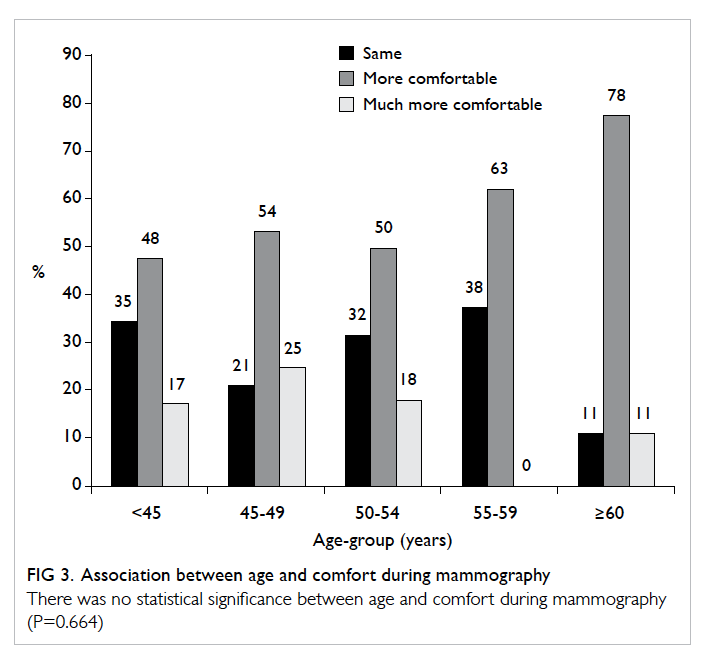
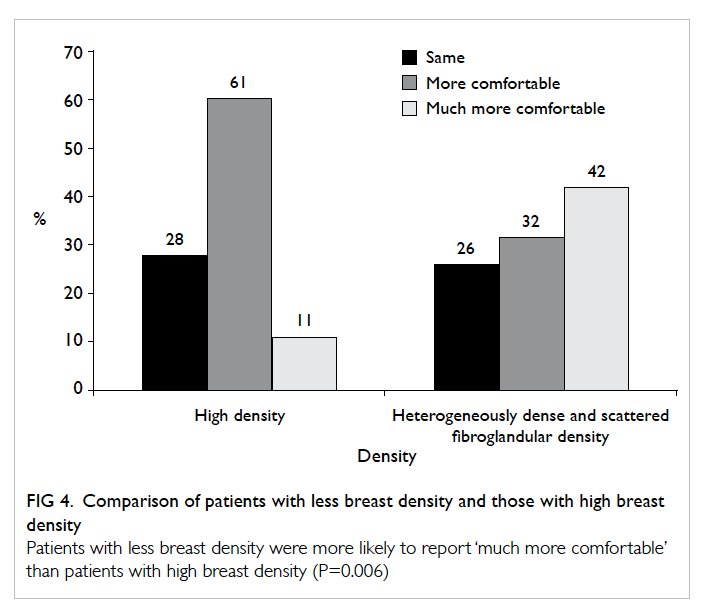
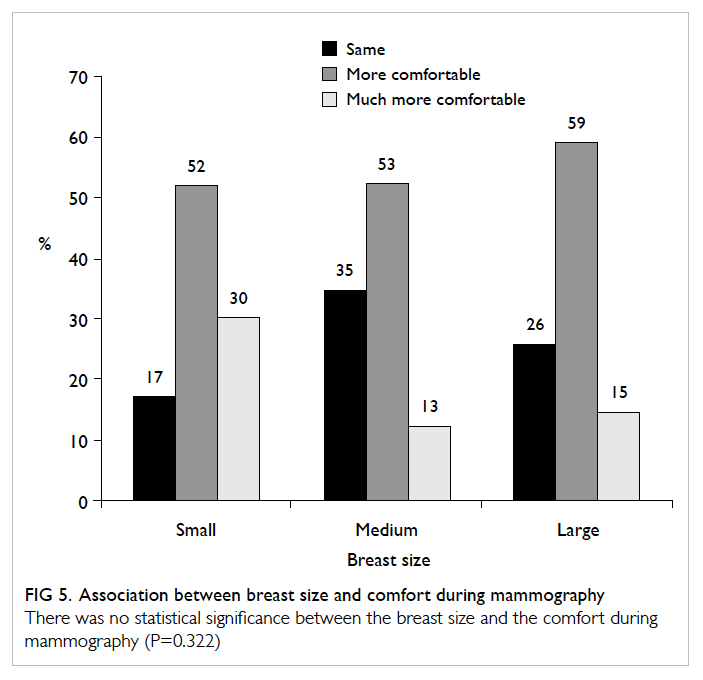
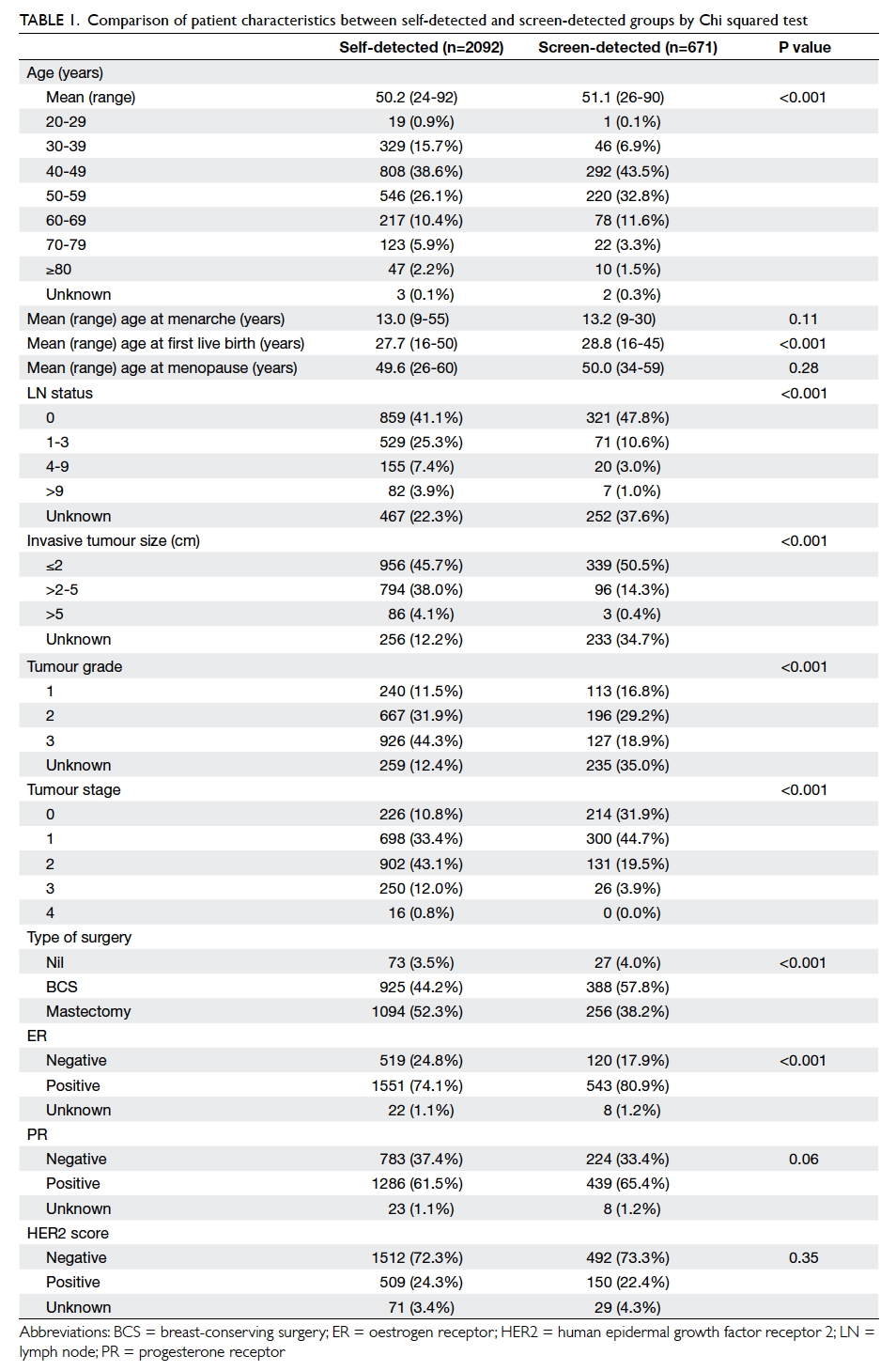
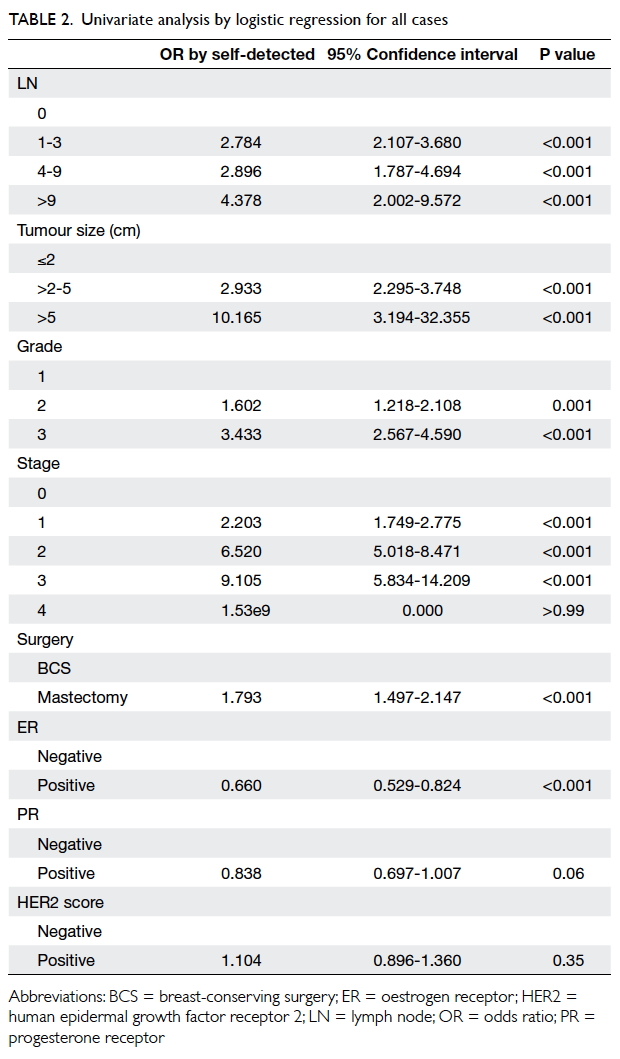
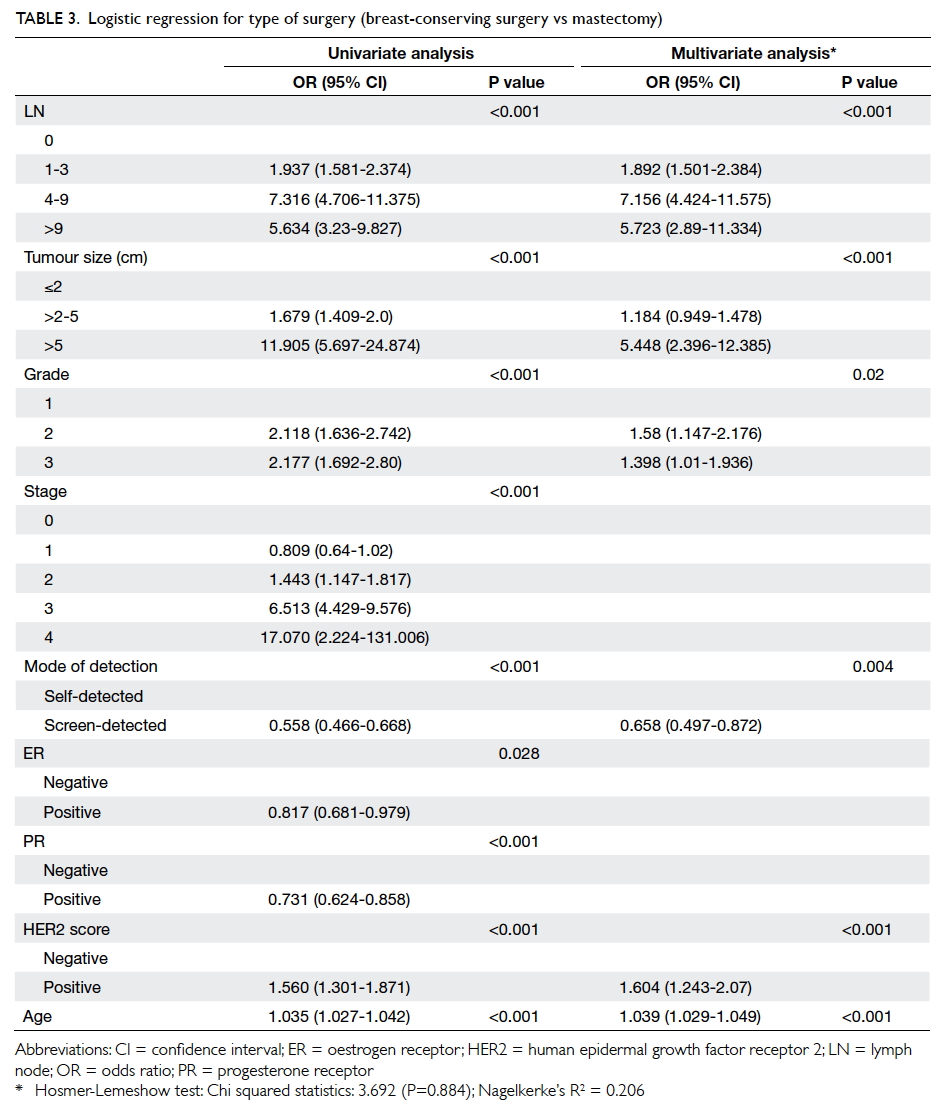
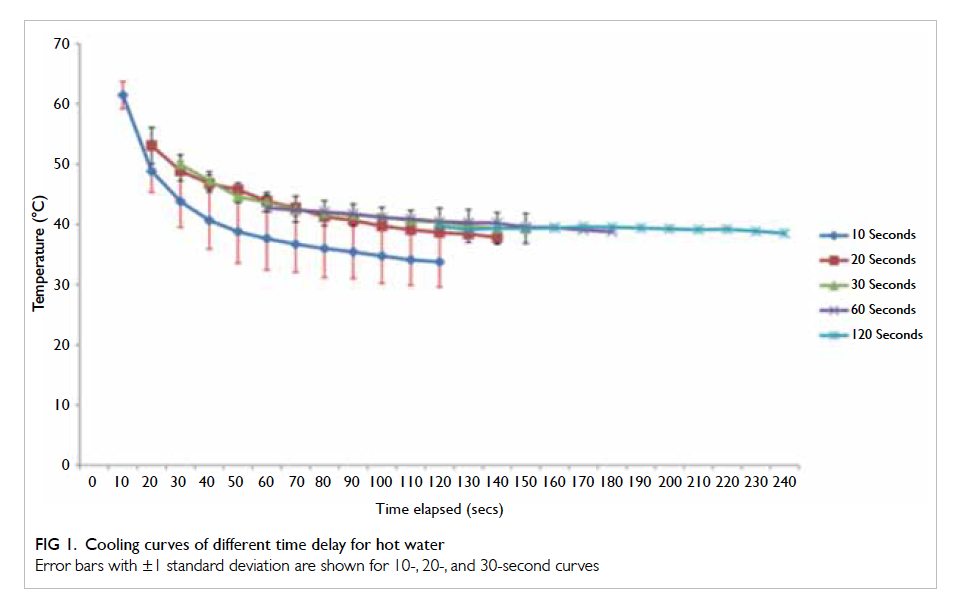
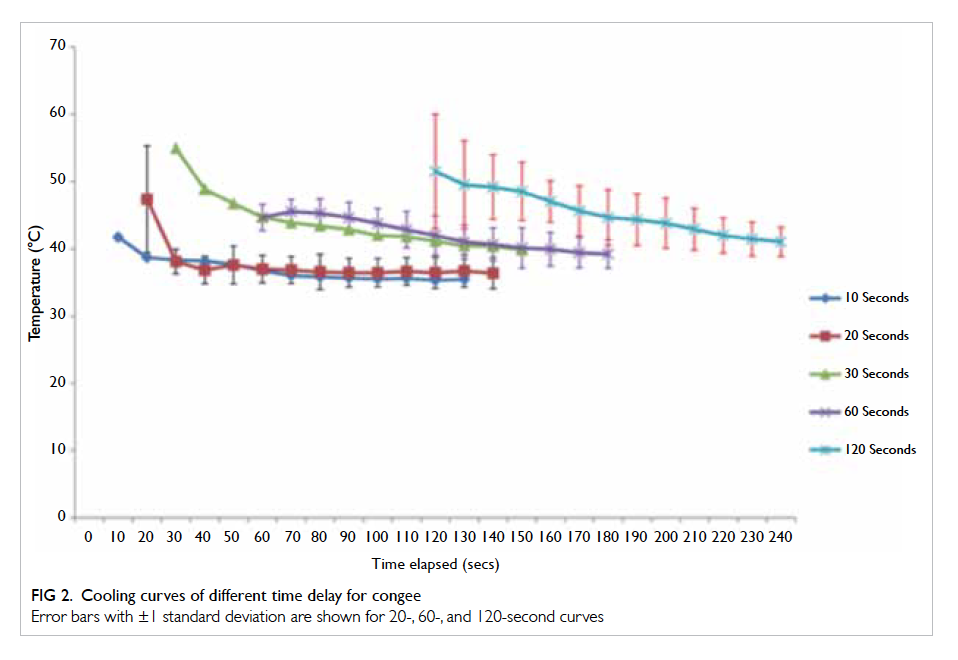
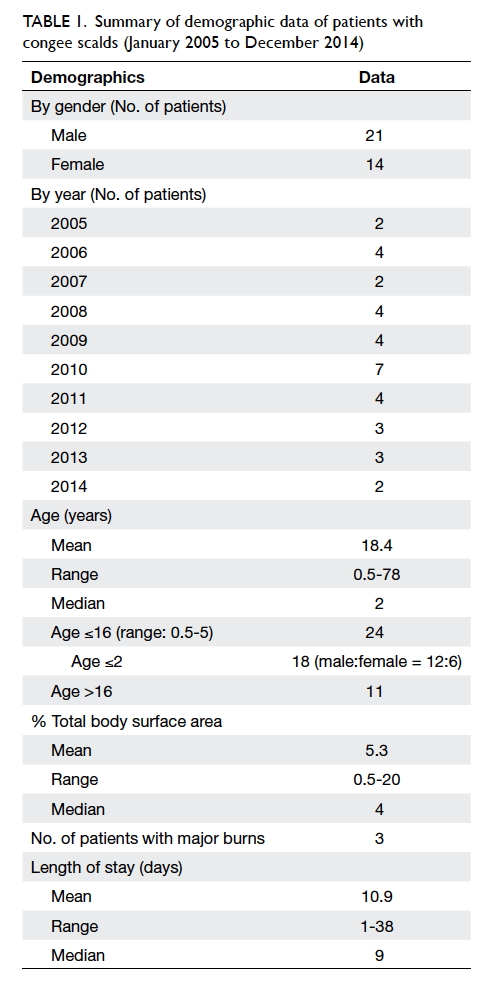
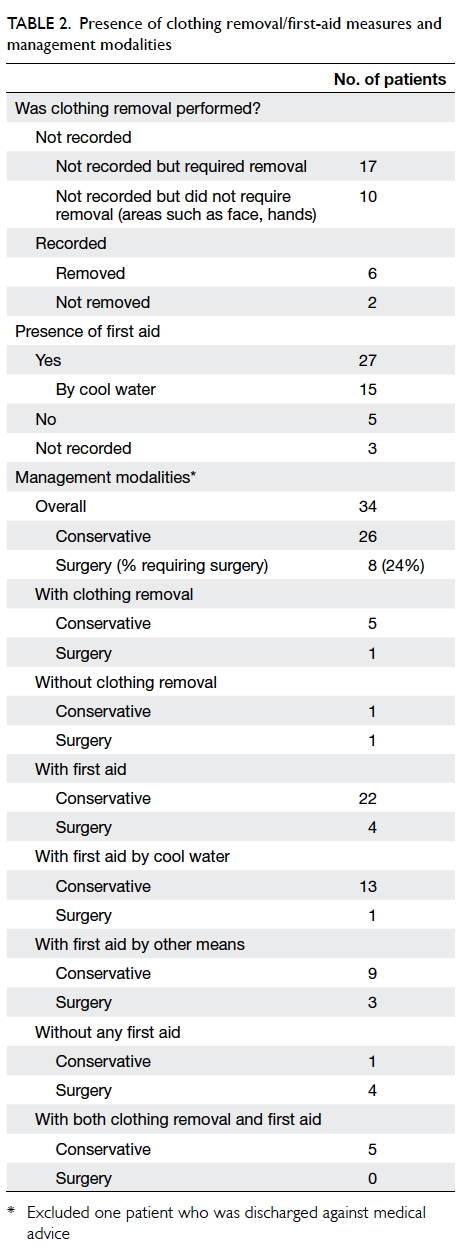
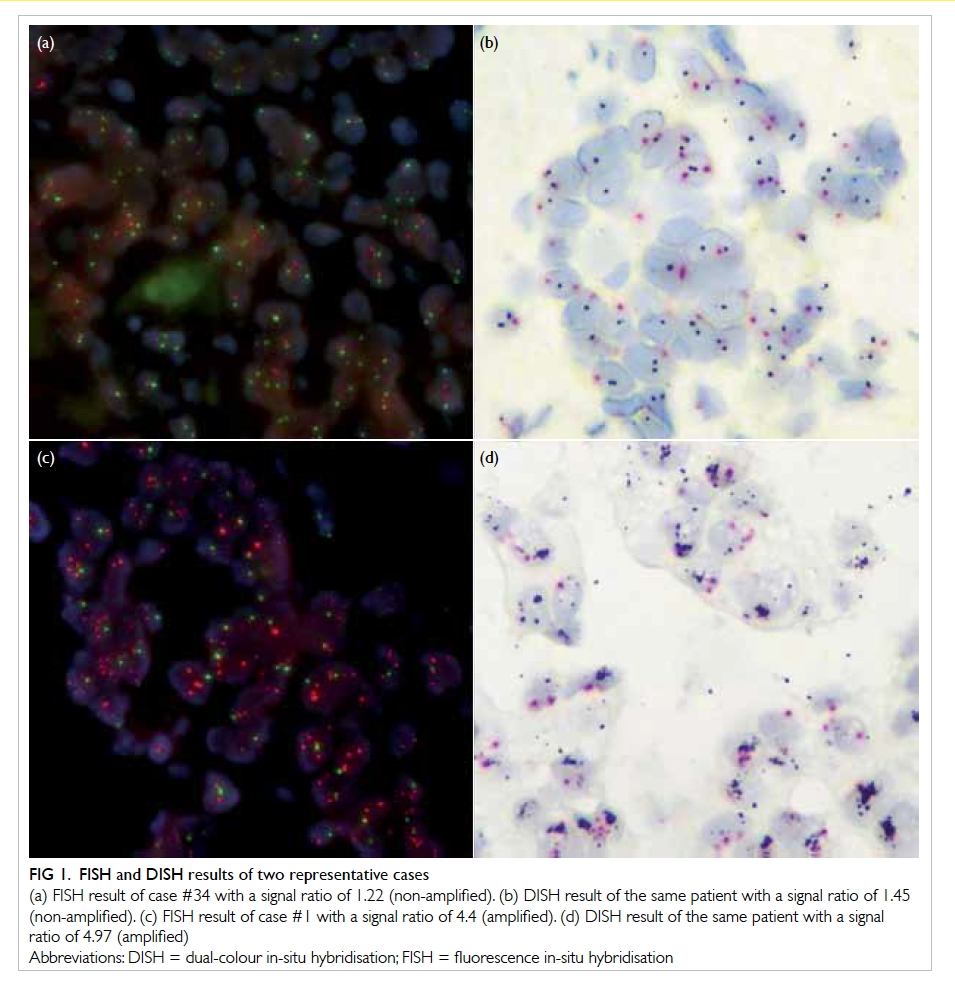
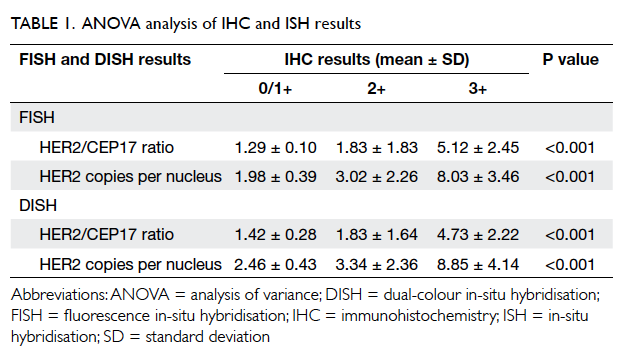
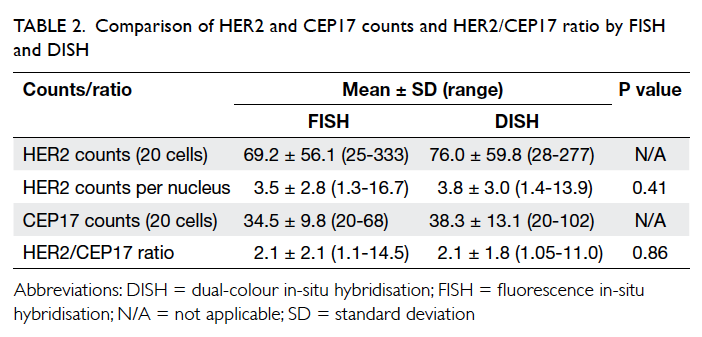
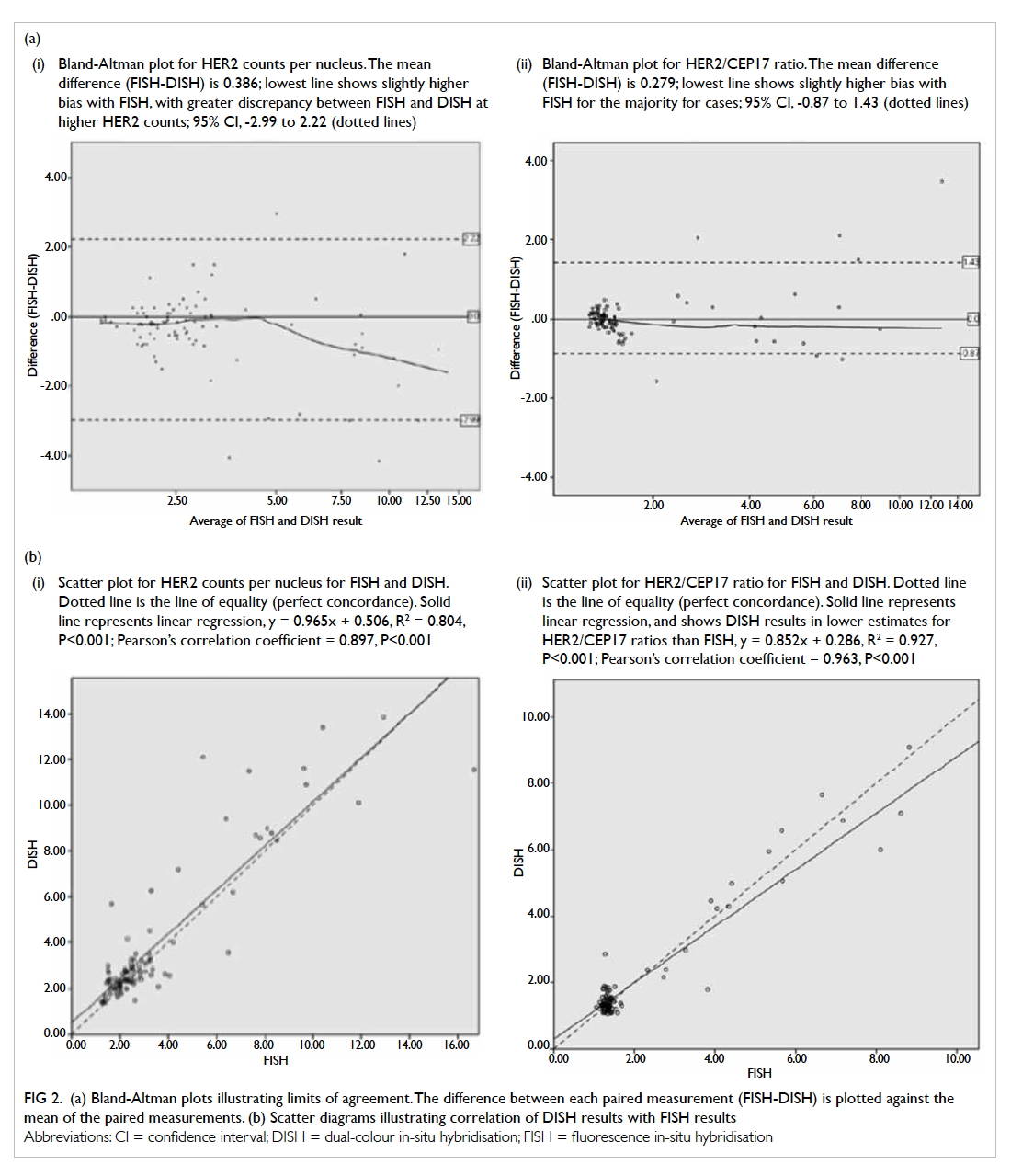
A prospective randomised controlled trial of octylcyanoacrylate tissue adhesive and standard suture for wound closure following breast surgery
Hong Kong Med J 2016 Jun;22(3):216–22 | Epub 22 Apr 2016
DOI: 10.12809/hkmj154513
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
A prospective randomised controlled trial of
octylcyanoacrylate tissue adhesive and standard
suture for wound closure following breast surgery
Clement TH Chen, FCSHK, FHKAM (Surgery)1;
Catherine LY Choi, FCSHK, FHKAM (Surgery)2;
Dacita TK Suen, FCSHK, FHKAM (Surgery)1;
Ava Kwong, FCSHK, FHKAM (Surgery)1
1 Department of Surgery, Queen Mary Hospital and Tung Wah Hospital,
Hong Kong
2 Private practice, Hong Kong
Corresponding authors: Dr Clement TH Chen (chenthc@ha.org.hk), Dr Ava Kwong (avakwong@hku.hk)
Abstract
Introduction: Several studies have demonstrated
that octylcyanoacrylate tissue adhesive provides
an equivalent cosmetic outcome as standard
suture for wound closure. This study aimed to compare
octylcyanoacrylate tissue adhesive with standard
suture for wound closure following breast surgery.
Methods: A prospective randomised controlled
trial was conducted in a public hospital in Hong
Kong. A total of 70 female patients, who underwent
elective excision of clinically benign breast lump
between February 2009 and November 2011, were
randomised to have wound closure using either
octylcyanoacrylate tissue adhesive or standard
wound suture following breast surgery. Wound
complications and cosmetic outcome were measured.
Results: Octylcyanoacrylate tissue adhesive
achieved wound closure in significantly less time
than standard suturing (mean, 80.6 seconds vs 344.6
seconds; P<0.001). There was no statistical difference
in wound condition or cosmetic outcome although
number of clinic visits, ease of self-showering,
and comfort of dressing significantly favoured
octylcyanoacrylate tissue adhesive.
Conclusions: Octylcyanoacrylate tissue adhesive
may be offered as an option for wound closure
following breast surgery.
New knowledge added by this study
- Use of octylcyanoacrylate (OCA) tissue adhesive in wound closure following breast surgery is feasible.
- OCA may be offered as an option for wound closure following breast surgery.
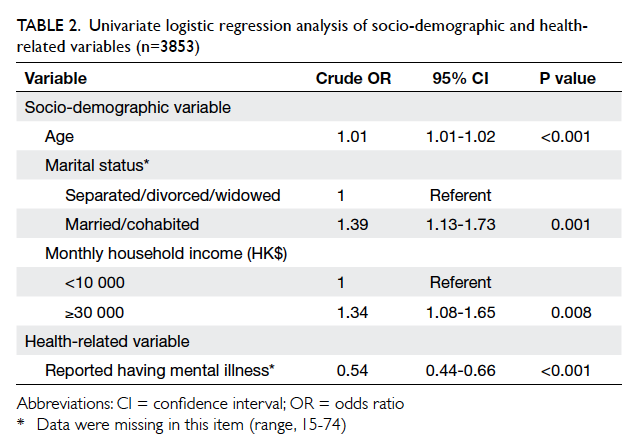
Introduction
In Hong Kong, thousands of patients undergo breast
surgery every year for both benign and malignant
conditions.1 Patients expect a good cosmetic
outcome and satisfactory postoperative wound
management. This is in addition to the expectation
of a cure, or in the case of breast cancer, complete
removal of lesions with optimal survival.
Several studies have demonstrated that
octylcyanoacrylate (OCA) tissue adhesive provides
an equivalent cosmetic outcome to wound suturing
in repair of lacerations,2 3 head and neck surgery,4 plastic surgery,5 and breast surgery.6 7 The OCA tissue adhesives are supplied as monomers in a liquid
form. They polymerise on contact with tissue anions,
forming a strong bond that holds the opposed
wound edges together. The OCA tissue adhesive
usually sloughs off with time. The wound epithelises
within 5 to 10 days and the adhesive does not require
removal.
In-vitro studies have shown that OCA
provides an effective antimicrobial barrier for the
first 72 hours after application.8 It is approved by the
US Food and Drug Administration as a topical skin
adhesive that protects the wound from bacteria. It
also facilitates postoperative wound care as patients
are allowed to shower immediately. There is no need
for suturing, staple removal, or dressings. Higher
patient satisfaction following skin closure with OCA
tissue adhesive compared with sutures has been
observed.6 Studies also show faster wound closure
with OCA.9
This study aimed to assess the outcome of
elective breast surgical incision repair with OCA
tissue adhesive compared with standard wound
suture (SWS). We compared the cosmetic outcome,
complication rates, and patient satisfaction score for
breast incisions in elective surgery closed with OCA
tissue adhesive versus SWS.
Methods
The study was in compliance with the Declaration
of Helsinki and ICH-GCP (International Conference
on Harmonisation, Good Clinical Practice). It was
reviewed and approved by the institutional review
boards.
Based on a randomised trial of OCA versus
SWS in breast surgery,5 patient satisfaction in an
OCA group has been reported to be significantly
higher than that of a SWS group. To detect a
difference with a power of 90% and α=0.05, 35
patients were needed for each arm.
A total of 70 female patients, who underwent
elective excision of clinically benign breast lump
between February 2009 and November 2011 in
this randomised controlled trial, were randomly
allocated to have wound closure using either OCA or
SWS with a continuous monofilament subcuticular
method. They were seen on the morning of the
surgery, consented, and randomly allocated to a study
arm. Each randomisation number was computer-generated, sealed in an envelope, and kept in a secure
designated place. At the time of wound closure, the
surgeon would call a third-party nurse to open the
sealed envelope that would determine the method
to be used for wound closure. The surgery was
performed by specialists or surgical trainees under
a specialist’s supervision. Two evaluation forms
were administered to collect information on wound
condition and cosmetic grading by different parties.
Postoperatively, the wound condition was
examined by a surgeon who was not involved in
the study. An evaluation form was completed to
note any indication of (1) wound infection, (2)
dehiscence, (3) oozing, and (4) discharge on day 0-1
(early postoperative period) and day 10-14 (first follow-up).
The cosmetic grading of the surgical wound
was checked on day 30 and 180 by an evaluator
(surgeons not involved in the study or the above
evaluations) who looked for any sign of (1) step-off
borders (edges not on same plane), (2) contour
irregularities (wrinkled skin near wound), (3) margin
separation (gap between sides), (4) edge inversion
(wound not properly everted), and (5) excessive
distortion (swelling/oedema/infection); and (6)
evaluated the overall appearance of the wound.
Patient evaluation of whether the appearance
of the wound was “good” or “bad” over a score of 1
(very bad) to 10 (very good) on day 30 and day 180
was also recorded.
Patients were also asked five questions about
self-care of the wound at the day-30 visit. Questions
were answered and rated on a 5-point Likert scale
(very good, good, neutral, bad, very bad) regarding
(1) pain, (2) ease of caring, (3) self-showering,
(4) frequency of hospital/clinic visits for wound
cleansing, and (5) comfort level of wound dressing.
Statistical analyses
Data were summarised with descriptive statistics.
Means and standard deviations (for numeric
variables) and numbers and percentages (for
categorical variables) were calculated where
appropriate. We checked the normality of the
data and found that it did not follow the normal
distribution. Therefore the Wilcoxon rank sum test
and Fisher’s exact test were applied to determine
any significant difference between the OCA and
SWS groups. All statistical analyses were done
using the Statistical Package for the Social Sciences
(Windows version 16.0; SPSS Inc, Chicago [IL], US)
and R version 3.0.2 (the R Foundation). All statistical
tests were two-sided and statistical significance was
considered at P<0.05.
Results
A total of 70 patients, half of whom were randomised
to receive OCA or SWS, were entered into this
study. One patient from the suture group was lost
to follow-up and excluded from subsequent analysis,
leaving a total number of 69 patients (35 for OCA
group and 34 for SWS group).
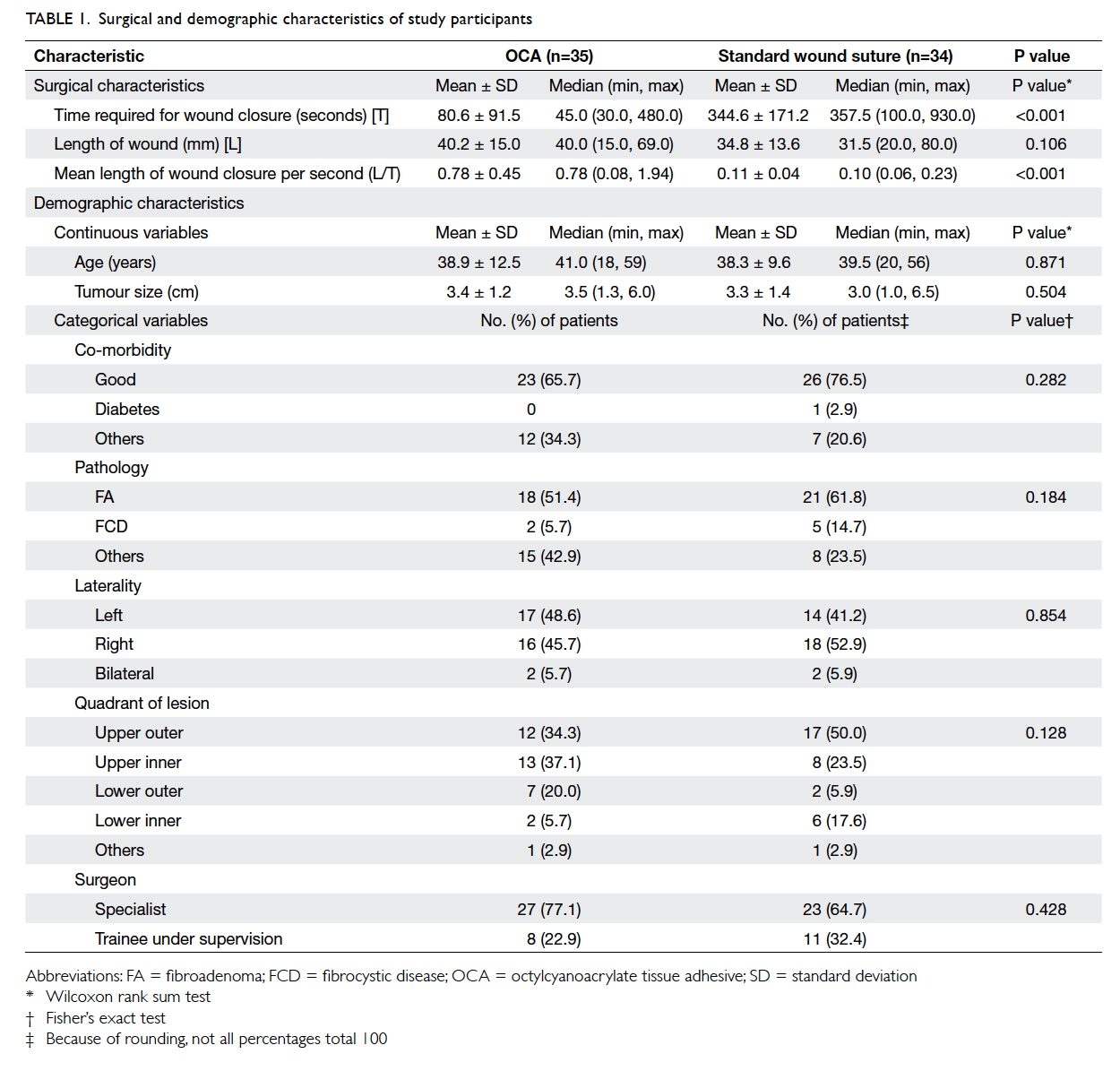
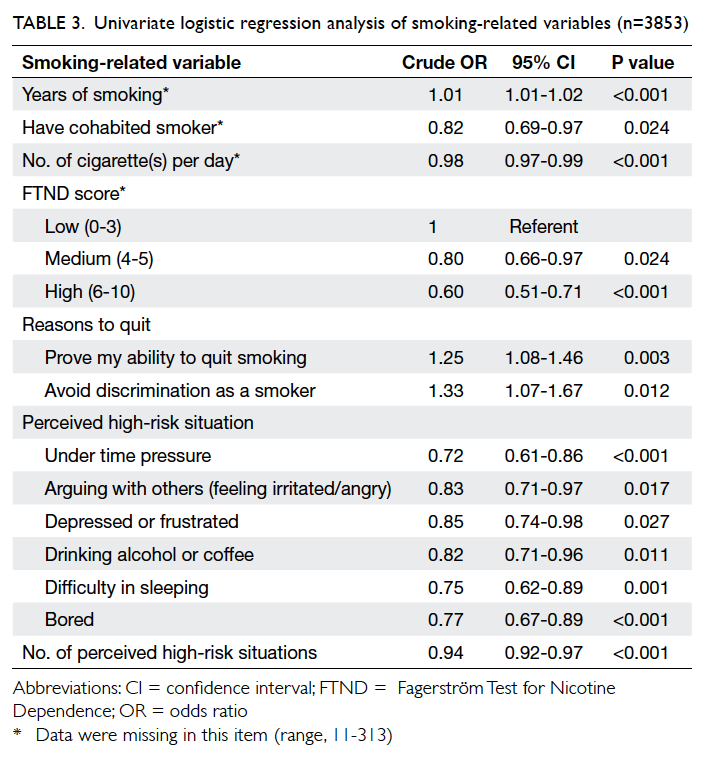
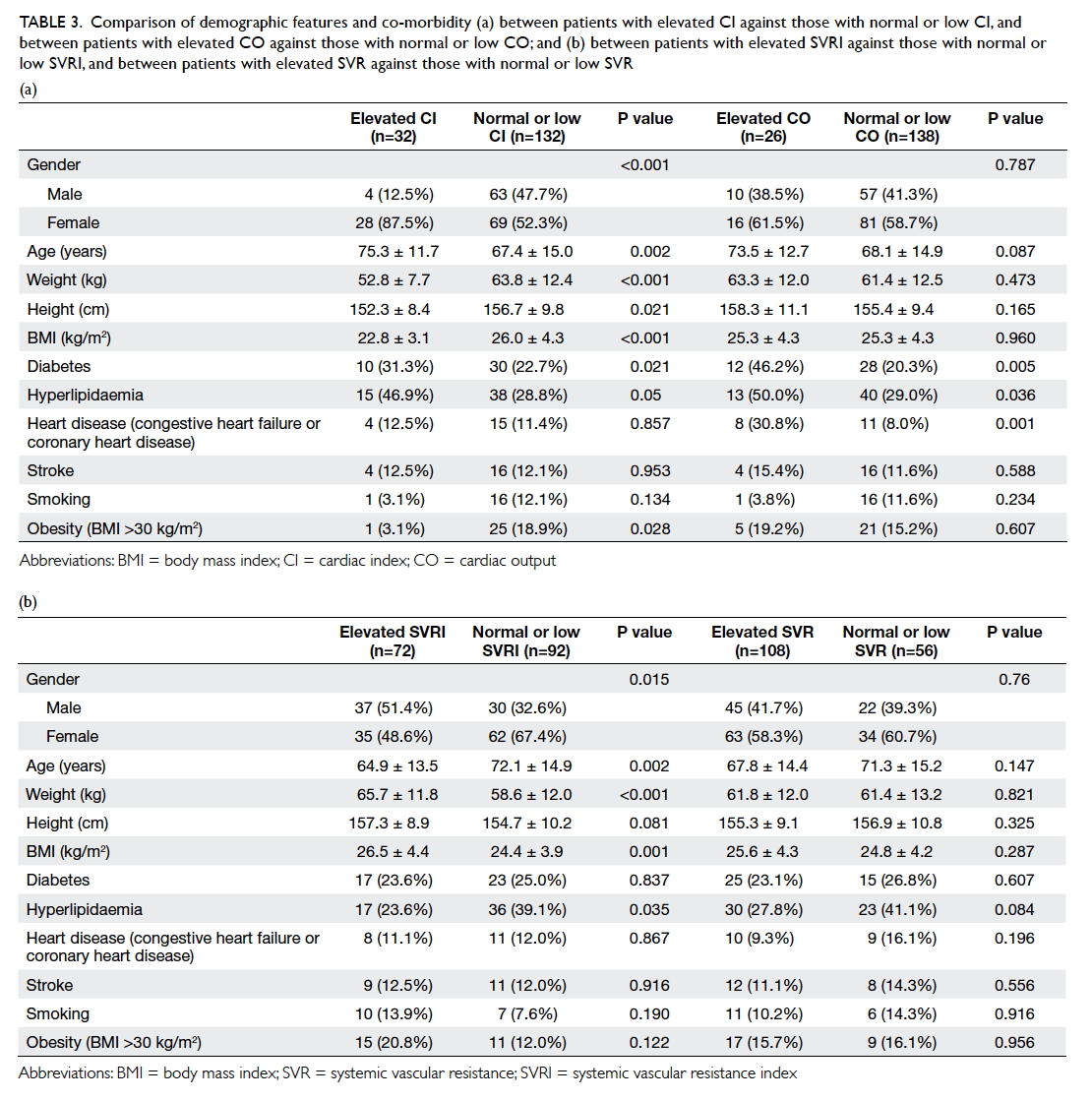
Demographic characteristics
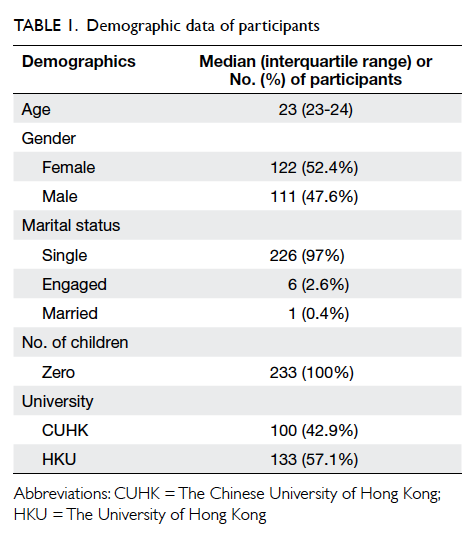
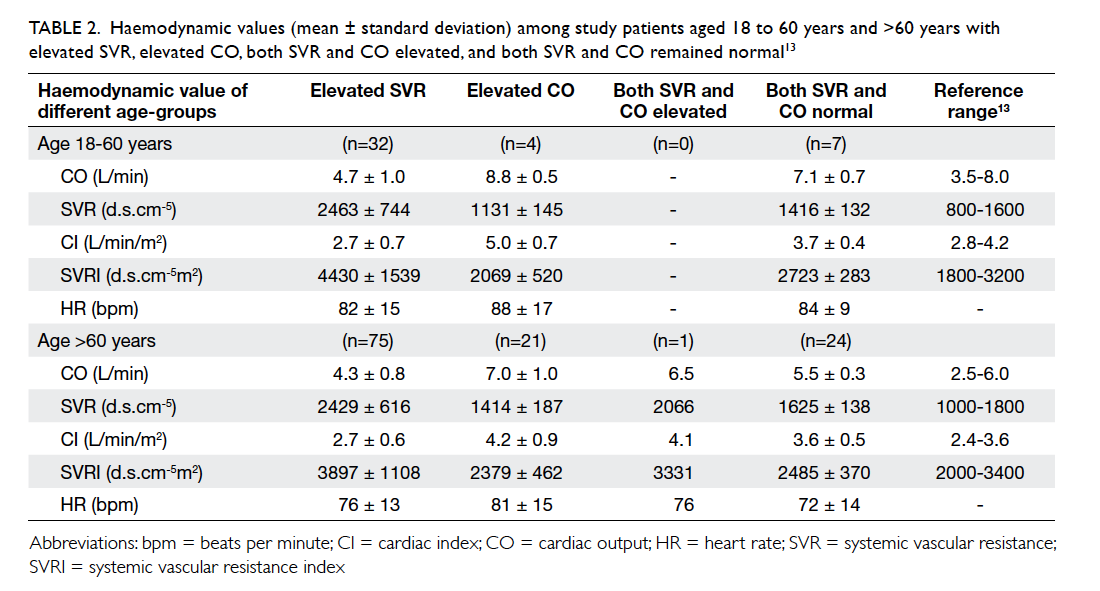
The demographics of the two groups were
comparable. There was no statistical difference in
terms of age, tumour size, co-morbidity including
diabetes, pathology, laterality and location of the
lesion, or the rank of the surgeon involved (Table 1).
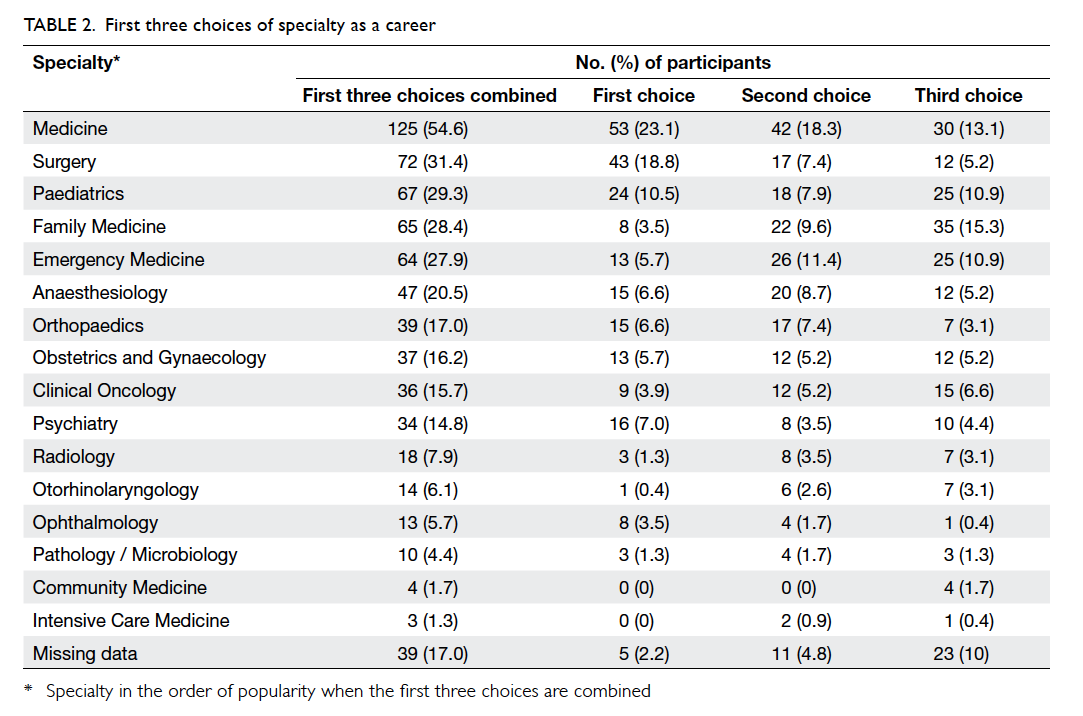
Use of OCA was associated with significantly
less time to complete the wound closure process
compared with suture (mean, 80.6 seconds vs 344.6
seconds; P<0.001; Table 1). With similar length of
surgical wound, OCA required 7.1 times less time to
close the wound than suture (P<0.001).
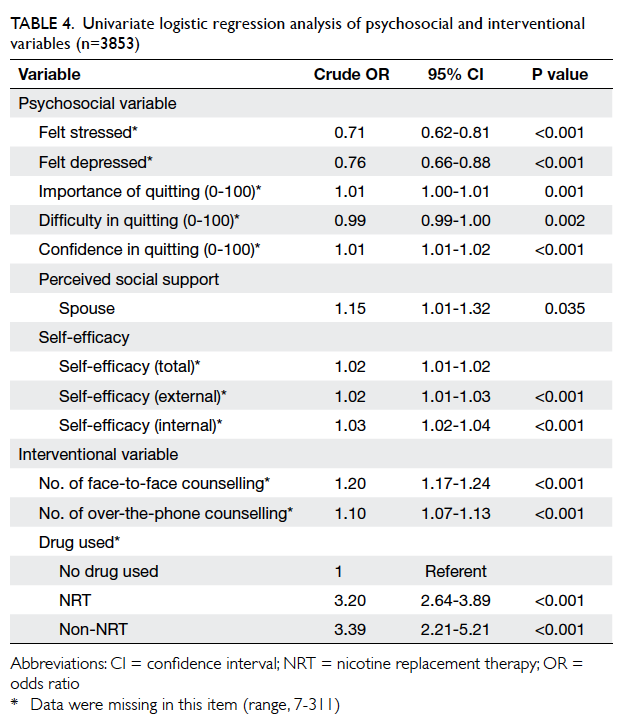
Wound conditions in early postoperative
period (day 0-1) and at first follow-up (day
10-14)
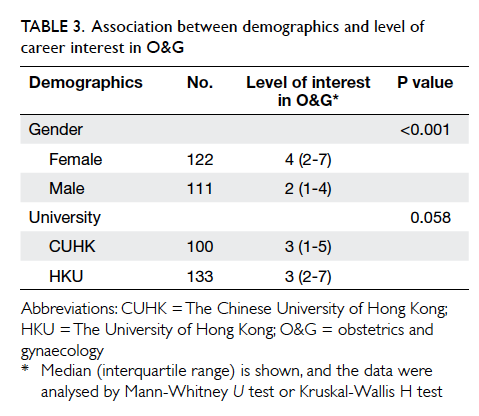
The occurrence of adverse wound condition is
shown in Table 2. There was no unfavourable
condition noticed upon first-day follow-up. Wound
complications on day 10-14 all occurred in the OCA
group.
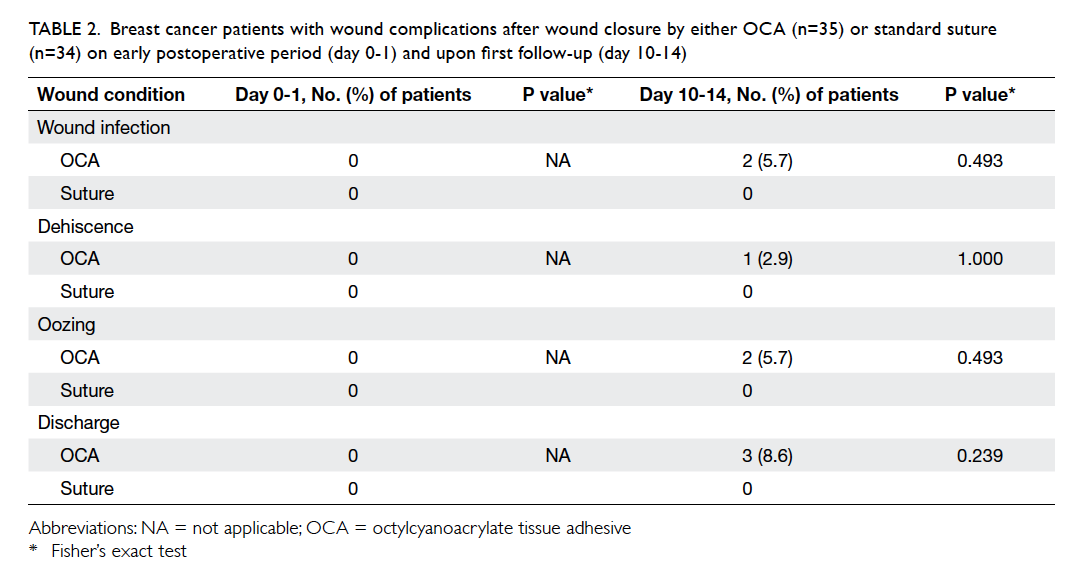
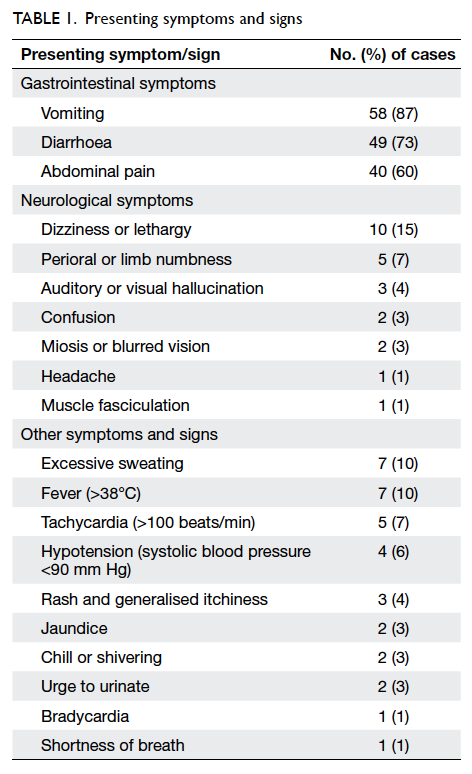
Table 2. Breast cancer patients with wound complications after wound closure by either OCA (n=35) or standard suture (n=34) on early postoperative period (day 0-1) and upon first follow-up (day 10-14)
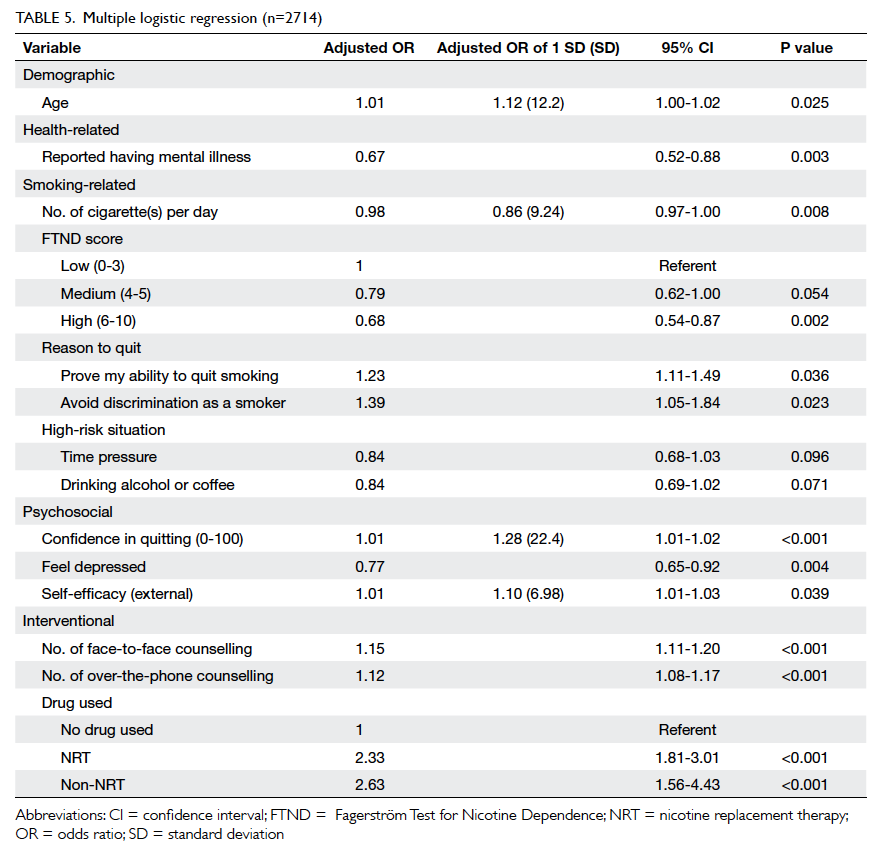
Cosmetic grading by an evaluator and
patients on day 30 and 180
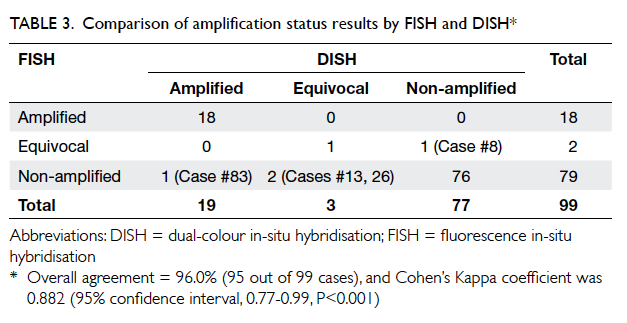
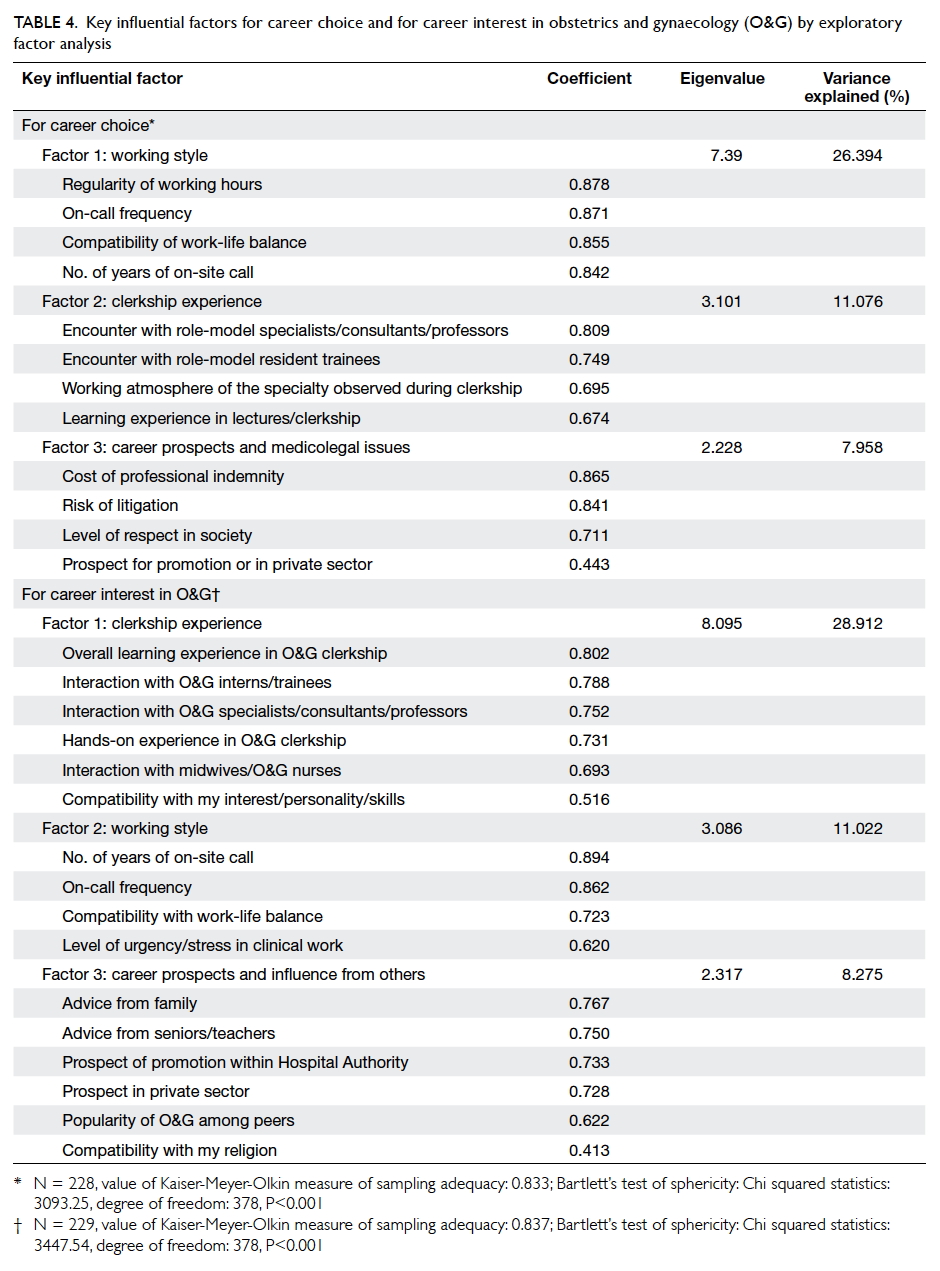
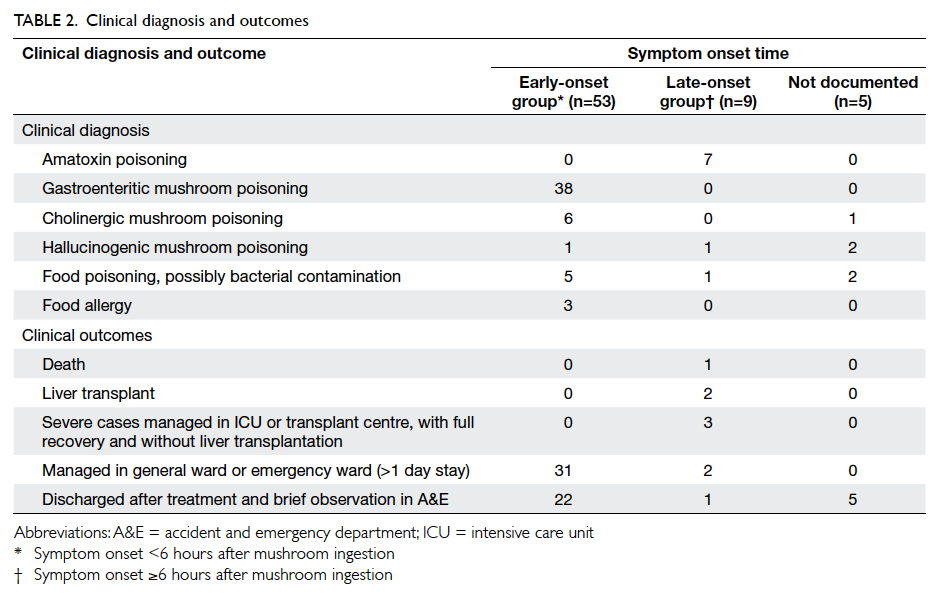
Table 3 summarises the incidence of any wound cosmetic problem on day 30 and 180. Cosmetic
problems were found only on day 30, and were not
confined to any one group. One patient from the
suture group felt that the overall appearance of the
surgical wound on day 30 was bad but subsequently
commented it was “good” on day 180. No bad
comments were received from any patient who had
undergone wound closure with OCA.
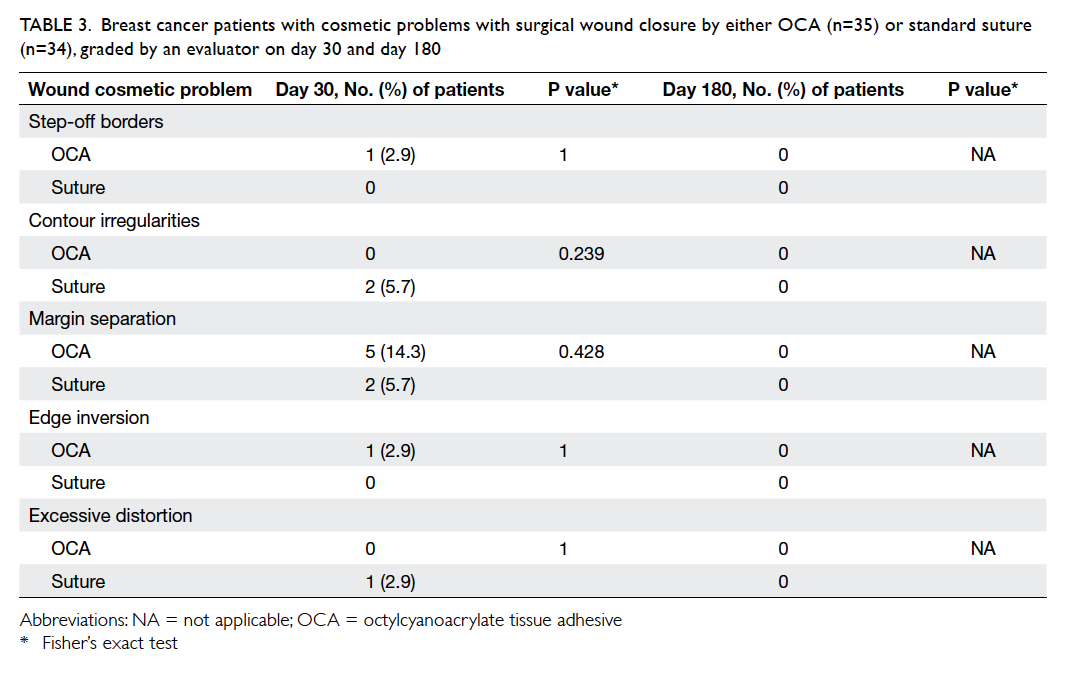
Table 3. Breast cancer patients with cosmetic problems with surgical wound closure by either OCA (n=35) or standard suture (n=34), graded by an evaluator on day 30 and day 180
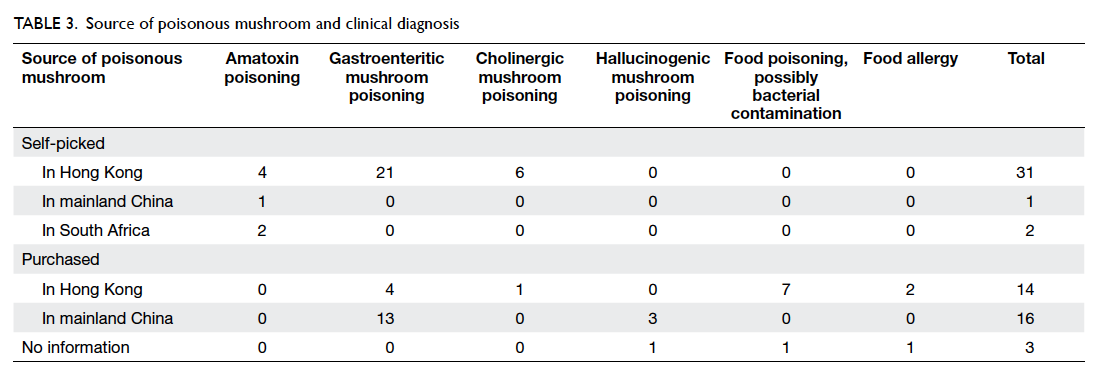
For the patient’s view of cosmetic outcome, a
higher score was given to wounds closed by sutures
compared with OCA on both day 30 and 180,
although the standard deviations were larger in the
OCA group, and the differences were not statistically
significant (Table 4). Higher scores were given on
day 180 compared with day 30 in both groups.
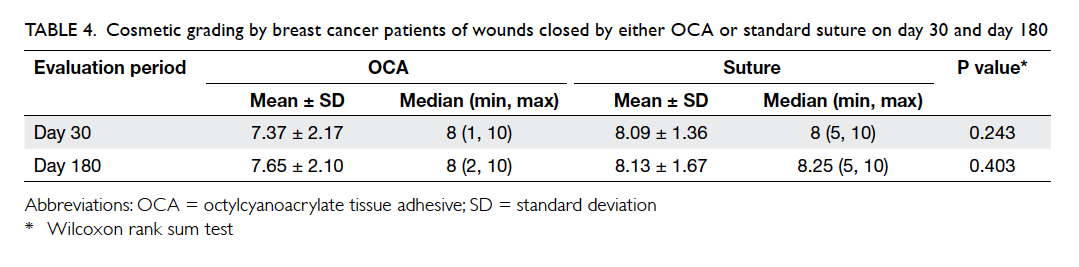
Table 4. Cosmetic grading by breast cancer patients of wounds closed by either OCA or standard suture on day 30 and day 180
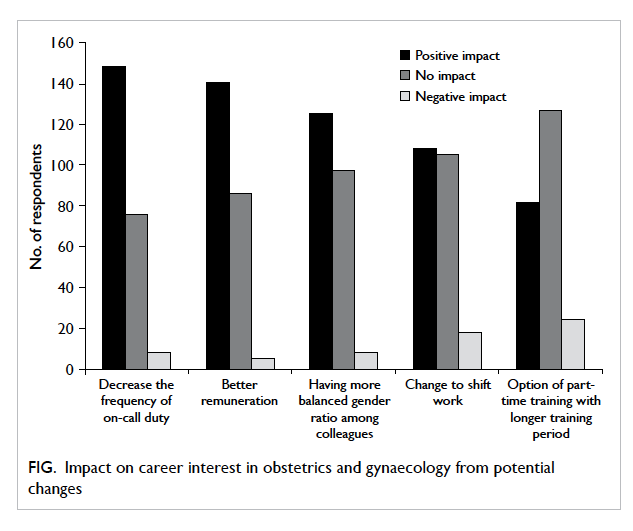
Patients’ opinion of different kinds of wound
management 30 days after surgery
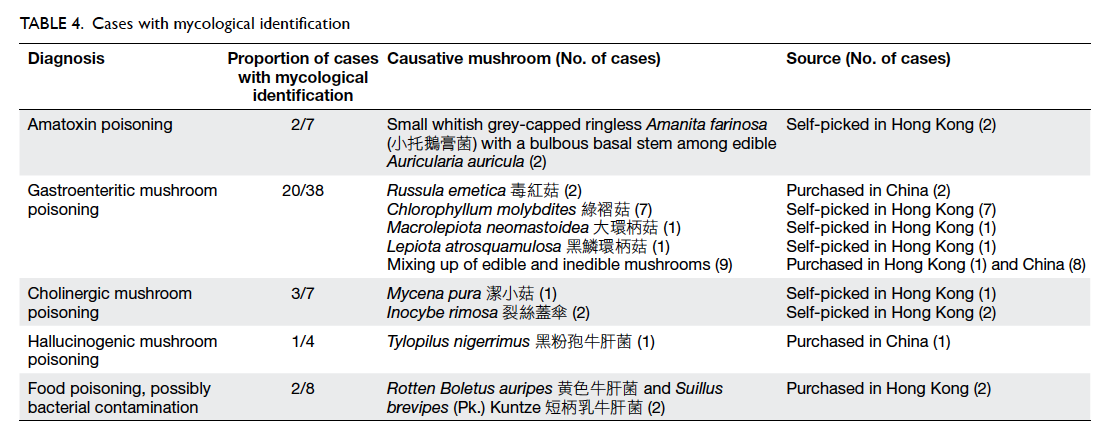
The actual number of hospital or clinic visits was
recorded. Patients in the OCA group required fewer
visits than those in the suture group (16 OCA vs 18
sutures, 1.19 ± 2.66 vs 2.50 ± 4.57; P=0.063). A higher
percentage of patients in the OCA group felt “very
good” on ‘self-showering’ (OCA vs suture, 66.7% vs
21.2%; P<0.001), ‘frequency of hospital/clinic visits
for wound cleansing’ (OCA vs suture, 66.7% vs 25.0%;
P=0.001), and ‘comfort level of wound dressing’
(OCA vs suture, 58.8% vs 18.2%; P=0.003) [Table
5]. More patients in the suture group rated “good”,
instead of “very good” for these three categories. The
same applied for ‘ease of caring’ although statistical
significance was not reached. For patients who
commented “bad” or “very bad”, the percentages
were generally higher from the OCA group. There
was no significant difference between the two groups
for reports about pain (P=0.564).
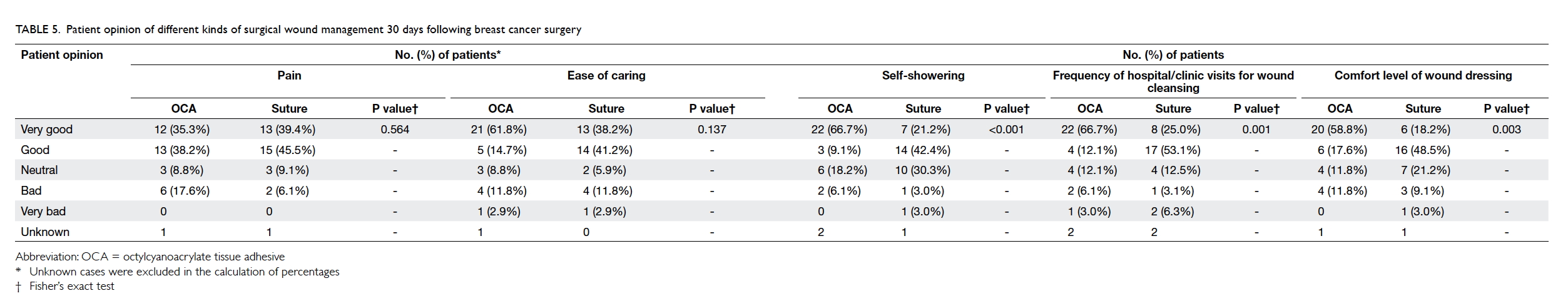
Table 5. Patient opinion of different kinds of surgical wound management 30 days following breast cancer surgery
Discussion
Tissue adhesive material has long been used in
wound closure in western countries, and offers
the advantages of faster closure, need for less
postoperative wound care, and higher patient
satisfaction.
In this study among Chinese women, we
demonstrated that time required for wound closure
was much less in the OCA group compared with the
SWS group (P<0.001). Although the difference was
significant, time required for wound closure was not
a significant concern for the surgeon.
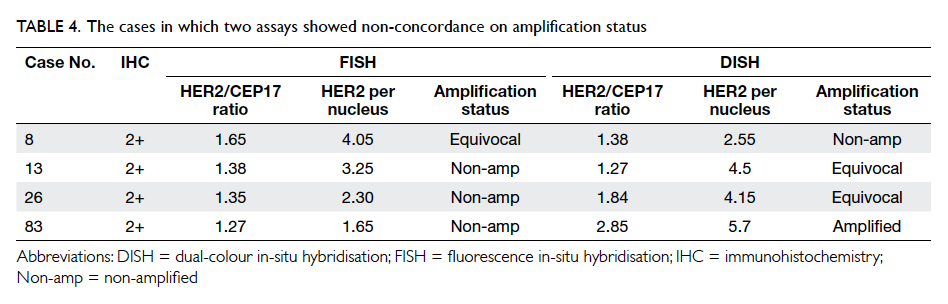
There were three instances of postoperative
complications in the OCA group. In two patients,
the surgery was performed by a trainee under
supervision, and in one by a specialist. One patient
required secondary suturing 3 weeks later. The other
two were treated conservatively with antibiotics. We
postulate that there is a learning curve for closure
with OCA, thus technical skill and experience of the
surgeon may play a role.
For cosmetic outcome, the score was
comparable in both groups, although slightly higher
in the SWS group. Nonetheless, the difference was
less than 1 point on a scale of 10. The standard
deviation in the OCA group was wider (OCA 2.17
vs SWS 1.36) at day 30 but was not statistically
significant.
For wound problems, margin separation
occurred on postoperative day 30 in five patients
in the OCA group compared with two in the SWS
group. There was no statistical difference in cosmetic
problem grading between the two study groups. It
should be noted that tissue adhesive wound repair
is a manual skill, just like suturing, and requires
practice and careful application. Factors such as
wound oozing or discharge may hinder proper
functioning of the tissue adhesive.
The skin of patients of Chinese or Asian descent
is more prone to keloid scarring and pigmentation,
thus the effect of using tissue adhesives may differ to
that of a western population. A previous study did not
show any difference in the rate of hypertrophic scar
formation.10 In our study, there was no hypertrophic
scar or keloid formation in either group.
There was a significant difference in preference
in terms of self-showering, frequency of hospital/clinic visits, and comfort level of dressing between OCA and SWS groups. These
factors affect patients since they impact on daily
activities and saving of time. On the other hand,
there was no statistical difference in degree of pain,
although the Phi value was very small, thus a larger
sample size may be required to detect any difference.
The sample size for all other values was adequate.
In terms of cost, a study in Hong Kong has
shown that tissue adhesive is more expensive than
SWS,11 but may be more cost-effective from a social
viewpoint in terms of superior cosmetic outcome
and overall patient satisfaction.
Strengths and weaknesses of this study
First, this was a prospective randomised study confined to closure of benign breast lump wounds and thus not necessarily applicable to all surgical
wounds. We did not determine the number of
eligible patients for the study, or note how many of
them refused to take part. This may have led to self-selection
bias. Second, the operation was performed
by different surgeons of different seniority, and may
have led to varying levels of surgical technique. This
was not shown to be statistically significant but the
sample size was not designed to reflect this. Lastly,
during wound evaluation, the surgeon might not have
been totally blind to the type of closure if sutures
or stitch marks were visible. This may be a cause
of blinding bias. The use of a scoring scale is also a
subjective evaluation, and may be influenced by the
patient’s mood and other factors.
Conclusions
The use of OCA should be discussed and offered to
patients as an option for wound closure in breast
surgery. It achieves faster wound closure, is not
inferior to standard wound closure, has a higher
comfort level, and requires less frequent clinic
visits. Understanding cost-effectiveness is essential
in medical care, thus OCA should be offered as an
option to be provided as a self-financed item in the
public sector where it is now widely available.
Acknowledgements
We thank Mr Jack Chau and Miss Fidelia Wong for
performing the early statistical analysis, and Mr Ling-hiu
Fung and Mr Wing-pan Luk of the Hong Kong
Sanatorium & Hospital for the later review and re-running
of the statistical analysis methods. We also
thank Ethicon, Johnson and Johnson Company for
providing the OCA study material.
Declaration
Johnson and Johnson provided the study material.
No other conflict of interests was declared by
authors.
References
1. Top ten cancers in 2013. Hong Kong Cancer Registry.
Available from: http://www3.ha.org.hk/cancereg/statistics.html. Accessed Jan 2016.
2. Singer AJ, Quinn JV, Clark RE, Hollander JE; TraumaSeal
Study Group. Closure of lacerations and incisions with
octylcyanoacrylate: a multicenter randomized controlled
trial. Surgery 2002;131:270-6. Crossref
3. Chow A, Marshall H, Zacharakis E, Paraskeva P,
Purkayastha S. Use of tissue glue for surgical incision
closure: a systematic review and meta-analysis of
randomized controlled trials. J Am Coll Surg 2010;211:114-25. Crossref
4. Maw JL, Quinn JV, Wells GA, et al. A prospective
comparison of octylcyanoacrylate tissue adhesive and
suture for the closure of head and neck incisions. J
Otolaryngol 1997;26:26-30.
5. Toriumi DM, O’Grady K, Desai D, Bagal A. Use of octyl-2-cyanoacrylate for skin closure in facial plastic surgery. Plast Reconstr Surg 1998;102:2209-19. Crossref
6. Gennari R, Rotmensz N, Ballardini B, et al. A prospective,
randomized, controlled clinical trial of tissue adhesive
(2-octylcyanoacrylate) versus standard wound closure in
breast surgery. Surgery 2004;136:593-9. Crossref
7. Nipshagen MD, Hage JJ, Beekman WH. Use of 2-octylcyanoacrylate
skin adhesive (Dermabond) for wound
closure following reduction mammaplasty: a prospective,
randomized intervention study. Plast Reconstr Surg
2008;122:10-8. Crossref
8. Quinn JV, Osmond MH, Yurack JA, Moir PJ. N-2-butylcyanoacrylate: risk of bacterial contamination with
an appraisal of its antimicrobial effects. J Emerg Med
1995;13:581-5. Crossref
9. Soni A, Narula R, Kumar A, Parmar M, Sahore M,
Chandel M. Comparing cyanoacrylate tissue adhesive and
conventional subcuticular skin sutures for maxillofacial
incisions—a prospective randomized trial considering
closure time, wound morbidity, and cosmetic outcome. J
Oral Maxillofac Surg 2013;71:2152.e1-8. Crossref
10. Wilson AD, Mercer N. Dermabond tissue adhesive versus
Steri-Strips in unilateral cleft lip repair: an audit of infection
and hypertrophic scar rates. Cleft Palate Craniofac J
2008;45:614-9. Crossref
11. Wong EM, Rainer TH, Ng YC, Chan MS, Lopez V. Cost-effectiveness
of Dermabond versus sutures for lacerated
wound closure: a randomised controlled trial. Hong Kong
Med J 2011;17 Suppl 6:4-8.