast cancer. No further
adjuvant chemotherapy was administered.
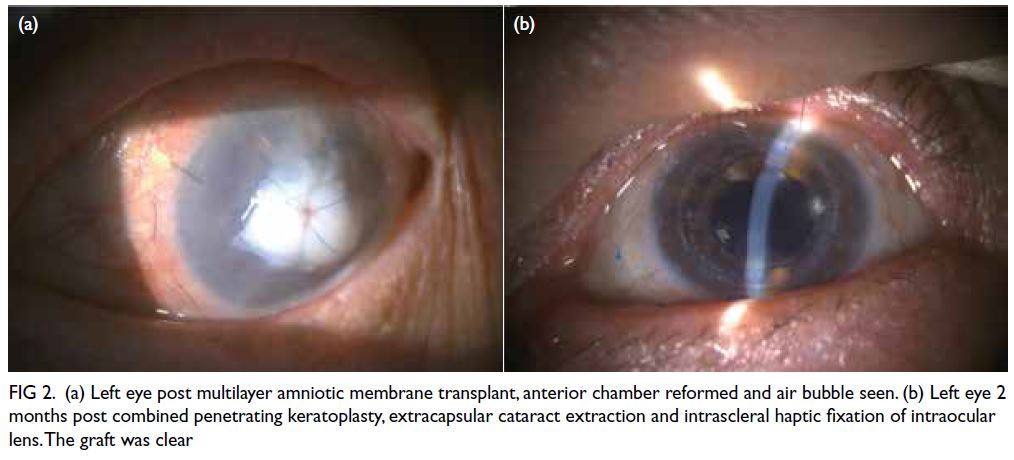
Figure 2. (a) Left eye post multilayer amniotic membrane transplant, anterior chamber reformed and air bubble seen. (b) Left eye 2 months post combined penetrating keratoplasty, extracapsular cataract extraction and intrascleral haptic fixation of intraocular lens. The graft was clear
Five months later, the patient’s general
health had stabilised. With the development of an intumescent cataract in her left eye, a triple
procedure of penetrating keratoplasty, extracapsular
cataract extraction and intrascleral haptic fixation of
intraocular lens (due to zonulysis) was performed.
Fortunately, the patient’s left eye visual
acuity recovered to 6/20 in the Snellen test and
was expected to further improve following suture
removal (Fig 2b). The patient did not develop any
further corneal erosion or melting in either eye after
drug discontinuation. Her right eye was well at the
latest follow-up and showed no sign of ocular surface
disease. The most recent Schirmer’s test in both eyes
was within normal limits.
Discussion
The top differential aetiologies in this case
were herpetic, neurotrophic, or drug-induced.
Although herpetic stromal keratitis does not
usually present at such a late stage, it can occur in
immunocompromised patients. Nonetheless, since
the patient denied a history of herpetic infection
and viral swabs were negative, this diagnosis
was unlikely. Neurotrophic keratopathy was also
excluded on the basis of bilaterally normal corneal
sensation. In light of the timing of symptom onset
following the chemotherapy/targeted therapy,
combined with a negative history of ocular surface
disease, we suspect the corneal perforation was most
likely drug-induced.
Carboplatin has not been reported to cause
ocular surface side-effects although it has been
rarely linked to optic neuropathy, retinal ischaemia,
and pigmentary maculopathy.1 Paclitaxel is a taxane-based
chemotherapeutic agent and aside from
cystoid macular oedema, has also been associated
with scintillating scotoma, photopsia, dry eye,
conjunctivitis, and limbal stem cell deficiency.1 2
Human epidermal growth factor receptor
2 (HER2) antibody drugs, pertuzumab and
trastuzumab, are used in the treatment of HER2-positive breast cancer and were administered
concurrently with chemotherapy in our patient.
Given the presence of HER2 receptors on the
ocular surface and lacrimal glands, ocular surface
disease can occur as a side-effect.2 The ocular side-effects
of trastuzumab have been well documented
and include conjunctivitis, dry eye, and marginal
keratitis.2 3 Nonetheless there have been limited case
reports of aggressive corneal melting associated with
trastuzumab monotherapy.4 Pertuzumab has been
associated with epiphora and conjunctivitis, but to
date there have been no reported cases of corneal
melting or perforation linked to pertuzumab or the
specific combination formulation of pertuzumab/trastuzumab targeted therapy. We hypothesise that
the concurrent use of taxane-based chemotherapy,
known for its ocular surface side-effects, in
combination with HER2-targeted therapy, may
have contributed to the aggressive corneal melting
observed in this case. Nonetheless the possibility
that the HER2 combination therapy (pertuzumab/trastuzumab) alone could have been responsible cannot be ignored.
It is of note that this new formulation drug
is given as a fixed-dose subcutaneous injection of
1200 mg pertuzumab and 600 mg trastuzumab for
one loading dose followed by 600 mg pertuzumab and
600 mg trastuzumab every 3 weeks for maintenance.
The conventional intravenous dose of trastuzumab
is based on body weight (8 mg/kg loading, 6 mg/kg
maintenance). It is difficult to compare doses since
the administration routes differ, although a phase 3
trial showed that the serum trough level of the drug
is slightly lower when administered subcutaneously.5 Therefore, the severe ocular presentation in this
case could not be explained by a higher dosage
or bioavailability of the anti-HER2 drugs since
bioavailability would be lower with the subcutaneous
route.5
Furthermore, in the phase 3 trial on this
new formulation combination targeted therapy
drug, it was also administered along with taxane
chemotherapy (similar to our patient), a common
regimen in hormone receptor positive breast cancer.5
All side-effects were recorded but no ocular surface
side-effects were reported.5 Hence, the potential
ocular surface side-effects are not listed in the package
insert for this relatively new combined subcutaneous
formulation of pertuzumab and trastuzumab.
In conclusion, our patient experienced
unilateral vision loss due to corneal perforation
while undergoing treatment with a combination
of chemotherapy and targeted therapy. Among the
drugs administered, paclitaxel and trastuzumab
are most frequently associated with ocular surface
side-effects, and their concurrent use may have
contributed to the severity of the presentation.
Patients receiving combined taxane chemotherapy
and HER2-targeted therapy—including newer
formulations such as combined subcutaneous
pertuzumab/trastuzumab—should be warned about
potential ocular surface complications. Early referral
for ophthalmic evaluation is recommended at the
onset of any ocular symptoms to prevent serious,
vision-threatening outcomes.
Author contributions
Concept or design: ALW Li, VWM Ho.
Acquisition of data: ALW Li, VWM Ho, DHT Wong.
Analysis or interpretation of data: ALW Li, VWM Ho.
Drafting of the manuscript: ALW Li, VWM Ho.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: ALW Li, VWM Ho, DHT Wong.
Analysis or interpretation of data: ALW Li, VWM Ho.
Drafting of the manuscript: ALW Li, VWM Ho.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
As an editor of the journal, KKW Li was not involved in the peer review process. Other authors have disclosed no conflicts of interest.
Funding/support
This study received no specific grant from any funding agency
in the public, commercial, or not-for-profit sectors.
Ethics approval
The study was approved by the Central Institutional Review
Board of Hospital Authority, Hong Kong (Ref No.: IRB 2024-
707). The patient provided written informed consent for
publication of this case report and unidentifiable information
and images.
References
1. Bader A, Begemann M, Al-Obaidi A, Habib MH, Anwer F,
Raza S. Ocular complications of antineoplastic therapies.
Future Sci OA 2023;9:FSO871. Crossref
2. Vitiello L, Lixi F, Coco G, Giannaccare G. Ocular surface
side effects of novel anticancer drugs. Cancers (Basel)
2024;16:344. Crossref
3. Kafa G, Horani M, Musa F, Al-Husban A, Hegab M, Asir N.
Marginal corneal infiltration following treatment for
metastatic breast cancer with triple chemotherapy of
trastuzumab, pertuzumab & docetaxel. Ocul Immunol
Inflamm 2023;31:431-6. Crossref
4. Barmas-Alamdari D, Chaudhary H, Baghdasaryan E, Dua P,
Cheela I. Trastuzumab-induced early corneal melt in
HER2-positive breast cancer: a case report and review. Am
J Case Rep 2024;25:e945488. Crossref
5. Tan AR, Im SA, Mattar A, et al. Fixed-dose combination of
pertuzumab and trastuzumab for subcutaneous injection
plus chemotherapy in HER2-positive early breast cancer
(FeDeriCa): a randomised, open-label, multicentre, noninferiority,
phase 3 study. Lancet Oncol 2021;22:85-97. Crossref

