Hong Kong Med J 2014 Aug;20(4):290–6 | Epub 25 Apr 2014
DOI: 10.12809/hkmj134071
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Acanthosis nigricans in obese Chinese children
HY Ng, MB, ChB, MRCPCH;
Jack HM Young, MB, ChB, MRCPCH;
KF Huen, FHKCPaed, FHKAM (Paediatrics);
Louis TW Chan, FHKCPaed, FHKAM (Paediatrics)
Department of Paediatrics and Adolescent Medicine, Tseung Kwan O
Hospital, Tseung Kwan O, Hong Kong
Corresponding author: Dr HY Ng (nghypatrick@gmail.com)
Abstract
Objectives: To investigate the demographic
characteristics and insulin resistance in local
overweight/obese Chinese children with and
without acanthosis nigricans, and the associations
of acanthosis nigricans with insulin resistance and
other cardiometabolic co-morbidities.
Design: Case series with cross-sectional analyses.
Setting: A regional hospital in Hong Kong.
Patients: Chinese children assessed between
January 2006 and December 2010 at Tseung Kwan O
Hospital for being overweight or obese.
Main outcome measures: The demographics,
anthropometric data, acanthosis nigricans status,
and biochemical results were analysed.
Results: A total of 543 overweight/obese children
were studied with 64% being boys and 29% had
insulin resistance. Adolescents aged 12 to 18 years,
compared with children aged 5 to 11 years, were
more likely to have acanthosis nigricans (63% vs
47%; P<0.001) and insulin resistance (37% vs 25%;
P=0.005). Compared with overweight children,
those who were obese were more likely to have
the two conditions: acanthosis nigricans (59% vs
44%; P=0.005) and insulin resistance (35% vs 19%;
P=0.001). Compared with those without acanthosis
nigricans, those with the condition had significantly
higher mean values for systolic blood pressures (P<0.001), 2-hour post-oral glucose tolerance
test glucose level (P=0.021), fasting insulin level
(P<0.001), homeostasis model of assessment–insulin resistance (P<0.001), fasting triglyceride
level (P<0.001), and alanine aminotransferase level
(P=0.002), but a lower high-density lipoprotein
cholesterol level (P<0.001). Those with acanthosis
nigricans were also more likely to have insulin
resistance (P<0.001), hypertension (P=0.021), fatty
liver (P=0.001), and abnormal glucose homeostasis
(P=0.003).
Conclusion: Obese Chinese children and adolescents
with acanthosis nigricans had a higher chance of
having insulin resistance and cardiometabolic co-morbidities.
Acanthosis nigricans is an important
clinical feature warranting early attention and
evaluation to facilitate timely interventions and
monitoring.
New knowledge added by this
study
- Hong Kong Chinese children with acanthosis nigricans were more likely to have insulin resistance, hypertension, fatty livers, and abnormal glucose homeostasis.
- In children, acanthosis nigricans is an important clinical sign warranting early attention and evaluation.
Introduction
Obesity was formally recognised as a global epidemic
by the World Health Organization (WHO) in 1997.1
During the past decades, the prevalence of being
overweight and obese has increased substantially. In
Hong Kong, 17% of children were overweight/obese
in 2005/6, which was a 5% increase since 1993, based
on International Obesity Task Force cut-offs.2
Overweight/obese children and adolescents
are more likely to have hyperinsulinaemia,
hypertension, and dyslipidaemia.3 The clustering
of cardiometabolic risk factors in these patients
tends to track into adult life.3 However, the Diabetes Prevention Program demonstrated that lifestyle
interventions could prevent or postpone the
onset of type 2 diabetes mellitus (DM) by 58% in
adults.4 Thus, identifying at-risk groups may allow
early interventions and prevention of potential
cardiometabolic complications.
Acanthosis nigricans (AN)—a hyperpigmented,
thickened, and velvety dermatosis at the nape of the
neck or axilla—is an easily identifiable physical sign.5
The American Diabetes Association includes it as an
indicator of DM risk in overweight youths entering
puberty.6 Yet, some authors have argued that it is not
an independent predictor of insulin resistance (IR) if
body mass index (BMI) is controlled for.7
Ethnic differences occur in obesity indices
and their associated risk factors include IR.8 Local
studies focusing on associations between AN with
IR and other cardiometabolic co-morbidities in
Chinese paediatric age-groups are sparse. In this
regional centre study, we describe the demographic
characteristics and IR in obese Chinese children
with and without AN, with a focus on exploring the
associations of AN with IR and other cardiometabolic
co-morbidities.
Methods
A retrospective study was conducted by recruiting
overweight/obese children and adolescents between
5 and 18 years of age who underwent obesity
assessment between January 2006 and December
2010 in a regional hospital in Hong Kong. Patients
were excluded if they had underlying metabolic
diseases, chronic diseases, or other medical
conditions resulting in obesity. Patients taking on
medications that would alter metabolic profiles were
also excluded.
Anthropometric data and AN status were recorded. Blood samples were collected. Ultrasound
liver scans were performed on patients with elevated
alanine aminotransferase (ALT) levels. Height was
measured to the nearest 0.1 cm using the Harpenden
stadiometer (Holtain; Crymych, UK)
and body weight to the nearest 0.1 kg with light
clothing using an electronic column scale (SECA-780; Seca Ltd, Hamburg, Germany). The BMI
(kg/m2) percentiles of 90th and 97th centiles
were used to define overweight and obesity, respectively.9 10
Local percentile standards were based on a local
population survey conducted in 1993.11 The BMI
z-score was calculated using this local age- and
gender-specific reference. Blood pressure (BP) was
measured using the standard oscillometric method
(BP-8800C; Colin Electronics, Komaki, Japan) in the
daytime with the children seated and rested. Average
BP was obtained from two measurements. The BP
z-score was calculated using the local BP reference.12
Participants were considered hypertensive if the
mean systolic BP z-score and/or diastolic BP (DBP) z-score
was/were greater than or equal to the 95th centiles
for age and gender.
The diagnosis of AN was made by
paediatricians; additional scoring for this entity was
not undertaken as not all authors agreed that specific
quantitative scales could improve the accuracy of IR
prediction.13
Blood samples for plasma glucose, insulin,
lipid profile, and liver enzymes following an
overnight fasting were obtained and a standard
oral glucose tolerance test (OGTT) was performed.
The homeostasis model of assessment (HOMA)–IR value was used to assess IR using the following
equation: fasting glucose (mmol/L) x fasting insulin
(µU/mL)/22.5.14 Any HOMA value of ≥4 was
considered to indicate IR. Glucose abnormalities
were defined according to criteria from the WHO.15
Abnormal glucose homeostasis was referred to any
combination of impaired fasting glucose, impaired
glucose tolerance, or DM on the basis of fasting or
2-hour plasma glucose levels in the OGTT.16 17 Fatty
liver was diagnosed by ultrasound scan affirmed by
the operational definition of non-alcoholic fatty liver
disease in the Asia-Pacific region.18
Statistical analyses
The statistical analyses were conducted using the
Statistical Product and Service Solutions (version
17.0 for Windows 7). Taking P<0.05 as statistically
significant, Student’s t test and Wilcoxon rank-sum
test were used to compare results with a normal and
skewed distribution, respectively. The Chi squared
test or Fisher’s exact test as appropriate were used
to analyse categorical variables. Multiple logistic
regression analysis was then performed to identify
independent factors associated with IR. To avoid
multicollinearity, body weight and height were not used in the model, since both variables correlated
highly with BMI. For the same reason, fasting insulin and glucose levels were not selected
for the model as the HOMA-IR was derived from
them. The model was simplified in a backward
stepwise fashion by removing variables with P values
of >0.1. Goodness-of-fit of the regression
model was tested with the Hosmer-Lemeshow test.
Results
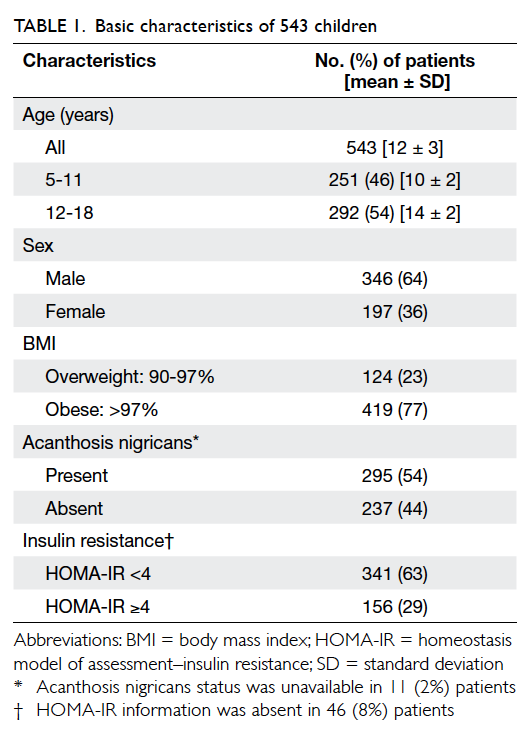
A total of 543 overweight/obese Chinese patients
were included in this study. They had a mean ± standard deviation age of 12 ± 3 years and 64%
(n=346) of them were boys. The majority (77%,
n=419) were obese with a BMI of >97%. In all, AN
was present in 54% (n=295) of the subjects and
29% (n=156) of them had IR. Relevant data are
summarised in Table 1.
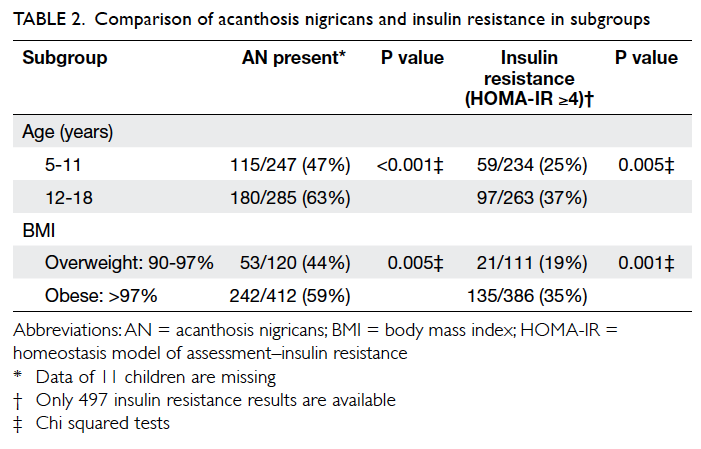
Table 2 illustrates that adolescents (aged 12-18
years), compared with younger children (aged 5-11
years), were more likely to have AN (63% vs 47%;
P<0.001) and IR (37% vs 25%; P=0.005). Obese
children, compared with overweight children, were
also more likely to have AN (59% vs 44%; P=0.005)
and IR (35% vs 19%; P=0.001).
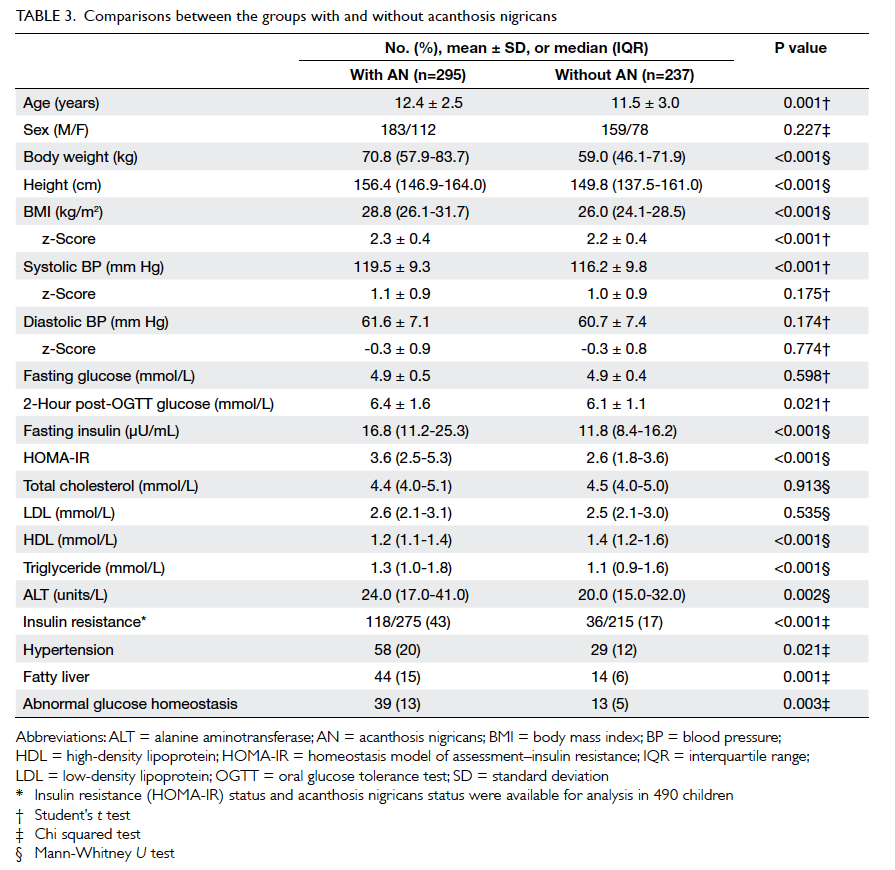
Table 3 shows baseline characteristics and
biochemical parameters in children with and
without AN. Apart from being older, the group with
AN had higher mean 2-hour post-OGTT glucose
(P=0.021), fasting insulin (P<0.001), triglyceride
(P<0.001), and ALT (P=0.002) levels, but lower mean
levels of high-density lipoprotein (HDL) cholesterol
(P<0.001). Their BMI (P<0.001), BMI z-score
(P<0.001), systolic blood pressure (SBP) [P<0.001],
and HOMA-IR values (P<0.001) were also higher.
Notably, the higher SBP, when converted to SBP
z-score (taking into account age and gender), was
no longer significant. Both DBP and DBP z-scores showed no differences
between the two groups. The presence of IR and
other cardiometabolic co-morbidities in subjects
with and without AN are also shown in Table 3.
The frequencies of IR, hypertension, fatty liver, and
abnormal glucose homeostasis were all significantly
higher in subjects with AN.
Further analysis of risk factors for IR using the multiple logistic regression model showed that the presence of AN (odds ratio [OR]=2.36; 95% confidence interval [CI], 1.46-3.80; P<0.001), older age (1.17; 1.07-1.28; P=0.001), higher triglyceride level (1.91; 1.33-2.74; P<0.001), and higher BMI z-score (6.95; 3.40-14.16; P<0.001) were significant independent variables predicting IR (Table 4). However, though HDL and 2-hour post-OGTT glucose level were borderline significant predictors for IR, their effect sizes were small. The Hosmer-Lemeshow test of goodness-of-fit was 0.315, indicating a good logistic regression model fit.
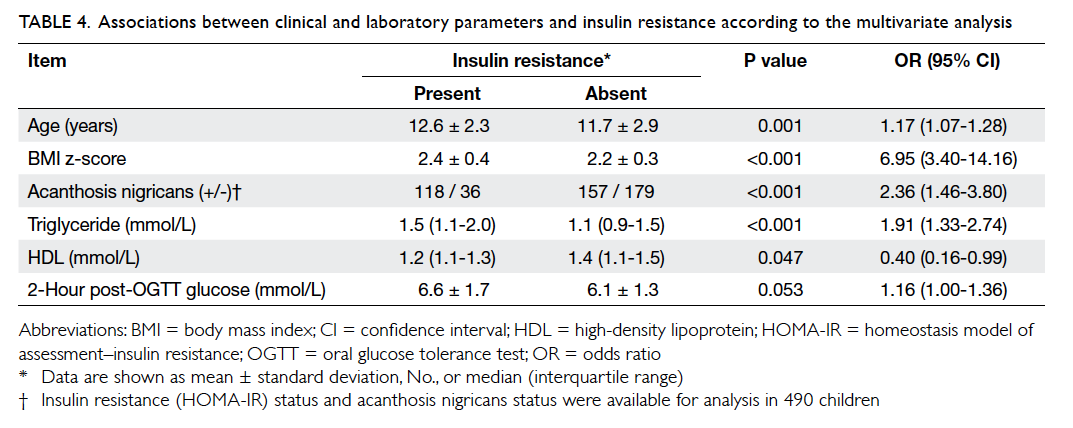
Table 4. Associations between clinical and laboratory parameters and insulin resistance according to the multivariate analysis
Discussion
Obesity is a public health problem that has become
epidemic worldwide. In the primary care setting,
identifying children with AN may allow early
implementation of interventions to prevent the
development of DM and other cardiometabolic
co-morbidities in overweight/obese children.16
Searching for AN over the neck is easy, non-intrusive.
and acceptable to the children.19 Presence of AN can
also be used as a grounds to initiate and reinforce
discussions about lifestyle modification.5 19 20
An observed AN frequency of 54% in our
subjects was consistent with data reported in the literature.21 22 Our adolescents were more likely
to have AN than younger children, in line with
hyperinsulinaemia being more severe among older
individuals.22 In our study, development of AN showed no gender preference, as in a study of 1412
unselected children by Stuart et al.23 In our cohort
and that in Nsiah-Kumi et al’s study,13 obese children
were more likely to have AN than overweight ones.
Whilst IR is a hallmark of obesity, it is also
associated with other metabolic derangements and
clinical or subclinical cardiovascular diseases.24 We
used the HOMA-IR value—a simple, validated, and
practical marker of IR in the paediatric population—to give a more physiological estimate of glucose
homeostasis,25 that was also shown to correlate well
with the hyperinsulinaemic-euglycaemic glucose
clamp technique, a gold standard for quantifying
insulin sensitivity.24 In a local community-based
cross-sectional study, it was shown that the mean
HOMA-IR value was lower among Hong Kong
Chinese adolescents than subjects in the United
States.8 Currently, there is no worldwide consensus
on defining IR among children. Some studies have chosen an HOMA-IR value as low as 2.7 while
others have shown that a value of 4 can be present
in pubertal children (because of the transient
physiological IR during puberty).13 Although we
do not have data about pubertal stage in our study
subjects, an HOMA-IR of ≥4 would be a conservative
but reasonable definition of IR, in parallel with the
threshold used in a multicentre trial in the United
States (Studies to Treat or Prevent Pediatric Type 2
Diabetes—STOPP-T2DM).26
In our study, 29% of our cohort had IR using
the cut-off HOMA-IR of ≥4, and the mean value
was higher among those with AN present (3.6
vs 2.6; P<0.001). Notably, IR was more common
among adolescents than young children (37%
vs 25%; P=0.005) as well as among obese than
overweight subjects (35% vs 19%; P=0.001). Goran
et al27 suggested that long-standing obesity and
the physiological IR during puberty accounted for
adolescents having more AN and IR. They found that
pubertal transition from Tanner I to Tanner III was
associated with a 32% reduction in insulin sensitivity
across different genders and ethnicities, and proved
that body fat was the predominant factor influencing
IR whereas total and visceral fat both contributed
independently to lower insulin sensitivity.27 Notably,
25% of our young (5-11 years old) overweight/obese
subjects already had IR, suggesting that the onset
of metabolic derangement might have started long
before adolescence and indicates that screening
should begin early during childhood.
In our cohort, IR and other cardiometabolic
co-morbidities were more prevalent among those
with AN. The relationship of AN with hypertension
may not be as strong as that with fatty liver and
abnormal glucose homeostasis. This might be
consistent with hypertension being more closely
related to obesity than to AN.28 Nevertheless, studies
assessing the relationship of BP and insulin levels
are conflicting.29 Some authors postulate that the
underlying pathophysiology is a common genetic
predisposition to both IR and hypertension, whilst
also involving other mechanisms.30
Dyslipidaemia is believed to play a central role
in the development of heart diseases. High level
of triglyceride and low level of HDL cholesterol
are commonly used criteria to define metabolic
syndrome both in children and adults.31 High
triglyceride levels and the IR index (HOMA-IR) were
strong, independent predictors of increased carotid
intima-media thickness, which was a non-invasive
measure of subclinical atherosclerosis in paediatric
research.32 Nevertheless, low HDL cholesterol
level carried an even greater relative risk than high
triglyceride levels.33 Compared with those without
AN, subjects with the condition had a higher mean
triglyceride level (P<0.001) but lower HDL level
(P<0.001), and hence their future cardiovascular health seems to be of great concern.
Fatty liver, or non-alcoholic fatty liver disease
(NAFLD), can be classified into isolated fatty liver
in which there is only accumulation of fat, and non-alcoholic
steatohepatitis (NASH) in which there is
fat accumulation and damage to liver cells. Presence
of the latter is associated with raised liver enzymes
and more abnormal ultrasound scans. Our subjects
with AN had higher levels of ALT (P=0.002) and
a higher proportion with fatty livers. In contrast,
Uwaifo et al34 reported that AN was not common
among a small cohort of 28 subjects with biopsy-proven
NASH, despite their high prevalence of IR.
These authors therefore questioned the use of AN
as an index of IR in patients with NASH. However,
in our study liver ultrasounds were only performed
in children with raised ALT levels. According to
Sartorio et al,35 the ALT level alone was insufficient
as a marker of NAFLD and the sensitivity of using its
level to predict NAFLD was as low as 41% (depending
on the cut-off used). Several prediction scores have
been developed for non-invasive liver steatosis
screening, but they have insufficient diagnostic
accuracy among obese children.36
For DM, incidence, prevalence, and disease
progression are believed to vary in different ethnic
groups. The overall frequency of abnormal glucose
homeostasis of 10% (8% impaired glucose tolerance
and 2% with DM; data not shown) was lower than in a
recent study by Brickman et al16 who reported a 29%
frequency of abnormal glucose homeostasis among
a group of 8-to-14 years old, mainly of Hispanic
and African American children with AN. Another
study from the United Kingdom found a higher
frequency of type 2 DM among African-Caribbean
and South Asian groups, while the Chinese and
white Caucasians had the lowest frequencies.37 The
reasons for such inter-ethnic differences are still
unclear but do not seem to be solely genetic, as
inter-generational social factors may also modify the
evolution and biology of the disease.37 Our results,
together with the recently reported sharp rise in
the incidence of type 2 DM in Hong Kong children
aged under 19 years after 2004,38 should alert our
health care professionals as to the importance of
early detection of potential predictors of abnormal
glucose metabolism such as AN.
Recently, the role of IR in cardiometabolic
derangements has attracted more attention.
Nevertheless, there is no prediction model for IR in
our local children and adolescents. Using multivariate
analysis, our study demonstrates that age, AN status,
triglyceride level, and BMI z-score are significant
independent variables associated with IR. Hopefully,
a simple and practical prediction model of IR with
acceptable sensitivity and specificity can be derived
by combining these clinical findings, anthropometric
measurements, and biochemical markers.
Limitations
Important limitations of this study included its
retrospective design, being single-centred, and
thus not being suitable for calculating population-based
rates. In addition, the stage of puberty (not
documented) may also influence IR. Moreover,
several relevant risk factors (family history of
metabolic derangement, maternal gestational DM,
duration of obesity, socio-economic status) were not
included in the analysis. As in other retrospective
studies, it was not possible to retrieve every single
item of data. Notably, AN status was unavailable in
11 (2%) patients while HOMA-IR information was
absent in 46 (8%) of the subjects, as fasting insulin
levels were not checked and might have contributed
to selection bias. Our study was clinic-based and
not population-based, and so an overestimate of
morbidity was a possibility. Besides, establishing
a relationship between cause and effect was not
possible due to the cross-sectional nature of the
study. Growth data collected in 1993 (HK1993)
are still widely used locally and seem appropriate
in Hong Kong.9 We adopted the operational BMI
cut-offs for daily use locally. However, the ideal
cut-offs for being overweight and having obesity
remain controversial, and various definitions and
operational values exist.39 These problems may also
limit direct comparisons between different studies
using different growth references and cut-offs.39
Conclusion
Local obese Chinese children with AN are at higher
risk of IR and cardiometabolic co-morbidities.
Primary care physicians should be vigilant for this
clinical sign. If present, early attention is necessary
to achieve early intervention. Further studies may
be necessary to evaluate the longitudinal risk
relationship between AN and cardiometabolic
outcomes.
References
1. Caballero B. The global epidemic of obesity: an overview. Epidemiol Rev 2007;29:1-5. CrossRef
2. So HK, Nelson EA, Li AM, et al. Secular changes in height, weight and body mass index in Hong Kong Children. BMC Public Health 2008;8:320. CrossRef
3. Bao W, Srinivasan SR, Wattigney WA, Berenson GS. Persistence of multiple cardiovascular risk clustering related to syndrome X from childhood to young adulthood: the Bogalusa Heart Study. Arch Intern Med 1994;154:1842-7. CrossRef
4. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-403. CrossRef
5. Kong AS, Williams RL, Smith M, et al. Acanthosis nigricans and diabetes risk factors: prevalence in young persons seen in southwestern US primary care practices. Ann Fam Med 2007;5:202-8. CrossRef
6. American Diabetes Association. Type 2 diabetes in children and adolescents. Diabetes Care 2000;23:381-9. CrossRef
7. Ice CL, Murphy E, Minor VE, Neal WA. Metabolic syndrome in fifth grade children with acanthosis nigricans: results from the CARDIAC project. World J Pediatr 2009;5:23-30. CrossRef
8. Kong AP, Choi KC, Ko GT, et al. Associations of overweight with insulin resistance, beta-cell function and inflammatory markers in Chinese adolescents. Pediatr Diabetes 2008;9:488-95. CrossRef
9. So HK, Nelson EA, Sung RY, Ng PC. Implications of using World Health Organization growth reference (2007) for identifying growth problems in Hong Kong children aged 6 to 18 years. Hong Kong Med J 2011;17:174-9.
10. Ng DK, Lam YY, Kwok KL, Chow PY. Obstructive sleep apnoea syndrome and obesity in children. Hong Kong Med J 2004;10:44-8.
11. Leung SS, Cole TJ, Tse LY, Lau JT. Body mass index reference curves for Chinese children. Ann Hum Biol 1998;25:169-74. CrossRef
12. Sung RY, Choi KC, So HK, et al. Oscillometrically measured blood pressure in Hong Kong Chinese children and associations with anthropometric parameters. J Hypertens 2008;26:678-84. CrossRef
13. Nsiah-Kumi PA, Beals J, Lasley S, et al. Body mass index percentile more sensitive than acanthosis nigricans for screening Native American children for diabetes risk. J Natl Med Assoc 2010;102:944-9.
14. Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412-9. CrossRef
15. Definition, diagnosis and classification of diabetes and its complications: report of a WHO consultation, part 1: diagnosis and classification of diabetes mellitus. Geneva, Switzerland: World Health Organization; 1999.
16. Brickman WJ, Huang J, Silverman BL, Metzger BE. Acanthosis nigricans identifies youth at high risk for metabolic abnormalities. J Pediatr 2010;156:87-92. CrossRef
17. Atabek ME, Pirgon O, Kurtoglu S. Assessment of abnormal glucose homeostasis and insulin resistance in Turkish obese children and adolescents. Diabetes Obes Metab 2007;9:304-10. CrossRef
18. Chitturi S, Farrell GC, Hashimoto E, et al. Non-alcoholic fatty liver disease in the Asia-Pacific region: definitions and overview of proposed guidelines. J Gastroenterol Hepatol 2007;22:778-87. CrossRef
19. Smith WG, Gowanlock W, Babcock K, et al. Prevalence of acanthosis nigricans in First Nations children in Central Ontario, Canada. Can J Diabetes 2004;28:410-4.
20. Kong AS, Williams RL, Rhyne R, et al. Acanthosis nigricans: high prevalence and association with diabetes in a practice-based research network consortium—a PRImary care Multi-Ethnic network (PRIME Net) study. J Am Board Fam Med 2010;23:476-85. CrossRef
21. Shalitin S, Abrahami M, Lilos P, Phillip M. Insulin resistance and impaired glucose tolerance in obese children and adolescents referred to a tertiary-care center in Israel. Int J Obes (Lond) 2005;29:571-8. CrossRef
22. Kluczynik CE, Mariz LS, Souza LC, Solano GB, Albuquerque FC, Medeiros CC. Acanthosis nigricans and insulin resistance in overweight children and adolescents. An Bras Dermatol 2012;87:531-7. CrossRef
23. Stuart CA, Pate CJ, Peters EJ. Prevalence of acanthosis nigricans in an unselected population. Am J Med 1989;87:269-72. CrossRef
24. Singh B, Saxena A. Surrogate markers of insulin resistance: a review. World J Diabetes 2010;1:36-47. CrossRef
25. Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics 2005;115:e500-3. CrossRef
26. Studies to Treat or Prevent Pediatric Type 2 Diabetes Prevention Study Group. Prevalence of the metabolic syndrome among a racially/ethnically diverse group of U.S. eighth-grade adolescents and associations with fasting insulin and homeostasis model assessment of insulin resistance levels. Diabetes Care 2008;31:2020-5. CrossRef
27. Goran MI, Ball GD, Cruz ML. Obesity and risk of type 2 diabetes and cardiovascular disease in children and adolescents. J Clin Endocrinol Metab 2003;88:1417-27. CrossRef
28. Sorof JM, Lai D, Turner J, Poffenbarger T, Portman RJ. Overweight, ethnicity, and the prevalence of hypertension in school-aged children. Pediatrics 2004;113:475-82. CrossRef
29. Jessup A, Harrell JS. The metabolic syndrome: look for it in children and adolescents, too! Clin Diabetes 2005;23:26-32. CrossRef
30. El-Atat FA, Stas SN, McFarlane SI, Sowers JR. The relationship between hyperinsulinemia, hypertension and progressive renal disease. J Am Soc Nephrol 2004;15:2816-27. CrossRef
31. Reinehr T, de Sousa G, Toschke AM, Andler W. Comparison of metabolic syndrome prevalence using eight different definitions: a critical approach. Arch Dis Child 2007;92:1067-72. CrossRef
32. Fang J, Zhang JP, Luo CX, Yu XM, Lv LQ. Carotid intima-media thickness in childhood and adolescent obesity relations to abdominal obesity, high triglyceride level and insulin resistance. Int J Med Sci 2010;7:278-83. CrossRef
33. Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab 2004;89:2583-9. CrossRef
34. Uwaifo GI, Tjahjana M, Freedman RJ, Lutchman G, Promrat K. Acanthosis nigricans in patients with nonalcoholic steatohepatitis: an uncommon finding. Endocr Pract 2006;12:371-9. CrossRef
35. Sartorio A, Del Col A, Agosti F, et al. Predictors of non-alcoholic fatty liver disease in obese children. Eur J Clin Nutr 2007;61:877-83. CrossRef
36. Koot BG, van der Baan-Slootweg OH, Bohte AE, et al. Accuracy of prediction scores and novel biomarkers for predicting nonalcoholic fatty liver disease in obese children. Obesity (Silver Spring) 2013;21:583-90. CrossRef
37. Oldroyd J, Banerjee M, Heald A, Cruickshank K. Diabetes and ethnic minorities. Postgrad Med J 2005;81:486-90. CrossRef
38. Huen KF, Low LC, Cheung PT, et al. An update on the epidemiology of childhood diabetes in Hong Kong. Hong Kong J Paediatr 2009;14:252-9.
39. Rolland-Cachera MF. Childhood obesity: current definitions and recommendations for their use. Int J Pediatr Obes 2011;6:325-31. CrossRef