EDITORIAL
Living well with kidney disease by patient and
care partner empowerment: kidney health for everyone everywhere
Kamyar Kalantar-Zadeh, MD, PhD1 #; Philip KT Li, MD2 #; Ekamol Tantisattamo, MD, MPH3; Latha Kumaraswami, BA4 #; Vassilios Liakopoulos, MD, PhD5 #; SF Lui, MD6 #; Ifeoma Ulasi, MD7 #; Sharon Andreoli, MD8 #; Alessandro Balducci, MD9 #; Sophie Dupuis, MA10 #; Tess Harris, MA11; Anne Hradsky, MA10; Richard Knight, MBA12; Sajay Kumar, BCom4; Maggie Ng13; Alice Poidevin, MA10; Gamal Saadi, MD14 #; Allison Tong, PhD15
1 The International Federation of Kidney Foundation–World Kidney Alliance (IFKF-WKA), Division of Nephrology and Hypertension and Kidney Transplantation, University of California Irvine, Orange, California, United States
2 Department of Medicine and Therapeutics, Carol & Richard Yu PD Research Centre, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong
3 Division of Nephrology, Hypertension and Kidney Transplantation, Department of Medicine, University of California Irvine School of Medicine, Orange, California, United States
4 Tanker Foundation, Chennai, India
5 Division of Nephrology and Hypertension, 1st Department of Internal Medicine, AHEPA Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece
6 Hong Kong Kidney Foundation and the International Federation of Kidney Foundations–World Kidney Alliance, The Jockey Club School of Public Health and Primary Care, The Chinese University of Hong Kong, Hong Kong
7 Renal Unit, Department of Medicine, College of Medicine, University of Nigeria, Ituku-Ozalla, Enugu, Nigeria
8 James Whitcomb Riley Hospital for Children, Indiana University School of Medicine, Indianapolis, Indiana, United States
9 Italian Kidney Foundation, Rome, Italy
10 World Kidney Day Office, Brussels, Belgium
11 Polycystic Kidney Disease Charity, London, United Kingdom
12 American Association of Kidney Patients, Tampa, Florida, United States
13 Hong Kong Kidney Foundation, Hong Kong
14 Nephrology Unit, Department of Internal Medicine, Faculty of Medicine, Cairo University, Giza, Egypt
15 Sydney School of Public Health, The University of Sydney, Sydney, New South Wales, Australia
# Members of the World Kidney Day Steering Committee
 Full
paper in PDF
Full
paper in PDF
Living with chronic kidney disease (CKD) is
associated with hardships for patients and their
care partners. Empowering patients and their care
partners, including family members or friends
involved in their care, may help minimise the burden
and consequences of CKD-related symptoms to
enable life participation. There is a need to broaden
the focus on living well with kidney disease and
re-engagement in life, including an emphasis on
patients being in control. The World Kidney Day
(WKD) Joint Steering Committee has declared
2021 the year of ‘Living Well with Kidney Disease’
in an effort to increase education and awareness on
the important goal of patient empowerment and
life participation. This calls for the development
and implementation of validated patient-reported
outcome measures to assess and address areas of life
participation in routine care. It could be supported
by regulatory agencies as a metric for quality care
or to support labelling claims for medicines and
devices. Funding agencies could establish targeted calls for research that address the priorities of
patients. Patients with kidney disease and their care
partners should feel supported to live well through
concerted efforts by kidney care communities
including during pandemics. In the overall wellness
programme for kidney disease patients, the need
for prevention should be reiterated. Early detection
with a prolonged course of wellness despite kidney
disease, after effective secondary and tertiary
prevention programmes, should be promoted.
World Kidney Day 2021 continues to call for
increased awareness of the importance of preventive
measures throughout populations, professionals,
and policy makers, applicable to both developed and
developing countries.
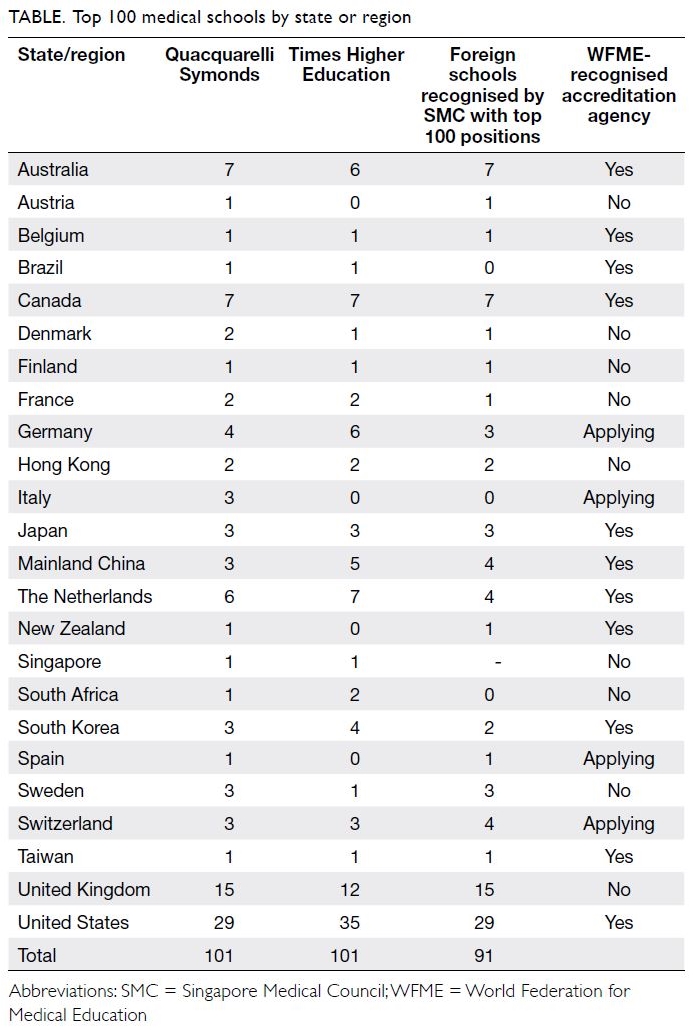
Patient priorities for living well: a
focus on life participation
Chronic kidney disease, its associated symptoms,
and its treatment, including medications, dietary and fluid restrictions, and kidney replacement
therapy can disrupt and constrain daily living, and
impair the overall quality of life of patients and their
family members. Consequently, this can also impact
treatment satisfaction and clinical outcomes.
1
Despite this, the past several decades have seen
limited improvement in the quality of life of people
with CKD.
1 To advance research, practice, and policy,
there is increasing recognition of the need to identify
and address patient priorities, values, and goals.
1
Several regional and global kidney health
projects have addressed these important questions
including the Standardised Outcomes in Nephrology
with more than 9000 patients, family members, and
healthcare professionals from over 70 countries.
2 3
Across all treatment stages, including CKD, dialysis
and transplantation, Standardised Outcomes in
Nephrology participating children and adults with
CKD consistently gave higher priority to symptoms
and life impacts than healthcare professionals.
2 3 In
comparison, healthcare professionals gave higher
priority to mortality and hospitalisation than
patients and family members. The patient-prioritised
outcomes are shown in the
Figure. Irrespective
of the type of kidney disease or treatment stage,
patients wanted to be able to live well, maintain their
role and social functioning, protect some semblance
of normality, and have a sense of control over their
health and well-being.
 Figure.
Figure. Conceptual framework of ‘Living Well with Kidney Disease’ based on patient centeredness and empowering patient with
focus on effective symptom management and life participation
Life participation, defined as the ability to do
meaningful activities of life including, but not limited
to, work, study, family responsibilities, travel, sport, social, and recreational activities, was established
a critically important outcome across all treatment
stages of CKD.
1 2 The quotations from patients with
kidney disease provided in the
Box demonstrates how
life participation reflects the ability to live well with
CKD.
4 According to the World Health Organization
(WHO), participation refers to “involvement in a life
situation.”
5 This concept is more specific than the
broader construct of quality of life. Life participation
places the life priorities and values of those affected
by CKD and their family at the centre of decision
making. The World Kidney Day Steering Committee
calls for the inclusion of life participation, a key
focus in the care of patients with CKD, to achieve
the ultimate goal of living well with kidney disease.
This calls for the development and implementation
of validated patient-reported outcome measures,
that could be used to assess and address areas of
life participation in routine care. Monitoring of
life participation could be supported by regulatory
agencies as a metric for quality care or to support
labelling claims for medicines and devices. Funding
agencies could establish targeted calls for research
that address the priorities of patients, including life
participation.
 Box.
Box. Quotations from patients with chronic kidney disease related to priorities for living well
Patient empowerment, partnership, and a paradigm shift towards a strengths-based approach to care
Patients with CKD and their family members including care partners should be empowered to
achieve the health outcomes and life goals that are
meaningful and important to them. The WHO defines
patient empowerment as “a process through which
people gain greater control over decisions or actions
affecting their health,”
6 which requires patients to
understand their role, to have knowledge to be able
to engage with clinicians in shared decision making,
skills, and support for self-management. For patients
receiving dialysis, understanding the rationale for a
lifestyle change, having access to practical assistance
and family support promoted patient empowerment,
while feeling limited in life participation undermined
their sense of empowerment.
7
The World Kidney Day Steering Committee
advocates for strengthened partnership with patients
in the development, implementation, and evaluation
of interventions for practice and policy settings, that
enable patients to live well with kidney diseases.
This needs to be supported by consistent, accessible,
and meaningful communication. Meaningful
involvement of patients and family members across
the entire research process, from priority setting and
planning the study through to dissemination and
implementation, is now widely advocated.
8 There
have also been efforts, such as the Kidney Health
Initiative, to involve patients in the development of
drugs and devices to foster innovation.
9
We urge for greater emphasis on a strengths-based
approach as outlined in the
Table, which
encompasses strategies to support patient resilience,
harness social connections, build patient awareness
and knowledge, facilitate access to support,
and establish confidence and control in self-management. The strengths-based approach is in
contrast to the medical model where chronic disease
is traditionally focussed on pathology, problems,
and failures.
10 Instead, the strengths-based approach
acknowledges that each individual has strengths and
abilities to overcome the problems and challenges
faced, and requires collaboration and cultivation of
the patient’s hopes, aspirations, interests, and values.
Efforts are needed to ensure that structural biases,
discrimination, and disparities in the healthcare
system also need to be identified, so all patients are
given the opportunity to have a voice.
 Table.
Table. Suggested strategies for ‘living well with chronic kidney disease’ using a strengths-based approach
The role of care partner
A care partner is often an informal caregiver who is
also a family member of the patient with CKD.
11 They
may take on a wide range of responsibilities including
coordinating care (including transportation to
appointments), administration of treatment
including medications, home dialysis assistance,
and supporting dietary management. Caregivers of
patients with CKD have reported depression, fatigue,
isolation, and also burnout. The role of the care
partner has increasingly become more important
in CKD care given the heightened complexity in
communicative and therapeutic options including
the expansion of telemedicine under the coronavirus
disease 2019 (COVID-19) pandemic and given the
goal to achieve higher life expectancy with CKD.
12
The experience of caring for a partially incapacitated
family member with progressive CKD can represent
a substantial burden on the care partner and may
impact family dynamics. Not infrequently, the career goals and other occupational and leisure aspects
of the life of the care partner are affected because
of CKD care partnership, leading to care partner
overload and burnout. Hence, the above-mentioned
principles of life participation need to equally apply
to care partners as well as all family members and
friends involved in CKD care.
Living with kidney disease in low-income
regions
In low and lower-middle-income countries including
in sub-Saharan Africa, Southeast Asia, and Latin
America, patient’s ability to self-manage or cope
with the chronic disease vary but may often be
influenced by internal factors including spirituality,
belief system, and religiosity, and external factors
including appropriate knowledge of the disease,
poverty, family support system, and one’s grit
and social relations network. The support system
comprising healthcare providers and caregivers plays
a crucial role as most patients rely on them in making
decisions, and for the necessary adjustments in their
health behaviour.
13 In low-income regions, where
there are often a relatively low number of physicians
and even lower number of kidney care providers
per population especially in rural areas, a stepwise
approach can involve local and national stakeholders
including both non-governmental organisations and
government agencies by (1) extending kidney patient
education in rural areas; (2) adapting telehealth
technologies if feasible to educate patients and train
local community kidney care providers, and (3)
implementing effective retention strategies for rural
kidney health providers including adapting career
plans and competitive incentives.
Many patients in low-resource settings present
in very late stage needing to commence emergency dialysis.
14 The very few fortunate ones to receive
kidney transplantation may acquire an indescribable
chance to normal life again, notwithstanding the
high costs of immunosuppressive medications in
some countries. For some patients and care partners
in low-income regions, spirituality and religiosity
may engender hope, when ill they are energised
by the anticipation of restored health and spiritual
wellbeing. For many patients, informing them
of a diagnosis of kidney disease is a harrowing
experience both for the patient (and caregivers)
and the healthcare professional. Most patients
present to kidney physicians (usually known as
“renal physicians” in many of these countries) with
trepidations and apprehension. It is rewarding
therefore to see the patient’s anxiety dissipate after
reassuring him or her of a diagnosis of simple
kidney cysts, urinary tract infection, simple kidney
stones, solitary kidneys, etc, that would not require
extreme measures like kidney replacement therapy.
Patients diagnosed with glomerulonephritis who
have an appropriate characterisation of their disease
from kidney biopsies and histology; who receive
appropriate therapies and achieve remission are
relieved and are very grateful. Patients are glad to
discontinue dialysis following resolution of acute
kidney injury or acute-on-chronic kidney disease.
Many patients with CKD who have residual
kidney function appreciate being maintained in a
relatively healthy state with conservative measures,
without dialysis. They experience renewed energy
when their anaemia is promptly corrected using
erythropoiesis-stimulating agents. They are happy
when their peripheral oedema resolves with
treatment. For those on maintenance haemodialysis
who had woeful stories from emergency femoral
cannulations, they appreciate the construction of
good temporary or permanent vascular accesses. Many patients in low-resource settings present in
very late stage needing to commence emergency
dialysis. Patients remain grateful for waking from a
uraemic coma or recovering from recurrent seizures
when they commence dialysis.
World kidney day 2021 advocacy
World Kidney Day 2021 theme on ‘Living Well with
Kidney Disease’ is deliberately chosen to have the
goals to redirect more focus on plans and actions
towards achieving patient-centred wellness. “Kidney
Health for Everyone, Everywhere” with emphasis
on patient-centred wellness should be a policy
imperative that can be successfully achieved if policy
makers, nephrologists, healthcare professionals,
patients, and care partners place this within the
context of comprehensive care. The requirement
of patient engagement is needed. The WHO in
2016 put out an important document on patient
empowerment
15:
“Patient engagement is increasingly recognised
as an integral part of healthcare and a critical
component of safe people-centred services. Engaged
patients are better able to make informed decisions
about their care options. In addition, resources may be
better used if they are aligned with patients’ priorities
and this is critical for the sustainability of health
systems worldwide. Patient engagement may also
promote mutual accountability and understanding
between patients and healthcare providers. Informed
patients are more likely to feel confident to report both
positive and negative experiences and have increased
concordance with mutually agreed care management
plans. This not only improves health outcomes but
also advances learning and improvement while
reducing adverse events.”
In the International Society of Nephrology
Community Film Event at World Congress of
Nephrology 2020, it is good to see a quote in the film
from patients:
“Tell me. I will forget; Show me. I will remember; Involve me. I will understand.”
The International Society of Nephrology
Global Kidney Policy Forum 2019 included a patient
speaker Nicki Scholes-Robertson from New Zealand:
“Culturally appropriate and sensitive patient
information and care are being undertaken in New
Zealand to fight inequities in kidney health, especially
in Maori and other disadvantaged communities.”
World Kidney Day 2021 would like to promote
to the policy makers on increasing focus and
resources on both drug and non-drug programmes
in improving patient wellness. Examples include
funding for erythropoiesis-stimulating agents and
antipruritic agents for managing anaemia and
itchiness, respectively, just name but a few.
16 17 Home
dialysis therapies have been consistently found to
improve patient autonomy and flexibility, quality of life in a cost-effective manner, enhancing life
participation. Promoting home dialysis therapies
should tie in with appropriate ‘assisted dialysis’
programmes to reduce patient and care partner
fatigue and burnout. Also, examples like self-management
programmes, cognitive behavioural
therapy, and group therapies for managing
depression, anxiety, and insomnia should be
promoted before resorting to medications.
18 The
principle of equity recognises that different people
with different levels of disadvantage require different
approaches and resources to achieve equitable
health outcomes. The kidney community should
push for adapted care guidelines for vulnerable and
disadvantaged populations. The involvement of
primary care and general physicians especially in low
and lower-middle-income countries would be useful
in improving the affordability and access to services
through the public sector in helping the symptom
management of patients with CKD and improve
their wellness. In the overall wellness programme
for kidney disease patients, the need for prevention
should be reiterated. Early detection with a prolonged
course of wellness despite kidney disease, after an
effective secondary prevention programme, should
be promoted.
19 Prevention of CKD progression can
be attempted by lifestyle and diet modifications
such as a plant-dominant low-protein diet and
by means of effective pharmacotherapy including
administration of sodium-glucose transport protein
2 inhibitors.
20 World Kidney Day 2021 continues
to call for increased awareness of the importance
of preventive measures throughout populations,
professionals, and policy makers, applicable to both
developed and developing countries.
19
Conclusions
Effective strategies to empower patients and their
care partners strive to pursue the overarching goal
of minimising the burden of CKD-related symptoms
in order to enhance patient satisfaction, health-related
quality of life, and life participation. World
Kidney Day 2021 theme on ‘Living Well with Kidney
Disease’ is deliberately chosen to have the goals to
redirect more focus on plans and actions towards
achieving patient-centred wellness. Notwithstanding
the COVID-19 pandemic that had overshadowed
many activities in 2020 and beyond, the World
Kidney Day Steering Committee has declared 2021
the year of ‘Living well with Kidney Disease’ in an
effort to increase education and awareness on the
important goal of effective symptom management
and patient empowerment. Whereas the World
Kidney Day continues to emphasise the importance
of effective measures to prevent kidney disease
and its progression,
18 patients with pre-existing
kidney disease and their care partners should feel
supported to live well through concerted efforts by kidney care communities and other stakeholders
throughout the world even during a world-shattering
pandemic as COVID-19 that may drain
many resources.
21 Living well with kidney disease is
an uncompromisable goal of all kidney foundations,
patient groups, and professional societies alike, to
which the International Society of Nephrology and
the International Federation of Kidney Foundation
World Kidney Alliance are committed at all times.
Author contributions
K Kalantar-Zadeh reports honoraria from Abbott, AbbVie,
ACI Clinical, Akebia, Alexion, Amgen, Ardelyx, Astra-Zeneca,
Aveo, BBraun, Cara Therapeutics, Chugai, Cytokinetics,
Daiichi, DaVita, Fresenius, Genentech, Haymarket Media,
Hospira, Kabi, Keryx, Kissei, Novartis, Pfizer, Regulus,
Relypsa, Resverlogix, Dr Schaer, Sandoz, Sanofi, Shire, Vifor,
UpToDate, and ZS-Pharma. PKT Li reports personal fees
from Fibrogen and Astra-Zeneca. G Saadi reports personal
fees from Multicare, Novartis, Sandoz, and Astra-Zeneca.
V Liakopoulos reports non-financial support from Genesis
Pharma. All other authors have disclosed no conflicts of
interest.
Funding/support
This editorial received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declaration
This article was published in Kidney International, volume
99, pages 278-284, Copyright World Kidney Day Steering
Committee (2021), and reprinted concurrently in several
journals. The articles cover identical concepts and wording,
but vary in minor stylistic and spelling changes, detail, and
length of manuscript in keeping with each journal’s style. Any
of these versions may be used in citing this article.
References
1. Tong A, Manns B, Wang AY, et al. Implementing core
outcomes in kidney disease: report of the Standardized
Outcomes in Nephrology (SONG) implementation
workshop. Kidney Int 2018;94:1053-68.
Crossref2. Carter SA, Gutman T, Logeman C, et al. Identifying
outcomes important to patients with glomerular disease and
their caregivers. Clin J Am Soc Nephrol 2020;15:673-84.
Crossref3. Hanson CS, Craig JC, Logeman C, et al. Establishing core
outcome domains in pediatric kidney disease: report of
the Standardized Outcomes in Nephrology-Children and
Adolescents (SONG-KIDS) consensus workshops. Kidney
Int 2020;98:553-65.
Crossref4. González AM, Gutman T, Lopez-Vargas P, et al. Patient and
caregiver priorities for outcomes in CKD: a multinational
nominal group technique study. Am J Kid Dis 2020;76:679-89.
Crossref5. World Health Organization, The International
Classification of Functioning, Disability and Health.
Towards a common language for functioning, disability
and health. 2002. Available from: https://www.who.int/classifications/icf/icfbeginnersguide.pdf. Accessed 11 Apr
2021.
6. World Health Organization. Health Promotion Glossary. 1998. Available from: https://www.who.int/publications/i/item/WHO-HPR-HEP-98.1. Accessed 11 Apr 2021.
7. Baumgart A, Manera KE, Johnson DW, et al. Meaning
of empowerment in peritoneal dialysis: focus groups
with patients and caregivers. Nephrol Dial Transplant
2020;35:1949-58.
Crossref8. PCORI. The Value of Engagement. Available from: https://
www.pcori.org/about-us/our-programs/engagement/public-and-patient-engagement/value-engagement. 2018. Accessed 1 Sep 2020.
9. Bonventre JV, Hurst FP, West M, Wu I, Roy-Chaudhury
P, Sheldon M. A technology roadmap for innovative
approaches to kidney replacement therapies: a catalyst for
change. Clin J Am Soc Nephrol 2019;14:1539-47.
Crossref10. Ibrahim N, Michail M, Callaghan P. The strengths based
approach as a service delivery model for severe mental
illness: a meta-analysis of clinical trials. BMC Psychiatry
2014;14:243.
Crossref11. Parham R, Jacyna N, Hothi D, Marks SD, Holttum S, Camic P. Development of a measure of caregiver burden
in paediatric chronic kidney disease: The Paediatric Renal
Caregiver Burden Scale. J Health Psychol 2014;21:93-205.
Crossref12. Subramanian L, Kirk R, Cuttitta T, et al. Remote
management for peritoneal dialysis: a qualitative study
of patient, care partner, and clinician perceptions and
priorities in the United States and the United Kingdom.
Kidney Med 2019;1:354-65.
Crossref13. Angwenyi V, Aantjes C, Kajumi M, De Man J, Criel B,
Bunders-Aelen J. Patients experiences of self-management
and strategies for dealing with chronic conditions in rural
Malawi. PLoS One 2018;13:e0199977.
Crossref14. Ulasi II, Ijoma CK. The enormity of chronic kidney disease
in Nigeria: the situation in a teaching hospital in South-East Nigeria. J Trop Med 2010;2010:501957.
Crossref15. World Health Organization. Patient engagement. Available
from: https://apps.who.int/iris/handle/10665/252269.
Accessed 11 Apr 2021.
16. Spinowitz B, Pecoits-Filho R, Winkelmayer WC, et al.
Economic and quality of life burden of anemia on patients
with CKD on dialysis: a systematic review. J Med Econ
2019;22:593-604.
Crossref17. Sukul N, Speyer E, Tu C, et al. Pruritus and patient reported
outcomes in non-dialysis CKD. Clin J Am Soc Nephrol
2019;14:673-81.
Crossref18. Gregg LP, Hedayati SS. Pharmacologic and psychological
interventions for depression treatment in patients with
kidney disease. Curr Opin Nephrol Hypertens 2020;29:457-64.
Crossref19. Li PK, Garcia-Garcia G, Lui SF, et al. Kidney health for
everyone everywhere—from prevention to detection and
equitable access to care. Kidney Int 2020;97:226-32.
Crossref20. Kalantar-Zadeh K, Li PK. Strategies to prevent kidney
disease and its progression. Nat Rev Nephrol 2020;16:129-30.
Crossref21. Kalantar-Zadeh K, Wightman A, Liao S. Ensuring choice
for people with kidney failure—dialysis, supportive care,
and hope. N Engl J Med 2020;383:99-101.
Crossref