© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Q Fever spondylodiscitis in the presence of
endovascular infections: a case report
Austin SL Lim, MD, DPBO; Azizul AB Sali, MD, MMed; Jason PY Cheung, MS, MD
Department of Orthopaedics and Traumatology, The University of Hong Kong, Hong Kong
Corresponding author: Prof Jason PY Cheung (cheungjp@hku.hk)
Case report
In areas endemic for tuberculosis infections, the
presence of infection on imaging and granulomatous
inflammation on histology is often sufficient to
start antituberculous pharmacotherapy. However,
this may not be appropriate in cases of non-tuberculous
granulomatous infection. Rarer causes
of spinal infection should be considered, especially
if the clinical response is suboptimal. We present
an unusual case of granulomatous spinal infection
caused by Coxiella burnetii.
A 74-year-old man from southern China with
no travel history presented with a 3-month history
of low back pain in the absence of neurological
deficit or constitutional symptoms. Co-morbidities
included hypertension, diabetes mellitus, and
hyperlipidaemia. There were no symptoms or signs
of endocarditis. Initial plain radiographs showed
lytic destruction of L4 and erosion of the L4-L5 disc
space. Laboratory investigations revealed raised
C-reactive protein (CRP) of 27.6 g/dL and erythrocyte
sedimentation rate of 32 mm/h. Blood culture, Widal
test for Salmonella, HIV testing and acid-fast bacilli
growth in sputum and blood were all negative. There
was no growth of fungus or brucellosis. Magnetic
resonance imaging and computed tomography (CT)
revealed L4/5 spondylodiscitis with psoas and
paravertebral abscesses (Fig 1), and a mycotic
infrarenal abdominal aortic aneurysm measuring
7.3 × 7.6 × 5.7 cm. He underwent endovascular stenting of the aneurysm. A CT-guided aspiration
of the psoas abscess was negative on Ziehl–Neelsen
and Grocott staining and tuberculous polymerase
chain reaction. Histology confirmed granulomatous
inflammation. After discussion with the microbiologist,
the patient was prescribed intravenous ceftriaxone
2 g per day and metronidazole 1 g per day but
switched to ertapenem 1 g per day due to a poor
clinical response. These antibiotics were used as
empirical therapy. Symptoms subsided and his CRP
normalised to <0.35 g/dL. He was discharged home
and prescribed lifelong amoxicillin/clavulanic acid
375 mg 3 times daily, with levofloxacin 500 mg once
daily for the mycotic aneurysm.
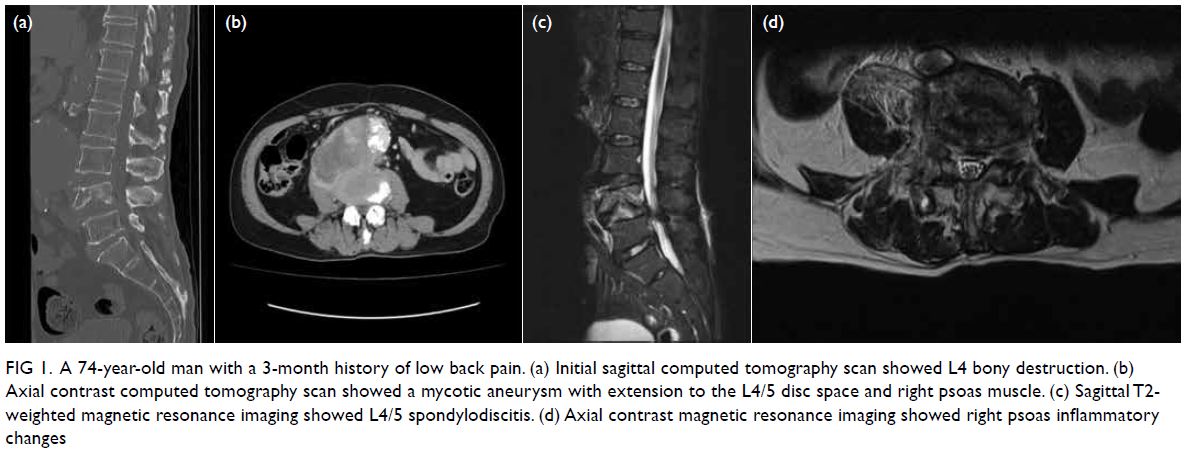
Figure 1. A 74-year-old man with a 3-month history of low back pain. (a) Initial sagittal computed tomography scan showed L4 bony destruction. (b) Axial contrast computed tomography scan showed a mycotic aneurysm with extension to the L4/5 disc space and right psoas muscle. (c) Sagittal T2-weighted magnetic resonance imaging showed L4/5 spondylodiscitis. (d) Axial contrast magnetic resonance imaging showed right psoas inflammatory changes
At 6 months after discharge he presented with
recurrent back pain along with radicular symptoms.
Inflammatory markers were elevated with CRP
3.5 g/dL and erythrocyte sedimentation rate 81 mm/h.
The same cultures and serology were negative.
Plain radiographs and CT showed increased bony
destruction of L4. Magnetic resonance imaging
revealed persistent retroperitoneal abscess with
progressive spondylodiscitis (Fig 2). A CT-guided
drainage of his right psoas abscess yielded negative
culture results. Due to the neurological deterioration,
an L3-L5 laminectomy with posterior instrumented
spinal fusion was performed. Anterior column
reconstruction was attempted by the vascular
surgeon but scarring prevented safe access to the
spinal column without aneurysmal injury. The L4-L5 disc material revealed granulomatous inflammation
and methicillin-resistant Staphylococcus aureus. He
was treated as co-infection with tuberculosis and
methicillin-resistant Staphylococcus aureus, and
was given isoniazid 300 mg, rifampicin 450 mg,
and ethambutol 800 mg once daily, and linezolid
600 mg every 12 hours. His inflammatory markers
normalised but back pain persisted.
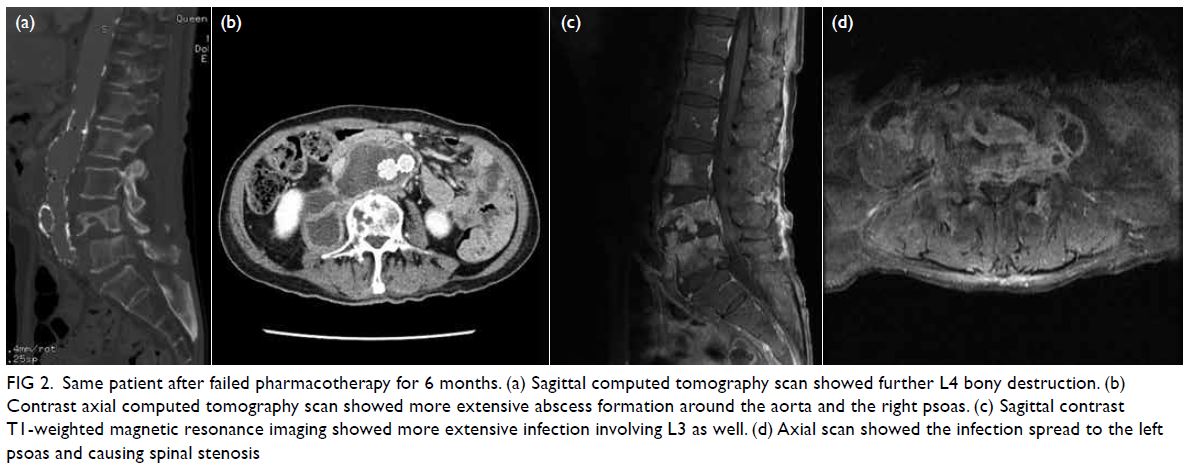
Figure 2. Same patient after failed pharmacotherapy for 6 months. (a) Sagittal computed tomography scan showed further L4 bony destruction. (b) Contrast axial computed tomography scan showed more extensive abscess formation around the aorta and the right psoas. (c) Sagittal contrast T1-weighted magnetic resonance imaging showed more extensive infection involving L3 as well. (d) Axial scan showed the infection spread to the left psoas and causing spinal stenosis
Serial radiographs showed implant failure
with further destruction of L4 (Fig 3) due to the
lack of anterior column support. Revision posterior
instrumented fusion was performed 3 months
after the initial surgery from T10 to the pelvis with
cement augmentation after removal of the loosened
L3-L5 implants. Intra-operative findings were friable
tissue and osteoporotic bone. Histology still showed
granulomatous inflammation but other cultures were
negative. Due to the persistent infection with negative
cultures, serology for C burnetii was performed and
revealed increased phase I and phase II polyvalent
antibodies with titre results of 3200 for both. The
patient was diagnosed with chronic Q fever and was
given lifelong hydroxychloroquine sulphate 200 mg
every 8 hours and doxycycline hyclate 100 mg every
12 hours owing to the presence of the endovascular
stent and spinal instrumentation. Serial monitoring
of Q fever serology was performed every 6 months.
At 2 years after surgery, he remains symptom free
with no implant loosening.
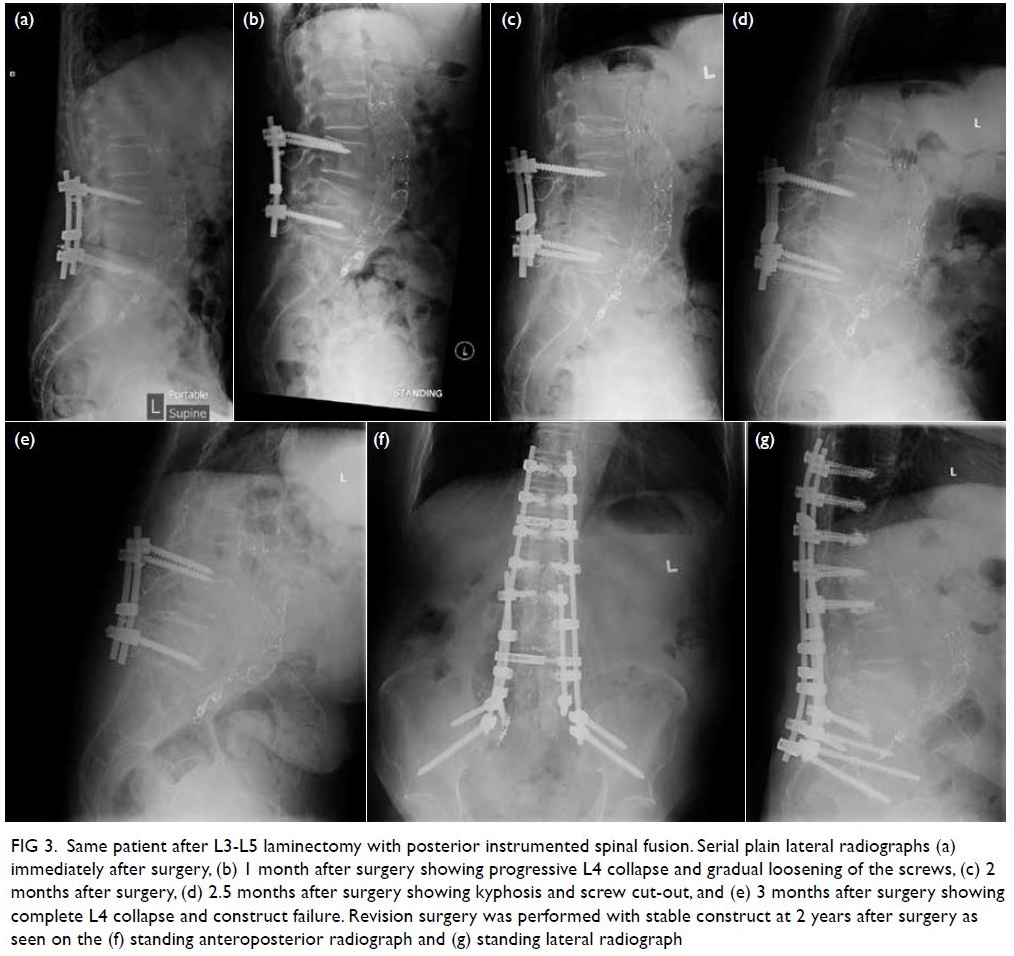
Figure 3. Same patient after L3-L5 laminectomy with posterior instrumented spinal fusion. Serial plain lateral radiographs (a) immediately after surgery, (b) 1 month after surgery showing progressive L4 collapse and gradual loosening of the screws, (c) 2 months after surgery, (d) 2.5 months after surgery showing kyphosis and screw cut-out, and (e) 3 months after surgery showing complete L4 collapse and construct failure. Revision surgery was performed with stable construct at 2 years after surgery as seen on the (f) standing anteroposterior radiograph and (g) standing lateral radiograph
Discussion
First described in 1937, Q fever is caused by
C burnetii and can be found worldwide with
an overall prevalence of 10%.1 2 Inoculation is
through direct contact, ingestion or inhalation of
contaminated materials. Traditionally it is thought
that contact with cows, goats, sheep or cats causes
this disease, but it is not always the case. In chronic Q
fever, endocarditis is the most common presentation, seen in 60% to 70% of cases. Other manifestations
include hepatitis, pericarditis, myocarditis, vascular
infections, and osteoarticular infection.3 Diagnosis
is usually by serological testing but can also be on
smears or frozen tissue with Giemsa staining that
reveals doughnut granulomas. Although serology
shows only indirect evidence of infection, it is a
simpler and reliable technique. In acute Q fever,
testing is performed for antibodies against phase II
antigens. In chronic Q fever, testing is for antibodies
against phase I antigens. Diagnosis is confirmed if
immunoglobulin G titre exceeds 1/800. Cultures are
not commonly performed due to its high infectivity
and is not available in our laboratory.
Osteoarticular Q fever infections are rare. One
study from France showed that only 7.3% of all their
patients with Q fever manifested with osteoarticular
infection.4 The most common form of Q fever
osteoarticular infection is chronic osteomyelitis. This
is usually a result of contamination from a previous
open fracture or prosthetic joints and vascular grafts.4
Adults usually present with an infected prosthetic
joint and children with multifocal osteomyelitis.1
A review of this case revealed that the patient had
previously travelled to Guangdong province in China
and had contact with farm animals. This was the only
potential source of infection from his history. A high
index of suspicion is needed for diagnosis, as seen in this
case, because a delay in treatment can lead to multiple
surgeries. Despite being in an endemic area for spinal
tuberculosis,5 other rarer causes of spondylodiscitis
with granulomatous inflammation must be considered.
These include other bacteria such as Brucellosis,
melioidosis, actinomycosis, and Bartonella infections.
Spirochetes, fungi, toxoplasmosis, and viruses such as
infectious mononucleosis, cytomegalovirus, measles,
and mumps are also potential causes. The presence
of endovascular infection with spondylodiscitis with
granulomatous inflammation and negative cultures
should raise the alarm for potential Q fever. Patients are commonly misdiagnosed and treated with prolonged
antimicrobials and surgery without improvement.
Author contributions
Concept or design: JPY Cheung.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: JPY Cheung.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: JPY Cheung.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
As an editor of the journal, JPY Cheung was not involved in the peer review process. Other authors have disclosed no
conflicts of interest.
Funding/support
This case report received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki and provided informed consent for all treatments
and procedures and consent for publication.
References
1. El-Mahallawy HS, Lu G, Kelly P, et al. Q fever in China:
a systematic review, 1989-2013. Epidemiol Infect
2015;143:673-81. Crossref
2. Fantoni M, Trecarichi EM, Rossi B, et al. Epidemiological
and clinical features of pyogenic spondylodiscitis. Eur Rev
Med Pharmacol Sci 2012;16 Suppl 2:2-7.
3. Landais C, Fenollar F, Constantin A, et al. Q fever
osteoarticular infection: four new cases and a review of the
literature. Eur J Clin Microbiol Infect Dis 2007;26:341-7. Crossref
4. Melenotte C, Protopopescu C, Million M, et al. Clinical
features and complications of Coxiella burnetii infections
from the French National Reference Center for Q fever.
JAMA Netw Open 2018;1:e181580. Crossref
5. Chan-Yeung M, Noertjojo K, Tan J, Chan SL, Tam CM. Tuberculosis in the elderly in Hong Kong. Int J Tuberc
Lung Dis 2002;6:771-9.

