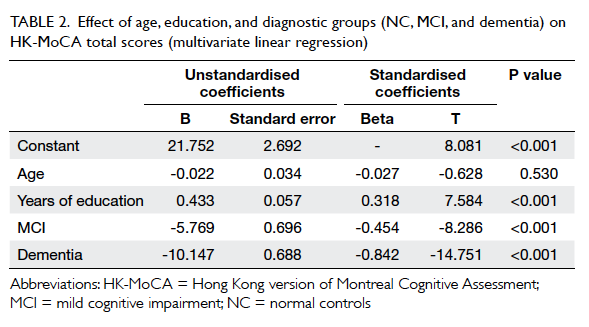
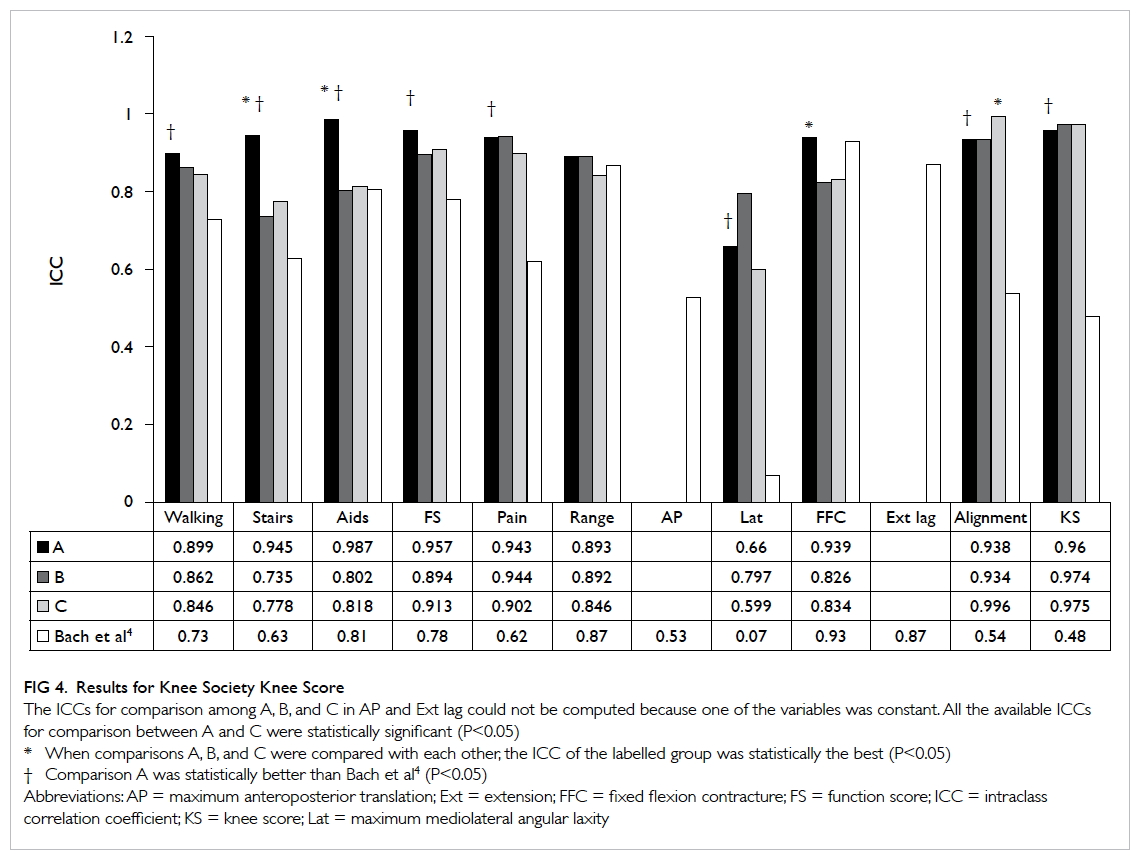
Improving the emergency department management of post-chemotherapy sepsis in haematological malignancy patients
Hong Kong Med J 2015 Feb;21(1):10–5 | Epub 10 Oct 2014
DOI: 10.12809/hkmj144280
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Improving the emergency department management of post-chemotherapy sepsis in haematological malignancy patients
HF Ko, MB, BS, FHKAM (Emergency Medicine)1; SS Tsui, APN1; Johnson WK Tse, APN, BSN HD (Nursing)1; WY Kwong, MB, ChB1; OY Chan, MB, BS2; Gordon CK Wong, MB, BS, FHKAM (Emergency Medicine)1
1 Accident and Emergency Department, Queen Elizabeth Hospital, Jordan, Hong Kong
2 Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong
Corresponding author: Dr HF Ko (frankhko@hotmail.com)
Abstract
Objective: To review the result of the implementation
of treatment protocol for post-chemotherapy sepsis
in haematological malignancy patients.
Design: Case series with internal comparison.
Setting: Accident and Emergency Department,
Queen Elizabeth Hospital, Hong Kong.
Patients: Febrile patients presenting to the Accident
and Emergency Department with underlying
haematological malignancy and receiving
chemotherapy within 1 month of Accident and
Emergency Department visit between June 2011 and
July 2012. Similar cases between June 2010 and May
2011 served as historical referents.
Main outcome measures: The compliance rate
among emergency physicians, the door-to-antibiotic
time before and after implementation of the protocol,
and the impact of the protocol on Accident and
Emergency Department and hospital service.
Results: A total of 69 patients were enrolled in
the study. Of these, 50 were managed with the
treatment protocol while 19 patients were historical
referents. Acute myeloid leukaemia was the most
commonly encountered malignancy. Overall, 88%
of the patients presented with sepsis syndrome. The
mean door-to-antibiotic time of those managed
with the treatment protocol was 47 minutes versus
300 minutes in the referent group. Overall, 86% of
patients in the treatment group met the target door-to-antibiotic time of less than 1 hour. The mean
lengths of stay in the emergency department (76 minutes vs
105 minutes) and hospital (11 days vs 15 days) were shorter
in those managed with the treatment protocol versus
the historical referents.
Conclusion: Implementation of the protocol can
effectively shorten the door-to-antibiotic time
to meet the international standard of care in
neutropenic sepsis patients. The compliance rate was
also high. We proved that effective implementation
of the protocol is feasible in a busy emergency
department through excellent teamwork between
nurses, pharmacists, and emergency physicians.
New knowledge added by this
study
- A well-written, easily available treatment protocol together with stocking of antibiotics in the emergency department can effectively shorten the door-to-antibiotic (DTA) time from 300 minutes to 47 minutes.
- In this study, 86% of patients met the target DTA time of less than 1 hour.
- Orchestrated efforts between nurses, pharmacists, and physicians are crucial for implementation of the protocol in one of the busiest emergency departments in the region.
Introduction
Cancer patients receiving chemotherapy sufficient
to cause myelosuppression and adverse effects on
the integrity of gastro-intestinal mucosa are at high
risk of invasive infections. Patients with profound,
prolonged neutropenia are at particularly high risk
of serious infections. Prolonged neutropenia is most
likely to occur in patients undergoing induction
chemotherapy for acute leukaemia. More than 80% of
those with haematological malignancies will develop
fever during more than one chemotherapy cycle.1
Since neutropenic patients are unable to mount a
strong inflammatory response to infections, fever may
be the only sign. Infection in neutropenic patients
can progress rapidly, leading to serious complications
and even death with a mortality rate ranging from
2% to 21%.2 3 It is critical to recognise neutropenic
fever patients early and initiate empirical, broad-spectrum antibiotics. Major international guidelines
advocate early administration of empirical antibiotics
within 1 hour of emergency department (ED)
presentation, sometimes even without cytological
proof of neutropenia.4 5 6 7 However, management of
febrile neutropenic patients varies across different
EDs, and even among different physicians. A recent
audit performed in the EDs of the United Kingdom
showed that only 26% of the audited patients received
intravenous antibiotics within the target time of
1 hour.8 Another study in French EDs showed that
management of febrile neutropenia was inadequate
and severity was under-evaluated in the critically ill.9
In order to improve and standardise the care of post-chemotherapy
sepsis in haematological malignancy
patients, the Accident and Emergency Department
(A&E) and Department of Medicine of Queen
Elizabeth Hospital (QEH) initiated a treatment
protocol in 2011. This is the first hospital in Hong
Kong to implement such a treatment protocol.
It included febrile patients with haematological
malignancy who had received chemotherapy within
1 month of ED visit. These patients were identified
at triage station and provided with a fast-track
consultation. The ED physician would verify the
history and perform a thorough physical examination
and targeted investigations. Empirical antibiotics
were administered after taking appropriate culture
samples aiming at a door-to-antibiotic (DTA) time
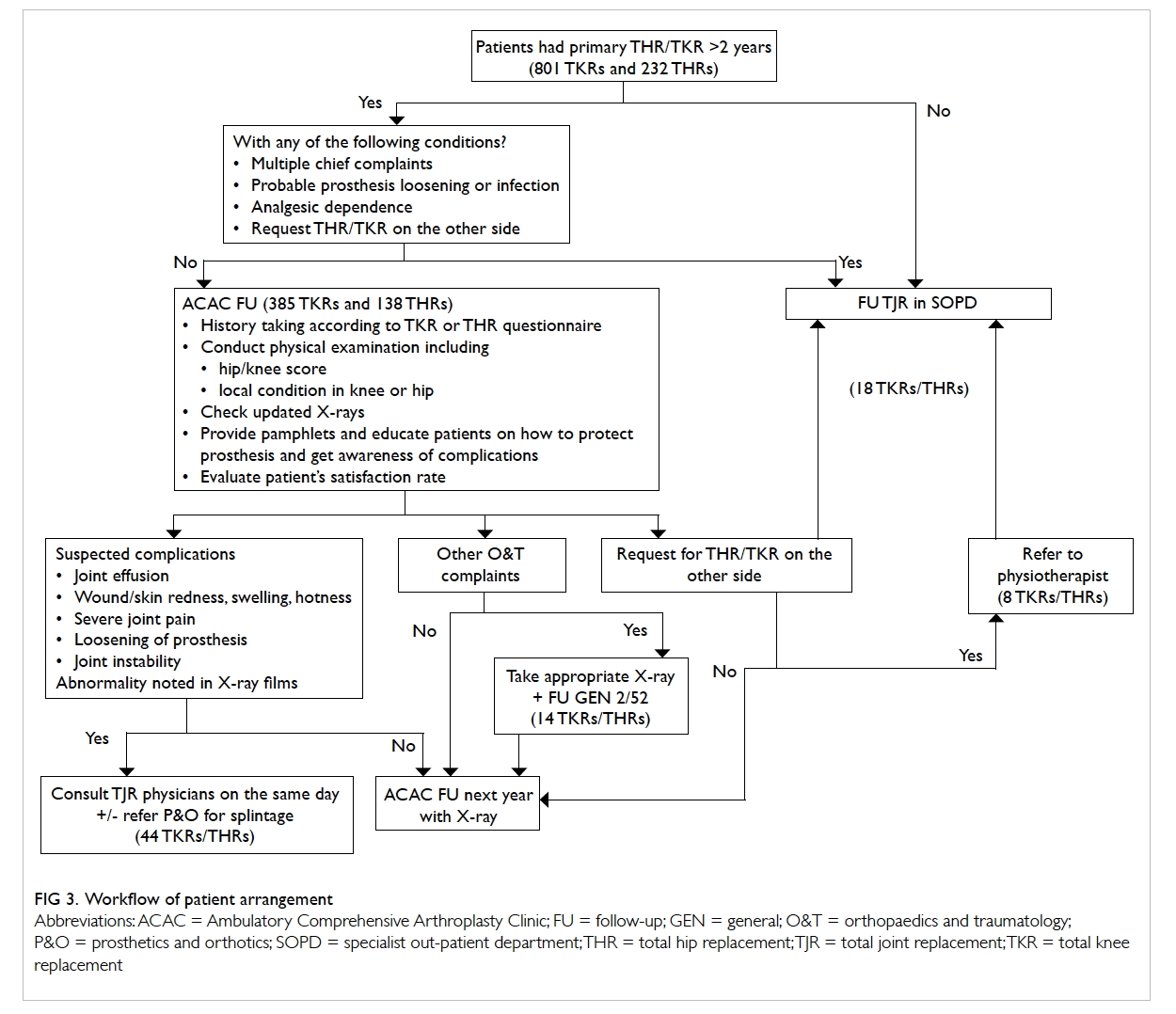
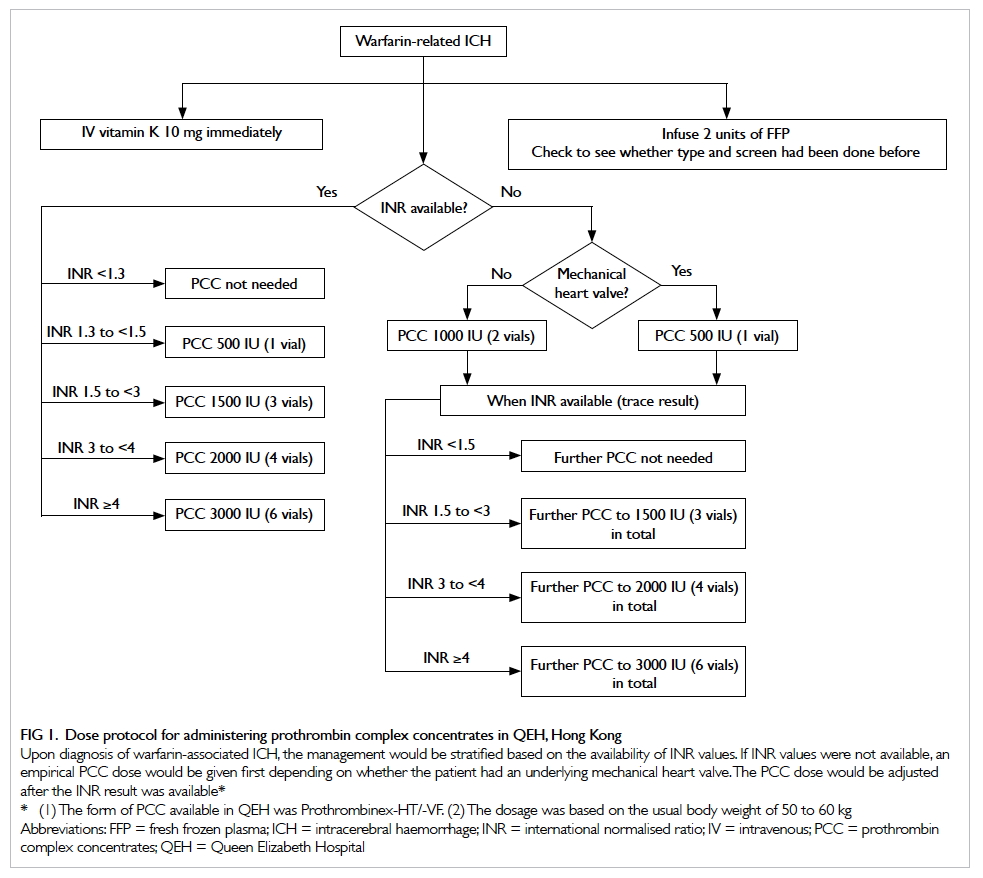
of less than 1 hour (Fig).
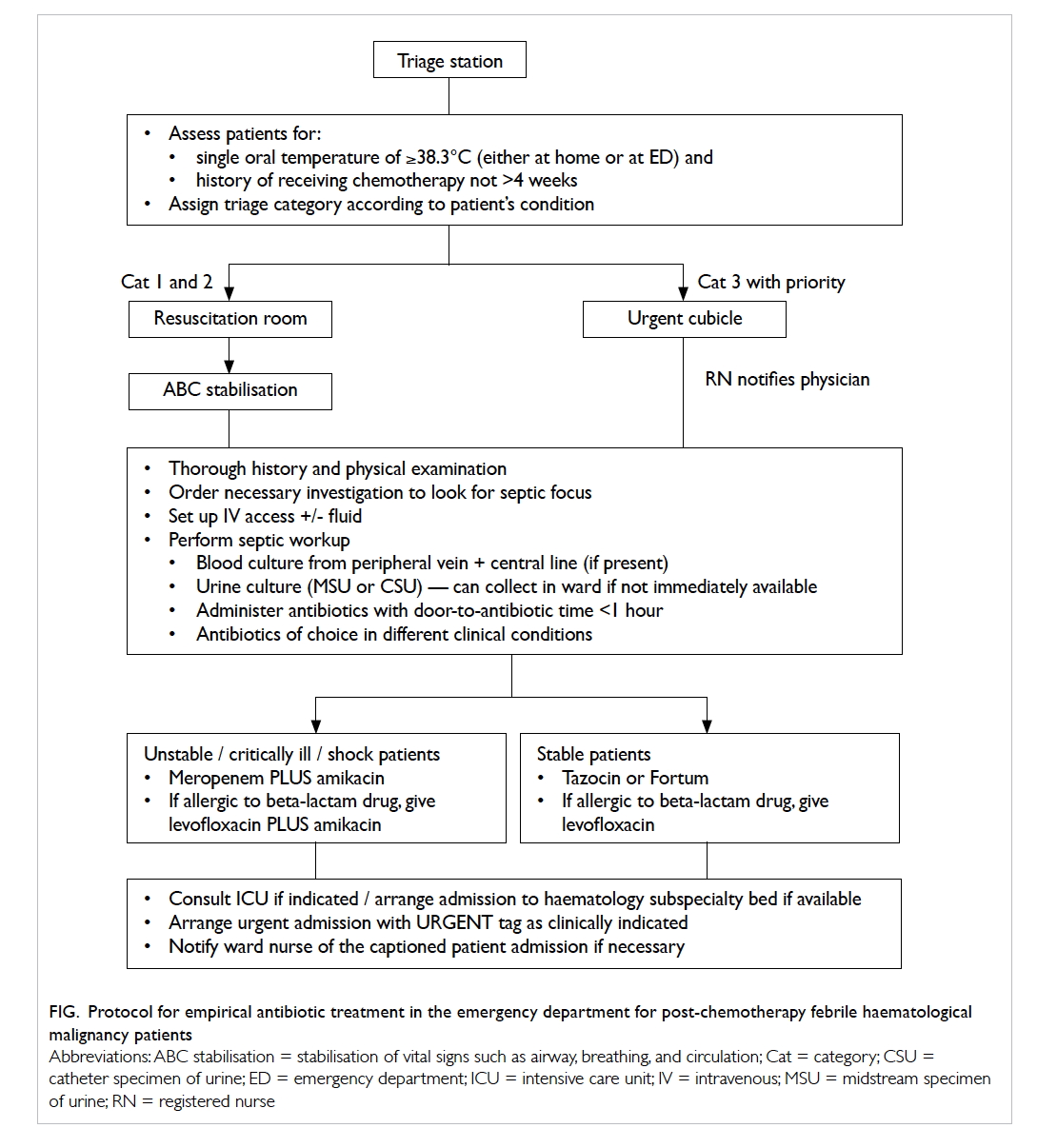
Figure. Protocol for empirical antibiotic treatment in the emergency department for post-chemotherapy febrile haematological malignancy patients
Local publication on post-chemotherapy
patients mainly focused on solid tumour patients,
in-patient management and their outcomes.10
There is a paucity of literature concerning the initial
ED management of haematological malignancy
patients. The objective of this study was to examine
the protocol compliance rate among ED physicians,
the DTA time before and after implementation of the
protocol, and the impact of the protocol on A&E and
hospital services. It also serves to provide invaluable
epidemiological data regarding the haematological
malignancy patients in Hong Kong.
Methods
This is a before-and-after study of the impact of a
protocol on the management of post-chemotherapy
sepsis in haematological malignancy patients. A
2-year retrospective chart review was conducted.
The first chart review was performed from June
2010 to May 2011. These patients were admitted
through ED to the haematological ward prior
to implementation of the protocol and served as
historical referents. Data were retrieved from the
admission book of the haematology ward. A diagnosis
of post-chemotherapy fever or neutropenic fever
was shortlisted. Cases that were admitted through
ED were analysed. The second year started from
June 2011. The intervention group included patients
recruited in the protocol. There were two patients
who fulfilled the inclusion criteria but were excluded
from the study since they refused any investigation
or treatment in ED despite explanation. The charts
were reviewed by two emergency physicians and
two senior nurses. Any discrepancy was resolved by
discussion among investigators. The protocol was
implemented on a 24-hour basis. According to the
protocol, fever was defined by a single measurement
of oral temperature of >38.3°C either at the triage
station or self-reported at home. Neutropenia was
defined as absolute neutrophil count (ANC) of
<1 x 10-9 /L. Sepsis was defined by Bone criteria11
(ie >2 out of 4 of the following: leukocyte count
<4 or >12 x 10-9 /L, respiratory rate >20/min, oral
temperature >38°C or <35°C, pulse >90 beats/min).
Door-to-antibiotic time was charted in the medical
record. Lengths of stay in the A&E and hospital
were retrieved from the Clinical Data Analysis and
Reporting System. The primary outcome was mean
DTA time. Secondary outcomes included compliance
of the ED physician with the protocol, mean ED
length of stay, mean hospital length of stay, and the
adverse outcome rate. Adverse outcomes included
occurrence of a serious medical complication or
death during index admission; these criteria are
commonly cited in oncology literature.12 Adverse
outcome was charted from patients’ medical record
during the index admission.
Chi squared tests were performed when
comparing categorical parameters between the
protocol and referent groups. Student’s t tests were
performed for parametric variables. All statistical
analyses were performed using the Statistical
Package for the Social Sciences (Windows version
17; SPSS Inc, Chicago [IL], US). A P value of less
than 0.05 was regarded as statistically significant.
The study was conducted in the A&E of
QEH, Hong Kong, a tertiary referral centre for
haematological malignancy patients. The A&E of
QEH is an urban ED with a daily attendance of 500
and is one of the busiest EDs in Hong Kong. This
study was approved by the chief of service of the department.
Results
A total of 69 patients were recruited; 19 patients were
referents while 50 belonged to the protocol group.
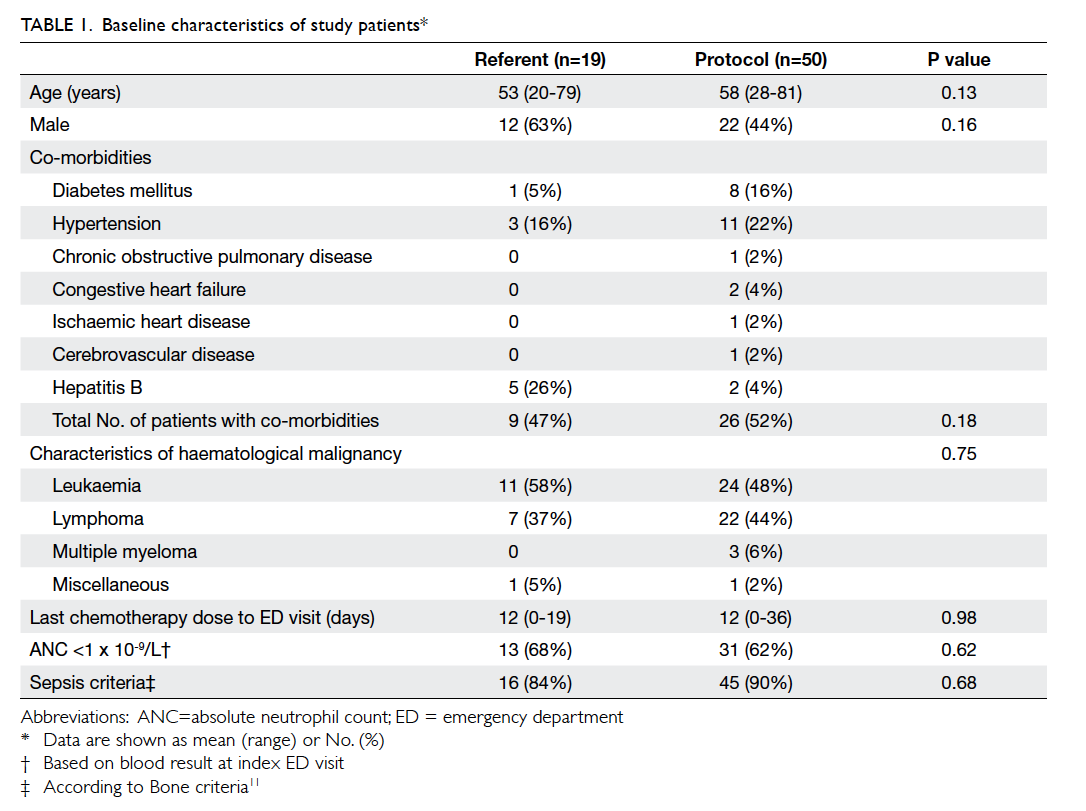
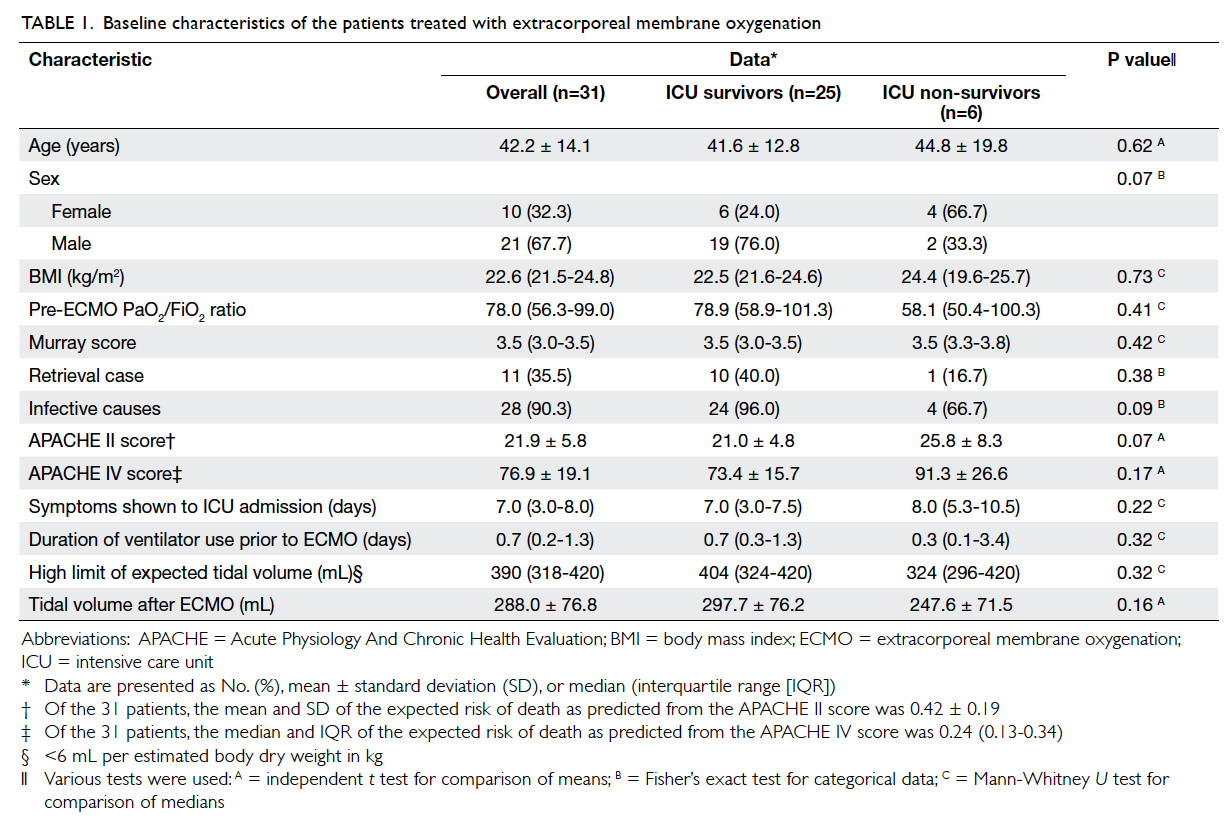
Baseline demographic data are shown in Table 1.
Overall, 49% of the patients were male. Their mean age
was 56 years (range, 20-81 years). Leukaemia was
the most commonly encountered haematological
malignancy, accounting for 51% of cases (n=35/69).
Among these, acute myeloid leukaemia was the most
prevalent subtype. Lymphoma was the second most
common haematological malignancy, making up
42% (n=29/69) of the cases. The mean duration of
the last chemotherapy dose to ED visit was 12 days
in both groups of patients. At least one co-morbidity
was present in 47% of patients in the referent
group and in 52% of patients in the protocol group
(P=0.18).
During the index ED visit, the mean door-to-consultation
time was 15 and 12 minutes in referent
group and protocol group, respectively (P=0.40).
Overall, 88% (n=16/19 in referent and n=45/50 in
protocol group) of the patients fulfilled the sepsis
criteria; 64% (n=44/69) had ANC of <1 x 10-9 /L,
although the result was not known at the time of
consultation. All protocol group patients received
antibiotics after blood cultures were taken during
their ED stay compared to none in the control group.
Tazobactam-piperacillin (Tazocin; Pfizer, Taiwan)
was the most commonly prescribed antibiotic in ED.
The mean DTA time in the protocol group was 47
minutes compared to 300 minutes in referent group
(P<0.05). Overall, 86% (n=43/50) of protocol group
patients could achieve the target DTA time of less
than 1 hour (P<0.05). The shortest time required
for antibiotic administration in the referent group was 70 minutes. The mean length of stay in
ED was 105 minutes in the referent group versus 76
minutes in the protocol group (P=0.46). The major
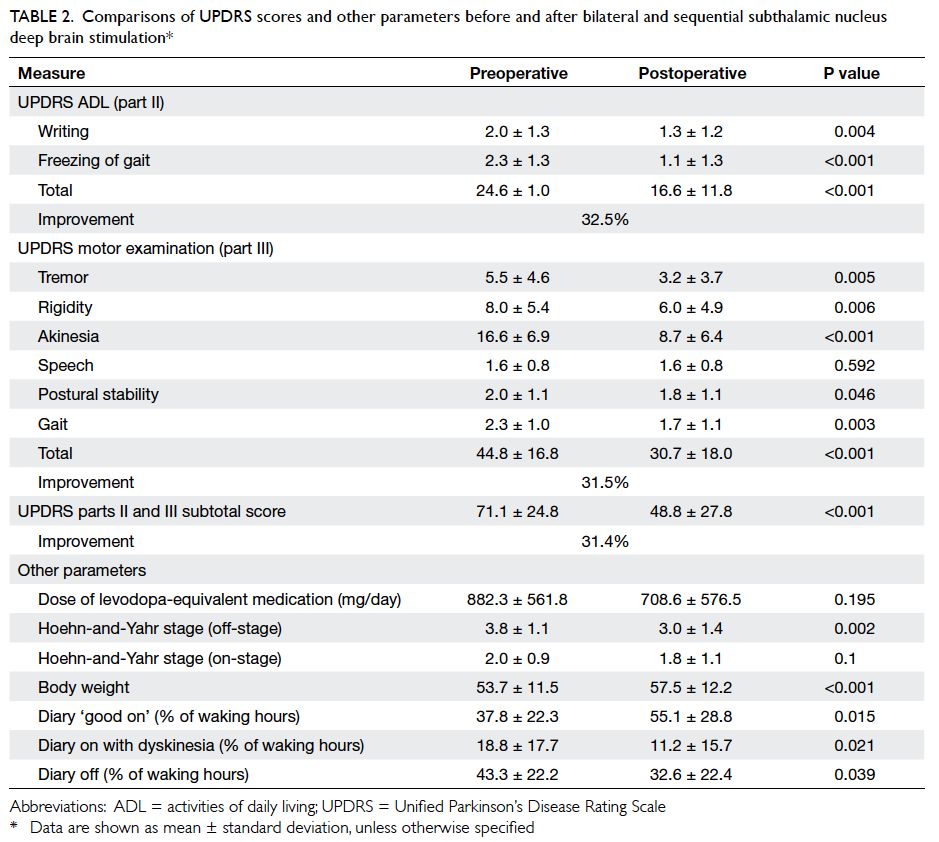
outcomes are shown in Table 2.
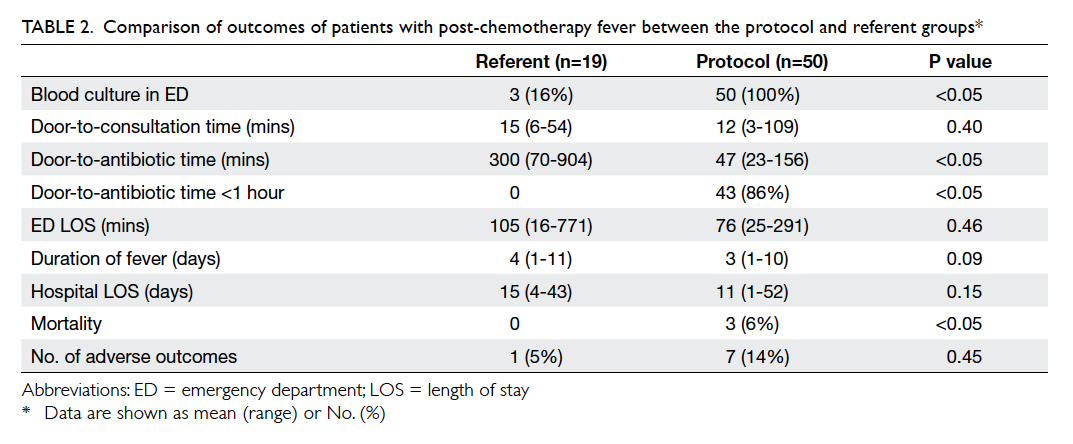
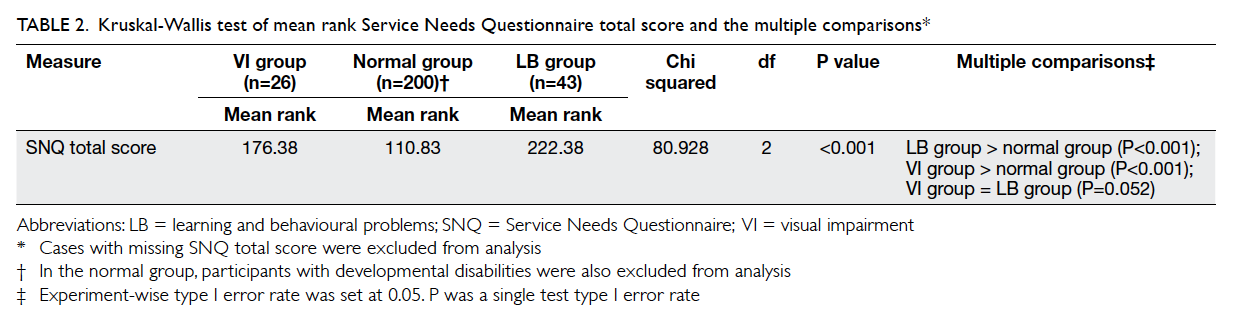
Table 2. Comparison of outcomes of patients with post-chemotherapy fever between the protocol and referent groups
The duration of fever, which was defined as
oral temperature of >38°C for 24 hours, was 4 days
in the referent group and 3 days in the protocol
group (P=0.09). One patient from the referent group
suffered from septic shock and required intensive
care unit (ICU) admission; no patient from this
group died. Six patients in the protocol group had
adverse outcomes; three had septic shock requiring
inotropic support, one of them required ICU
admission, while three patients died during index
admission. Adverse event rate was 5% in the referent
group versus 14% in the protocol group (P=0.45).
Overall, 25% (n=17/69) of patients had bacteraemia.
Escherichia coli was recovered in five samples of
which two were extended-spectrum beta-lactamase
(ESBL)–producing bacteria. Streptococcus mitis
was the second most common pathogen and was
found in four samples. Overall, 43% (n=30/69)
of the patients had microbiologically documented
infection. The mean length of hospital stay was 15
days in the referent group compared with 11 days in the
protocol group (P=0.15).
Discussion
Chemotherapy-induced sepsis is a medical
emergency that requires urgent assessment and
treatment with antibiotics. Our study shows that
88% of post-chemotherapy febrile patients fulfilled
the sepsis criteria. Overall, 25% of patients had
bacteraemia, a rate similar to that reported in
the literature.4 Hence, prompt identification and
early administration of broad-spectrum empirical
antibiotics is the cornerstone of management. In
a retrospective study of 2731 patients with septic
shock (only 7% of whom were neutropenic), each
hour delay in initiating effective antimicrobials
decreased survival by around 8%.13 Another cohort
study showed that the in-hospital mortality among
adult patients with severe sepsis or septic shock
decreased from 33% to 20% when time from triage
to appropriate antimicrobial therapy was ≤1 hour
compared with >1 hour.14 Our protocol suggested
Tazocin as the first-line antibiotic, in accordance
with the 2010 Infectious Diseases Society of America
guideline.4 However, the rising trend of ESBL E coli
infection may raise concern of antibiotic resistance.
A larger-scale cohort study should be carried out
to update the local microbiology prevalence and
amend the empirical antibiotic recommendations
accordingly.
Implementation of the protocol in our
department could significantly reduce the mean
DTA time from 300 minutes to 47 minutes (P<0.05).
Furthermore, 86% of patients could achieve the target
DTA time of <1 hour. The result was satisfactory
when compared with similar studies conducted in
Europe and North America where reported median
DTA ranged from 154 minutes to 3.9 hours.15 16 17
Audits from the UK report that only 18% to 26% of
patients receive initial antibiotic within the target
DTA of 1 hour.8 According to the authors, the
most common reasons for failure to comply with
this time frame included failure to administer the
initial dose of the empirical antibacterial regimen
until the patient has been transferred from ED to
the inpatient ward, prolonged time between arrival
and clinical assessment, lack of awareness of the
natural history of neutropenic fever syndrome and
its evolution to severe sepsis and shock, failure of the
ED to stock appropriate antibacterial medications,
and non-availability of neutropenic fever protocols
in the ED for quick reference.8 The last two points
were further supported by studies. A chart review of
201 febrile neutropenic patients in Canada showed
that the electronic clinical practice guideline could
decrease the DTA time by 1 hour (3.9 hours vs 4.9
hours).16 Another retrospective observational study
of timeliness of antibiotic administration in severely
septic patients presenting to a US community ED
showed that storing key antibiotics could decrease
the mean DTA time by 70 minutes (167 minutes
vs 97 minutes).18 The percentage of severely septic
patients receiving antibiotics within 3 hours of arrival
to the ED increased from 65% pre-intervention to
93% post-intervention.18 Before the implementation
of this protocol, multiple briefing sessions were held
with nurses and physicians to increase awareness
about prompt treatment of post-chemotherapy fever.
Antibiotics were stocked in the ED and were readily
available. The protocol could be easily downloaded
from the department website. Regular collaboration
existed between the nursing manager and the
pharmacist to replenish the antibiotic stock. Thus,
successful implementation of the protocol involved
a joint effort by different parties.
There was a trend towards reducing the
duration of fever and length of hospital stay in the
intervention group. Although this does not imply
causation, especially in view of the small sample size,
the correlation makes one ponder whether a delay
in antibiotic delivery indeed increases the length of
hospital stay. Similar correlation was demonstrated
in a UK review.15 However, we could not demonstrate
an impact on mortality and adverse outcome. The
reason may partly be related to the small sample
size, heterogeneous nature of haematological
malignancies, and overall low incidence of mortality
(4%) in our study as compared to 49.8% in-hospital
mortality rate reported in an 11-year review.19 Our
result shows that the length of ED stay was similar
between control and intervention groups, thus,
demonstrating that this protocol did not add further
burden to the overcrowding ED.
This study has two limitations that need to be
discussed. First, the study used a retrospective chart
review design and there were inherent challenges
with missing information and poor documentation.
Second, prior to implementation of the protocol,
the febrile haematological malignant patients were
often instructed to either attend the day ward or
A&E; this partly explains the relatively small case
number in the control group. In addition, we relied
on the diagnosis coding for case identification in the
control group; some cases might have been missed as
a result of error in coding. Even if they attended ED,
the lack of awareness and reluctance of physicians
to prescribe antibiotics led to a significant delay
in administration of the first dose of antibiotic.
Although the number of control cases was small,
mean DTA time of 300 minutes echoed the same in
a similar study performed overseas.16 Efforts were
made to use accepted chart review methods to assess
outcomes that were automatically recorded in the
electronic ED information systems, and to examine
the nurse records of antibiotic administration.
Conclusion
Implementation of a treatment protocol in post-chemotherapy
febrile haematological malignancy
patients can significantly shorten the mean DTA
time to <1 hour, which is now the standard of care
worldwide. The key to effective implementation lies
in orchestration of efforts between administrators,
physicians, nurses, and pharmacists. We can prove
that the protocol is feasible even in a busy urban ED.
Acknowledgement
We would like to thank Ms Kelly Choy for statistical
analysis.
Declaration
No conflicts of interests were declared by authors.
References
1. Klastersky J. Management of fever in neutropenic patients
with different risks of complications. Clin Infect Dis
2004;39 Suppl 1:S32-7. CrossRef
2. Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 Update
of recommendations for the use of white cell growth
factors: an evidence-based clinical practice guideline. J
Clin Oncol 2006;24:3187-205. CrossRef
3. Herbst C, Naumann F, Kruse EB, et al. Prophylactic
antibiotics or G-CSF for the prevention of infections
and improvement of survival in cancer patients
undergoing chemotherapy. Cochrane Database Syst Rev
2009;(1):CD007107.
4. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice
guideline for the use of antimicrobial agents in neutropenic
patients with cancer: 2010 update by the Infectious Diseases
Society of America. Clin Infect Dis 2011;52:e56-93. CrossRef
5. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis
campaign: international guidelines for management
of severe sepsis and septic shock: 2012. Crit Care Med
2013;41:580-637. CrossRef
6. Bate J, Gibson F, Johnson E, et al. Neutropenic sepsis:
prevention and management of neutropenic sepsis in
cancer patients (NICE Clinical Guideline CG151). Arch
Dis Child Educ Pract Ed 2013;98:73-5. CrossRef
7. Flowers CR, Seidenfeld J, Bow EJ, et al. Antimicrobial
prophylaxis and outpatient management of fever and
neutropenia in adults treated for malignancy: American
Society of Clinical Oncology clinical practice guideline. J
Clin Oncol 2013;31:794-810. CrossRef
8. Clarke RT, Warnick J, Stretton K, Littlewood TJ. Improving
the immediate management of neutropenic sepsis in
the UK: lessons from a national audit. Br J Haematol
2011;153:773-9. CrossRef
9. André S, Taboulet P, Elie C, et al. Febrile neutropenia in
French emergency departments: results of a prospective
multicentre survey. Crit Care 2010;14:R68. CrossRef
10. Hui EP, Leung LK, Poon TC, et al. Prediction of outcome
in cancer patients with febrile neutropenia: a prospective
validation of the Multinational Association for Supportive
Care in Cancer risk index in a Chinese population and
comparison with the Talcott model and artificial neural
network. Support Care Cancer 2011;19:1625-35. CrossRef
11. Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis
and organ failure and guidelines for the use of innovative
therapies in sepsis. The ACCP/SCCM Consensus
Conference Committee. American College of Chest
Physicians/Society of Critical Care Medicine. Chest
1992;101:1644-55. CrossRef
12. Klastersky J, Paesmans M, Rubenstein EB, et al. The
Multinational Association for Supportive Care in Cancer
Risk Index: a multinational scoring system for identifying
low-risk febrile neutropenic cancer patients. J Clin Oncol
2000;18:3038-51.
13. Kumar A, Roberts D, Wood KE, et al. Duration of
hypotension before initiation of effective antimicrobial
therapy is the critical determinant of survival in human
septic shock. Crit Care Med 2006;34:1589-96. CrossRef
14. Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time
to antibiotics on survival in patients with severe sepsis
or septic shock in whom early goal-directed therapy was
initiated in the emergency department. Crit Care Med
2010;38:1045-53. CrossRef
15. Sammut SJ, Mazhar D. Management of febrile neutropenia
in an acute oncology service. QJM 2012;105:327-36. CrossRef
16. Lim C, Bawden J, Wing A, et al. Febrile neutropenia in EDs:
the role of an electronic clinical practice guideline. Am J
Emerg Med 2012;30:5-11, 11.e1-5.
17. Nirenberg A, Mulhearn L, Lin S, Larson E. Emergency
department waiting times for patients with cancer with
febrile neutropenia: a pilot study. Oncol Nurs Forum
2004;31:711-5. CrossRef
18. Hitti EA, Lewin JJ 3rd, Lopez J, et al. Improving door-to-antibiotic
time in severely septic emergency department
patients. J Emerg Med 2012;42:462-9. CrossRef
19. Legrand M, Max A, Peigne V, et al. Survival in neutropenic
patients with severe sepsis or septic shock. Crit Care Med
2012;40:43-9. CrossRef
Find HKMJ in MEDLINE: