Hong Kong Med J 2017 Aug;23(4):395–403 | Epub 7 Jul 2017
DOI: 10.12809/hkmj166186
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Hypersensitivity to antipyretics: pathogenesis, diagnosis, and management
QU Lee, MB, ChB, FHKAM (Paediatrics)
Department of Paediatrics and Adolescent Medicine, Princess Margaret Hospital, Laichikok, Hong Kong
Corresponding author: Dr QU Lee (leequnui@gmail.com)
Abstract
Antipyretics are commonly prescribed drugs
and hypersensitivity occurs at rates of 0.01%
to 0.3%. Hypersensitivity can be due to
immune mechanisms that include type I to IV
hypersensitivity. Type I hypersensitivity results from
specific immunoglobulin E production following
sensitisation on first exposure. Subsequent exposures
elicit degranulation of mast cells, culminating an
immediate reaction. Non–type I hypersensitivity
is a delayed reaction that involves various effector
cells, resulting in maculopapular rash, fixed drug
eruptions, drug reaction with eosinophilia and
systemic symptoms, and Stevens-Johnson syndrome/toxic epidermal necrolysis. Antipyretics also cause
non-immune hypersensitivity via cyclooxygenase
inhibition. Apart from hypersensitivity to parent
compounds, hypersensitivity to excipient has been
reported. Clinical manifestations of antipyretic
hypersensitivity involve the skin, mucosa, or multiple
organs. Diagnosis of hypersensitivity requires
a detailed history taking and knowledge of any
underlying disorders. Differential diagnoses include
infection, inflammatory conditions, and antipyretics
acting as co-factors of other allergens. Investigations
include specific immunoglobulin E assays,
lymphocyte transformation test, basophil activation
test, and skin prick test. Lack of standardisation and
a scarcity of available commercial reagents, however,
limit the utility of these tests. A drug provocation
test under close supervision remains the gold
standard of diagnosis. A trial of the culprit drug
or other structurally different antipyretics can be
considered. Patients with confirmed hypersensitivity
to antipyretics should consider either avoidance
or desensitisation. Other theoretical options
include subthreshold or low-dose paracetamol,
cyclooxygenase-2 inhibitors, pre-medication with
antihistamines with or without a leukotriene receptor
antagonist, co-administration of prostaglandin
E2 analogue, traditional Chinese medicine, or
desensitisation if antipyretics are deemed desirable.
Safety and efficacy of unconventional treatments
warrant future studies.
Introduction
Antipyretics (APs) are widely consumed drugs. In
2013, the National Institute for Health and Care
Excellence advised that paracetamol and ibuprofen
can be prescribed for febrile children in distress.1
In a national cross-sectional study in France, more
than 80% of health care professionals resorted to AP to
manage fever in children. Paracetamol was the first-choice
AP among 88% of health care professionals while
ibuprofen, a non-steroidal anti-inflammatory drug
(NSAID), was preferred by 11%.2
Diclofenac sodium
and mefenamic acid have also been advocated as
APs for children.3 4
What makes use of APs truly
ubiquitous is their non-prescription, over-the-counter
availability. Widespread consumption often
entails an increased chance of adverse drug reaction
(ADR). Paracetamol and NSAIDs are two of the
most common drugs to cause an allergic or pseudo-allergic
reaction, secondary to general anaesthetic
agents and beta-lactam antibiotics.5
Prevalence
of NSAID hypersensitivity ranges from 0.1% to
0.3%.6
Hypersensitivity reactions to ibuprofen
occur at 0.01%.7
The epidemiology of paracetamol
hypersensitivity is unclear. This is understandable
since prescription data for over-the-counter drugs
are difficult to obtain. Nevertheless between 1982
and 1991, the Spanish Drug Monitoring System
estimated the incidence of ADR to paracetamol to
be less than 1 per 100 000 inhabitants below the age
of 15 years. Among the reported ADRs, 30% were
related to skin eruption, urticaria, or itchiness.8
The real incidence might have been higher, had
unreported cases been included. This is a review
of the pathogenesis, diagnosis, and management of
hypersensitivity to APs.
Types of hypersensitivity reactions to antipyretics
Hypersensitivity reactions to APs are idiosyncratic
responses of the body towards drugs given at a
therapeutic dose. Around two thirds of patients
with NSAID or paracetamol hypersensitivity are
single reactors, while one third are cross-reactors.9
Reaction may either be to the active ingredient or to excipients. Hypersensitivity to APs can manifest
as an immune-mediated reaction that stems from
an immunoglobulin (Ig) E–mediated (immediate)
reaction or a non–IgE-mediated (delayed) reaction.
Unlike other drugs, hypersensitivity to APs can also
be non–immune-mediated.
Immune-mediated hypersensitivity
Type I hypersensitivity
Type I hypersensitivity to APs, or an IgE-mediated
reaction, is selective in nature. It presents with
single NSAID-induced urticaria/angioedema or
anaphylaxis (SNIUAA) or hypersensitivity to NSAIDs
with structural similarity but tolerance to NSAIDs
from different classes. Ibuprofen and paracetamol
are two common causes of SNIUAA.10
Severity ranges from localised urticaria, mucosal swelling,
and angioedema to anaphylaxis. Susceptible patients
become sensitised to an AP on first exposure, with
the production of drug-specific IgE. Specific IgE
molecules become attached to high-affinity IgE
receptors on mast cells or basophils. Re-exposure to
the same AP or cross-reacting drugs leads to cross-linking
of adjacent IgE receptors and subsequent
degranulation of vasoactive inflammatory mediators
like histamine and tryptase.11
Patients with SNIUAA
against ibuprofen produce IgE against specific
antigen determinants of the drug. Hence they may
react to arylpropionic acids with similar chemical
structure but tolerate NSAIDs from other groups,
such as acetic acids.7
Similarly, patients with selective
hypersensitivity to paracetamol confirmed by IgE
tests or oral challenge can tolerate other NSAIDs.12
Non–type I hypersensitivity
Maculopapular eruptions
According to the revised Gell and Coombs
classification, maculopapular eruption (MPE) is a
type IV-c, T-cell–mediated delayed hypersensitivity
reaction.13
It is said to be the most common delayed
drug rash due to an AP. Implicated drugs include
ibuprofen, diclofenac, and paracetamol.14
Such MPE
manifests as a morbilliform or scarlatiniform rash
that starts on the trunk with subsequent spread to
the limbs. Onset of MPE ranges from within 7 to 14
days of first consumption of the drug, but may take
only 2 to 3 days in patients with prior sensitisation.
The reaction of MPE involves skin-homing T
lymphocytes, drug-specific cells that express
cutaneous lymphocyte antigen. Around two thirds
of the T-cells are CD4+, while one third are CD8+.
Having resided in the dermo-epidermal junction, these
cells release perforin and granzyme B, two mediators
of keratinocyte apoptosis, via their ability to induce
pore formation in the cell membrane.15
Histological
changes include intracellular, intercellular and
dermal papilla oedema, dislodgment of epidermal
basal cells, hydropic degeneration, spongiosis of
the lower epidermis, and dyskeratosis and necrosis
of keratinocytes. Inflammatory infiltration by
T-cells is seen at the dermo-epidermal junction and
eosinophils in the perivascular region.16
Fixed drug eruption
Fixed drug eruption (FDE) is a peculiar type of T-cell–mediated delayed drug hypersensitivity. It starts
with solitary, well-circumscribed macules that erupt
anywhere on the skin or mucosa, usually over the
lips, palms, soles, groins, or glans penis. With time,
the lesions evolve into plaques that recur at the same
site on re-exposures to the same drug. The interval
between drug intake and FDE is around 30 minutes
to 8 hours. The eruption resolves spontaneously after
cessation of the culprit, leaving hyperpigmentation
at the affected site. Pathologically, migration and
residence of drug-specific effector-memory CD8+
T-cells in the epidermal side of the dermo-epidermal
junction of the affected area account for the
recurrence of eruption at the same site. Upon drug
re-exposure, quiescent CD8+ cells become activated
and secrete interferon-γ and cytotoxic granules into
the local microenvironment.17 Paracetamol is one of
the most common causes of FDE, as are mefenamic
acid, ibuprofen, and aspirin.18
Drug reaction with eosinophilia and systemic symptoms
Drug reaction with eosinophilia and systemic
symptoms (DRESS) is classified as a type IV-b
delayed hypersensitivity reaction with eosinophil
involvement. It is characterised by fever, exfoliative
dermatitis, lymphadenopathy, haematological
abnormalities (hypereosinophilia, atypical
lymphocytes), and organ dysfunction. The interval
between drug consumption and onset of symptoms
is quite prolonged, ranging from 3 weeks to 3
months. The pathophysiology of DRESS involves
viral reactivation (eg human herpes type 6) and
T-cell activation, two determining factors with a
mutual causal relationship.19
FOXP3+ (forkhead
box P3) regulatory T-cells are activated early in the
course of DRESS, but are subsequently deactivated
and become deficient, culminating in the emergence
of autoimmune diseases commonly seen in the
aftermath of DRESS. Ibuprofen and paracetamol
have rarely been associated with DRESS.20 21
Stevens-Johnson syndrome/toxic epidermal necrolysis
Stevens-Johnson syndrome/toxic epidermal
necrolysis (SJS/TEN) is a type IV-c delayed
hypersensitivity reaction to infections or drugs
including APs. The interval between intake of the
culprit drug and SJS/TEN is shorter than that of
DRESS, ranging from 1 to 21 days.22
Skin lesions
in SJS/TEN are typically target-like with central
necrosis, bullae formation, or purpuric lesions.
In SJS, less than 10% of the body surface area
is involved, whereas in TEN, more than 30% is
involved. Gentle rubbing of ‘normal’ skin causes
separation of the epidermis (Nikolsky sign). Mucosal
and eye inflammation is present in 90% and 60%
of cases, respectively. Severe cases culminate in
corneal scarring, respiratory distress syndrome,
pneumonia, and respiratory failure.23
A caveat in the
diagnosis is that the prodromal phase of SJS/TEN
may be mistaken as symptoms of a febrile illness,
with consequent administration of APs. In the event
that SJS/TEN occur secondary to other causes,
subsequent appearance of skin and mucosal lesions
may impart the wrong impression of AP as the
causative agent. In SJS/TEN, CD4 T-cells accumulate
in the dermis while CD8 T-cells predominate in
the epidermis. T-cell infiltration causes massive
apoptosis of the keratinocytes via the toxic action of
perforin, granzyme, and Fas/Fas ligand interaction.24
Of note, SJS/TEN due to NSAIDs is exceedingly rare.
The incidence for ibuprofen was 0.013 per 1 000 000
as opposed to 0.032 per 1 000 000 for oxicams.25
Compared with controls, the relative risk of
paracetamol and ibuprofen for SJS/TEN in children
ranges from 5 to 11.26
It is also noteworthy that APs
are often prescribed together with antibiotics to
treat infection, with the latter two factors (antibiotics and infection) potentially
related to SJS/TEN.27
Acute generalised exanthematous pustulosis
Acute generalised exanthematous pustulosis
(AGEP) is a rare type IV-d drug hypersensitivity
with sterile subcorneal pustule formation. Onset
of pustules occurs around 1 day after drug intake.
Most patients present with fever. Non-follicular
small pustules with an erythematous base start on
the face or intertriginous area and subsequently
become generalised. The pustules, which are
itchy or burning, persist for 4 to 30 days before
desquamation.28 Histological characteristics include
papillary oedema, perivascular infiltration by
neutrophils, and drug-specific T-cells and epidermal
keratinocyte necrosis. Interleukin-8, a neutrophil
chemoattractant, is expressed by drug-specific T-cells.
Presence of human leukocyte antigens (HLAs)-DR within the inflammatory infiltrate suggests
the role of a major histocompatibility complex
in causing this peculiar type of drug eruption.29 Among NSAIDs, only the oxicams are significantly
associated with AGEP, with a multivariate odds ratio
of 8.4. Paracetamol is not considered at an increased
risk of causing AGEP.30
Organ-specific delayed hypersensitivity
Of note, NSAIDs can cause an allergic inflammatory
response in different organs. Cases of NSAID-induced
hepatitis, pneumonitis, nephritis, and
aseptic meningitis have been reported.6
Non–immune-mediated hypersensitivity: cyclooxygenase inhibition
Three types of non-immune drug hypersensitivity to
NSAIDs have been described: NSAID-exacerbated
respiratory disease (NERD), NSAID-exacerbated
cutaneous disease (NECD), and NSAID-induced
urticaria/angioedema (NIUA). In NERD, patients
usually have asthma, rhinosinusitis, and/or nasal
polyps. Aspirin or other NSAIDs may precipitate
nasal congestion, rhinorrhoea, bronchial
obstruction, or dyspnoea within 30 to 180 minutes
of ingestion. Urticaria, angioedema, and flushing of
the upper thorax may occur. Patients with NECD
usually have underlying chronic spontaneous
urticaria. Aspirin or NSAIDs may cause flare-up of
urticaria and angioedema in 12% to 30% of patients
with chronic spontaneous urticaria. On the other
hand, NIUA occurs primarily in patients without
underlying disease. Immediate reactions that occur
less than 15 minutes following consumption and late
reactions that occur after several hours have been
described.10
Non-immune hypersensitivity to NSAIDs is
the result of cyclooxygenase (COX) inhibition, a
pharmacological property common to all NSAIDs
that accounts for their propensity to cause cross-reactivity.
Three COXs—COX-1, COX-2, and
COX-3—have been identified, and NSAIDs like
ibuprofen inhibit all three COXs. On the contrary,
paracetamol is a weak inhibitor of COX-1 and COX-2, especially at a low dose, and preferentially inhibits
COX-3.31 In susceptible patients, inhibition of
COX leads to overproduction of pro-inflammatory
cysteinyl leukotrienes by mast cells and eosinophils
but depletion of the homeostatic and anti-inflammatory
prostaglandin E2 (PGE2).31 Imbalance
of leukotrienes and prostaglandins culminates in
inflammation in the skin, nasal cavities, sinuses,
and airway mucosa.32 Accumulation of leukotrienes
in the skin results in urticaria and angioedema
characterised by dermal oedema, and lymphatic
dilation involving perivascular or interstitial cellular
infiltration.33
Recent genetic studies have further elucidated
the pathogenesis of NSAID hypersensitivity due to
COX inhibition, explaining why it only occurs in
some patients. Candidate genes are responsible for
various enzymes, receptors, or mediators involved
in dysregulation of arachidonic acid metabolism,
initiation of immune response, dysfunction of
epithelial cells, biochemical signalling, effector
function in inflammatory cells, and aspirin
metabolism.34 Studies revealed that HLAs are
associated with NSAID hypersensitivity, for instance,
subjects with HLA DPB1*0301 are at a higher risk of
developing NERD.35 Aside from genes, methylation
profiles of DNA have been associated with NERD,
underscoring the role of epigenetics.36
Hypersensitivity to excipients
Discussion of hypersensitivity to APs is incomplete
without mentioning the role of excipients that act as
vehicles of drugs. It was thought that an excipient,
being ostensibly inert, should not cause ADR. Recent
reports of excipient hypersensitivity, however,
have cast doubt on that.37 Common paracetamol
preparations come in the form of tablets, syrup, and
suppositories. As with other drugs, excipients in
paracetamol contain preservatives, colouring, sugar,
and ethanol. Parabens and benzoates, two potential
allergens, are preservatives widely used in various
paracetamol preparations.
Different excipients are added to produce
different formulations. For instance, one type of
paracetamol syrup contains propylene glycol,
methyl hydroxybenzoate, propyl hydroxybenzoate,
xanthan gum, sorbitol solution 70%, sucrose,
mango flavouring, and purified water.38 There are
currently more than 90 registered manufacturers
of generic paracetamol in Hong Kong, producing a
stunning inventory of more than 900 paracetamol-containing
formulations in the drug registry of the
Department of Health.39 Patients hypersensitive to
the excipient of one product (eg paracetamol tablet)
may tolerate another form (eg paracetamol syrup)
or the same form of another brand. Unfortunately,
pharmaceutical companies may not disclose
excipient components of a drug in their entirety.
This makes thorough comparison between different
products difficult.
Diagnosis of hypersensitivity to antipyretics
History and clinical scoring system
Prudent management of hypersensitivity to APs
starts with an attempt to confirm or exclude the
diagnosis. As APs are usually prescribed for fever on
an as-required basis, clinicians should concentrate
on actual consumption rather than prescription.
Reactions that appear within 1 to 2 hours of AP
consumption constitute immediate hypersensitivity,
while reactions that appear several hours or beyond
are considered delayed hypersensitivity. Although
symptoms usually subside within 24 to 48 hours,
some may persist for up to 1 to 2 weeks.40
The number of previous exposures to an
AP should be noted. The same drug tolerated on
many occasions is unlikely to be the culprit. An
AP tolerated only once before may trigger an IgE-mediated
reaction the second time it is given to a
susceptible patient. An AP given for the first time can
still trigger a reaction via T-cell activation or COX
inhibition. Previous exposure may not be apparent
in case of poor recall or if the AP is given in the
context of polypharmacy. With details of the past and
present drug treatment, clinicians should estimate the
probability of AP hypersensitivity before attaching
the label. A validated scoring system can help classify
patients as definite, probable, possible, or doubtful
cases of ADR.41
The next step is to differentiate
between single-reactors and cross-reactors by
thorough history taking and collation of data from
various sources, including written and electronic
drug records.
Care is needed for proper drug identification, as
APs may have many trade names. Clinicians can refer
to the Drug Database of the Department of Health
for a comprehensive list of registered drugs from
different pharmaceutical companies.39 Over-the-counter
drugs should be carefully studied in history
taking. Patients should be encouraged to submit any
remaining drugs to hand for identification. Clinicians
should try to differentiate between hypersensitivity
to the active ingredients versus excipients. Patients
who react to different preparations of the same drug
are likely hypersensitive to the active ingredient,
while those who react only to some preparations may
be suffering from hypersensitivity to excipient(s).
A clinical history is valuable in predicting
hypersensitivity to APs: 17% of children with such
hypersensitivity have a positive family history. Such
children are more than 5 times likely to have NSAID
hypersensitivity compared with controls.9
Emergence
of an ADR within an hour of administration and a
history of hypersensitivity to multiple NSAIDs are
two other stronger predictors of challenge-proven
NSAID hypersensitivity.42
Clinicians should then differentiate between
various clinical manifestations. Urticarial rash and
angioedema are found in type I hypersensitivity
and reactions due to COX inhibition; whereas MPE
is erythematous, non-itchy, and flat lesions that
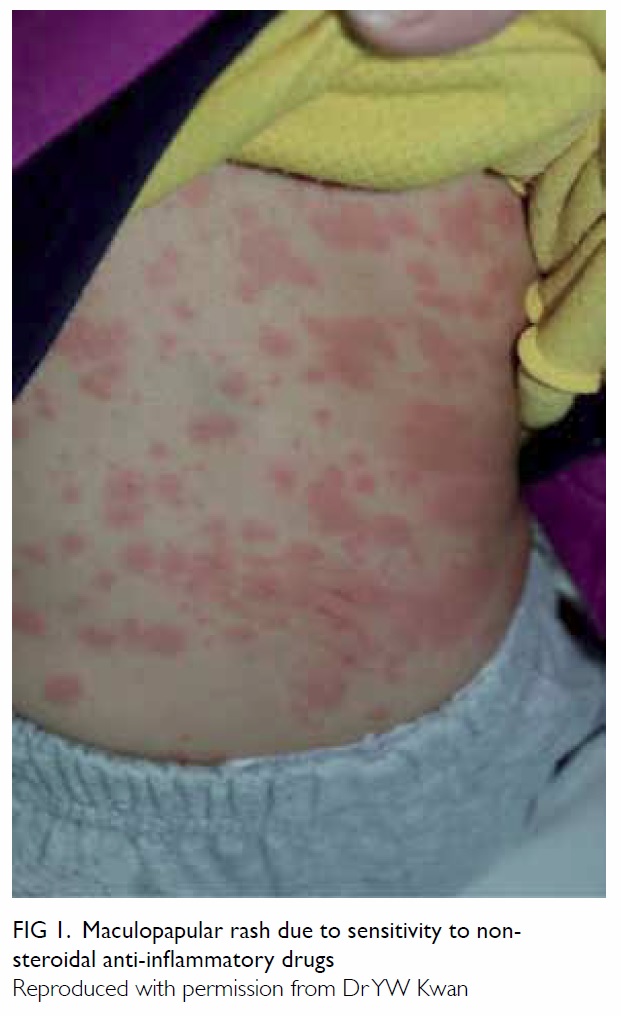
blanche on pressure (Fig 1). Isolated discoid lesions
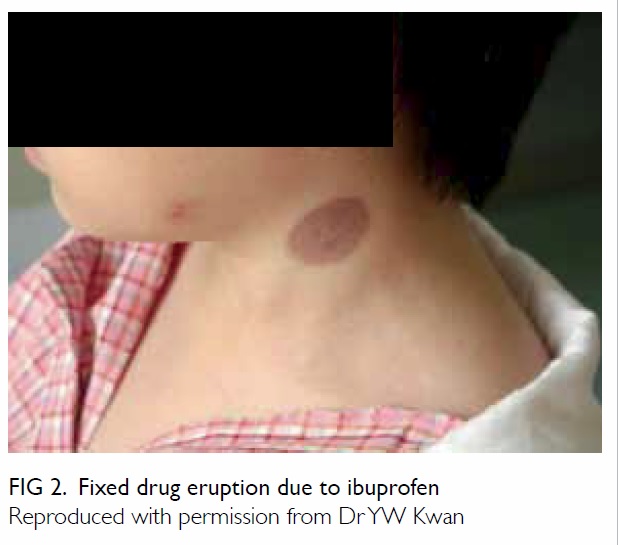
recurring at the same site are indicative of FDE (Fig 2). Presence of ‘red-flag signs’ signifies more sinister
diseases. Mucosal inflammation and ulcerations
associating with unremitting fever, intense skin
pain, and Nikolsky sign should raise concern about
possible development of SJS/TEN. Widespread
MPE associating with persistent fever, peripheral
eosinophilia, liver impairment but absence of
mucosal inflammation is suggestive of DRESS. In
NERD, patients typically have underlying chronic
rhinosinusitis, nasal polyps, and asthma complicated
by NSAID intolerance. Patients with NECD may
have chronic spontaneous urticaria.10
Differential diagnoses of hypersensitivity to
APs include hypersensitivity to concomitant drugs
and diseases with skin or mucosal manifestations,
eg viral infections, chronic urticaria, or Kawasaki
disease. On the other hand, SJS is related to infection
such as mycoplasma in 25% of affected children.27
As mentioned, AP may be given for fever control after
the onset of other symptoms. The febrile illness
that requires AP can also cause skin or mucosal
symptoms. One should also consider the possibility
that the AP is a co-factor of other allergens. A co-factor
may not cause allergy per se, but may lower
the threshold for allergic reaction to another
allergen. Common co-factors include exercise,
infection, menstruation, stress, alcohol, angiotensin-converting
enzyme inhibitors, and NSAID. Possible
mechanisms of co-factors include tight junction
dysregulation, increased gastrointestinal absorption
of allergens, and COX inhibition. The prevalence of
co-factor–dependent anaphylaxis related to NSAID
ranges from 1.2% to 4.7%.43
In-vivo tests
Aside from diagnosis of allergy to an aeroallergen
in patients with NERD, the skin prick test for AP is
probably useful only in the context of IgE-mediated SNIUAA. A negative skin prick test, however,
does not exclude hypersensitivity to APs as many
reactions are non–IgE-mediated. Moreover, with the
passage of time, even individuals with IgE-mediated
hypersensitivity may lose skin test positivity. An
intradermal test and atopic patch test may be helpful in diagnosing NSAID-induced delayed
hypersensitivity. These tests are generally specific but
not sensitive for diagnosis. Lack of standardisation
and a scarcity of available commercial reagents limit
their utility. Except for diagnosis of IgE-mediated
hypersensitivity to APs, skin tests seem to have little
diagnostic value.10
A drug provocation test (DPT), which works
independently of the underlying mechanism,
remains the gold standard for diagnosis of
hypersensitivity to APs and establishment of cross-reactivity.
As usual formulations are used, DPT is
more feasible than skin tests for AP. In a Turkish
paediatric study, only five (14%) of 36 children with
a history of single NSAID hypersensitivity reacted
positively to a DPT using the culprit drug. For 18
children with an alleged history of multiple NSAID
hypersensitivity, DPT was positive in eight (44%).
Among patients with NSAID hypersensitivity,
50% also reacted to paracetamol.9
Conversely, only
25% of patients with paracetamol hypersensitivity
develop cross-intolerance to NSAID.12
The negative
predictive value of DPT in children reaches 100% for
NSAIDs, so patients who pass a DPT can be safely
given the NSAID in future.45
A DPT is generally
not recommended during pregnancy, intercurrent
illness, or in patients with co-morbidities such as
cardiac, hepatic or renal disease, or uncontrolled
asthma. Contra-indications to DPT include a history
of SJS/TEN, DRESS, AGEP, systemic vasculitis,
drug-induced autoimmune diseases, and severe
anaphylaxis.46
A typical protocol for DPT starts with 1/50 to
1/20 of a single maximum dose of an AP, followed
by four to five incremental doses given at regular
intervals (eg 60 minutes) until the single maximum
dose is reached.9
Patients who pass a DPT on day
1 can be given a 2-day course on day 2 to ensure
full tolerance to the test drug. In case symptoms
or signs of ADR appear, DPT should be aborted
and anti-allergic treatment immediately given. The
threshold cumulative dose can then be determined.
For paracetamol, this ranges from 75 mg to 325 mg.47
The same procedure can be repeated at least 1
week later, using another AP from a structurally
unrelated class to determine cross-reactivity.48
For
instance, patients who fail a DPT for ibuprofen, an
arylpropionic acid, can undergo a subsequent DPT
for diclofenac, an acetic acid. A DPT should be
carried out in the hospital setting with resuscitation
facilities available and supervised by clinicians
experienced in managing drug hypersensitivity and
anaphylactic reaction.
In-vitro tests
Most in-vitro tests to date have not been validated or
standardised. Aside from research purposes they are
not routinely recommended for clinical use.
Serum specific immunoglobulin E test
Demonstration of specific IgE (sIgE) against a
NSAID in the serum theoretically aids diagnosis of
SNIUAA. Serum sIgE against paracetamol has been
demonstrated by some researchers.49
Compared
with skin prick test, however, serum sIgE against
NSAID is less useful. Sensitivity and specificity of
sIgE are not known.14
Basophil activation test
Detection of CD63 signifies activation of basophils
and forms the basis of the basophil activation test.
As a diagnostic tool for NIUA, basophil activation
test is relatively sensitive but not specific.50
Lymphocyte transformation test
As drug-specific T-lymphocytes are frequently
involved in NSAID hypersensitivity, a lymphocyte
transformation test (LTT) has been advocated as a
diagnostic tool. The test is based on measurement
of 3H-thymidine uptake by dividing T-cells. The
NSAIDs considered suitable for LTT include
diclofenac, mefenamic acid, and paracetamol.
Sensitivity of the LTT ranges from 60% to 70% with
specificity of approximately 85%. A positive LTT
is useful for diagnosis, but a negative test does not
exclude hypersensitivity. Involvement of a stringent
protocol and need for expert interpretation means
that LTT can be performed only by specialised
laboratories.51
Management of hypersensitivity to antipyretics
Acute management
The offending AP should be stopped and
antihistamine given. In case of anaphylactic reaction,
emergent treatment and resuscitation should be
performed. Oxygen, intramuscular adrenaline, and
antihistamine should be given. A severe cutaneous
adverse reaction should be managed in the intensive
care unit. Standard treatment includes intravenous
fluids, corticosteroid, intravenous Ig, and other
immunosuppressants.23
Follow-up
Management of suspected AP hypersensitivity starts
with thorough discussion with patients or caretakers
of the pros and cons of the AP as opposed to avoidance.
The aims of investigation include confirmation of
hypersensitivity and cross-reactivity, differentiation
between hypersensitivity to the active ingredient
versus excipients, and trial of safe alternatives.
Detailed review of drug history is of paramount
importance. Above all, DPT is pivotal to achieving the
aims of investigation. A combination of drug history
and DPT culminates in six alternative approaches to
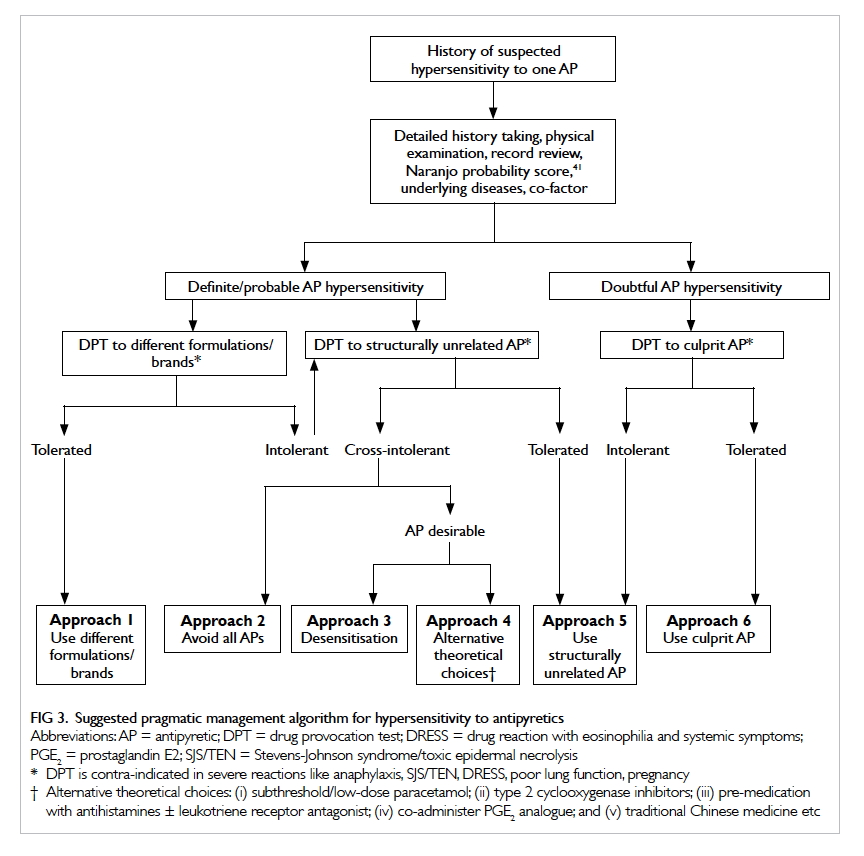
deal with hypersensitivity to APs (Fig 3).
Patients allergic to excipients in one AP may
tolerate a different brand or different formulation of
the same drug (approach 1). Detailed comparison of
constituents may reveal the excipient in question.
In case of doubt, DPT can be performed on the
alternative brand or formulation to confirm
tolerance. In case the patient reacts to different
formulations and brands of the same AP, a trial of AP
with unrelated structure can be considered (approach
5). A common example is to try ibuprofen in patients
with paracetamol hypersensitivity. As mentioned
before, three quarters of patients with paracetamol
hypersensitivity tolerate NSAIDs. Patients
hypersensitive to ibuprofen, an arylpropionic acid,
can consider DPT using paracetamol or an acetic
acid such as diclofenac.
Patients with cross-intolerance to paracetamol
and NSAIDs pose a management dilemma.
Avoidance of all APs seems logical (approach 2),
especially if the feverish patient is not ‘distressed’.
Nonetheless whether a patient is in distress or not
is a matter of subjective judgement. For cultural
reasons, it is exceedingly difficult to persuade Hong
Kong parents not to give APs to a child with a high
fever. In case fever control is deemed desirable by
either parents or physicians, viable solutions should
be sought. Desensitisation (approach 3) is another
viable option. A standard desensitisation protocol
has been established for aspirin.52 Desensitisation is
applicable to patients having NERD or NIUA.10 It is
contra-indicated in patients with a history of severe,
life-threatening drug reactions such as SJS/TENS or
DRESS. Nonetheless desensitisation should only be
carried out in medical facilities with resuscitation
equipment and expertise in drug allergy. Alternative
theoretical choices (approach 4) include subthreshold
or low-dose paracetamol,47 53 COX-2 inhibitors,54 pre-medication with antihistamines with or without
leukotriene receptor antagonist,55 co-administration
of a PGE2 analogue,56 and traditional Chinese
medicine.57 Future studies are needed to define
the safety and efficacy of these unconventional
treatments.
Patients with a mild or doubtful reaction to
an AP can consider a DPT, the gold standard to
diagnose or exclude hypersensitivity to the culprit
drug. Patients who react to the culprit AP during
DPT can either try a structurally unrelated AP
(approach 5) or try a different brand/formulation
(approach 1). Finally, patients who pass the DPT can
be given the culprit drug in future (approach 6), as
the test has a very high negative predictive value.10
Conclusion
It is arguable that APs may not be indicated in
the first place and should be avoided in patients
with hypersensitivity. Although APs should not
be prescribed simply for the sake of ‘temperature
control’, the need to mitigate patient discomfort
should not be disregarded.58 Patients with illnesses
such as heart failure, head injury, or sepsis present
special problems. Their limited reserve to withstand
the hypermetabolic state associated with febrile
episodes puts them at particular risk.59 For these
patients, APs seem beneficial. In case they have
hypersensitivity to APs, viable options should be
sought. Attempts to predict such hypersensitivity
are daunting. Disappointingly, prediction of severe
cutaneous adverse reactions to APs is virtually
impossible. However, the presence of a positive family
history, reaction within 1 hour of consumption, and
history of multiple NSAID hypersensitivities may
sound an alarm for the increased risk of genuine
immediate hypersensitivity to APs. Clinicians
need to strike a balance between ‘hypersensitivity
phobia’ for the sake of drug safety and liberal use
of APs to uphold patients’ rights. Knowledge of the
pathogenesis of AP hypersensitivity and meticulous
diagnostics are key to judicious management.
References
1. Davis T. NICE guideline: feverish illness in children—assessment and initial management in children younger
than 5 years. Arch Dis Child Educ Pract Ed 2013;98:232-5. Crossref
2. Bertille N, Pons G, Khoshnood B, Fournier-Charrière
E, Chalumeau M. Symptomatic management of fever in
children: a national survey of healthcare professionals’
practices in France. PLoS One 2015;10:e0143230. Crossref
3. Polman HA, Huijbers WA, Augusteijn R. The use
of diclofenac sodium (Voltaren) suppositories as an
antipyretic in children with fever due to acute infections: a
double-blind, between-patient, placebo-controlled study. J
Int Med Res 1981;9:343-8. Crossref
4. Khubchandani RP, Ghatikar KN, Keny S, Usgaonkar NG.
Choice of antipyretic in children. J Assoc Physicians India
1995;43:614-6.
5. Demoly P, Bousquet J. Epidemiology of drug allergy. Curr
Opin Allergy Clin Immunol 2001;1:305-10. Crossref
6. Sánchez-Borges M. Clinical management of nonsteroidal
anti-inflammatory drug hypersensitivity. World Allergy
Organ J 2008;1:29-33. Crossref
7. Sánchez-Borges M, Capriles-Hulett A, Caballero-Fonseca
F. Risk of skin reactions when using ibuprofen-based
medicines. Expert Opin Drug Saf 2005;4:837-48. Crossref
8. Carvajal A, Prieto JR, Alvarez Requejo A, Martin Arias
LH. Aspirin or acetaminophen? A comparison from data
collected by the Spanish Drug Monitoring System. J Clin
Epidemiol 1996;49:255-61. Crossref
9. Yilmaz O, Ertoy Karagol IH, Bakirtas A, et al.
Challenge-proven nonsteroidal anti-inflammatory drug
hypersensitivity in children. Allergy 2013;68:1555-61. Crossref
10. Kowalski ML, Asero R, Bavbek S, et al. Classification and
practical approach to the diagnosis and management of
hypersensitivity to nonsteroidal anti-inflammatory drugs.
Allergy 2013;68:1219-32. Crossref
11. Schnyder B, Pichler WJ. Mechanisms of drug-induced
allergy. Mayo Clin Proc 2009;84:268-72. Crossref
12. Rutkowski K, Nasser SM, Ewan PW. Paracetamol
hypersensitivity: clinical features, mechanism and role of
specific IgE. Int Arch Allergy Immunol 2012;159:60-4. Crossref
13. Pichler WJ. Drug hypersensitivity reactions: classification
and relationship to T-cell activation. In: Pichler WJ, editor.
Drug hypersensitivity. Basel: Karger; 2007: 168-89. Crossref
14. Kowalski ML, Makowska JS, Blanca M, et al.
Hypersensitivity to nonsteroidal anti-inflammatory drugs
(NSAIDs)—classification, diagnosis and management:
review of the EAACI/ENDA(#) and GA2LEN/HANNA*.
Allergy 2011;66:818-29. Crossref
15. Yawalkar N, Egli F, Hari Y, Nievergelt H, Braathen LR,
Pichler WJ. Infiltration of cytotoxic T cells in drug-induced
cutaneous eruptions. Clin Exp Allergy 2000;30:847-55. Crossref
16. Yawalkar N. Drug-induced exanthems. Toxicology
2005;209:131-4. Crossref
17. Shiohara T. Fixed drug eruption: pathogenesis and
diagnostic tests. Curr Opin Allergy Clin Immunol
2009;9:316-21. Crossref
18. Savin JA. Current causes of fixed drug eruption in the UK.
Br J Dermatol 2001;145:667-8. Crossref
19. Schrijvers R, Gilissen L, Chiriac AM, Demoly P.
Pathogenesis and diagnosis of delayed-type drug
hypersensitivity reactions, from bedside to bench and
back. Clin Transl Allergy 2015;5:31. Crossref
20. Roales-Gómez V, Molero AI, Pérez-Amarilla I, et al. DRESS
syndrome secondary to ibuprofen as a cause of hyperacute
liver failure. Rev Esp Enferm Dig 2014;106:482-6.
21. Tank ND, Karelia BN, Bhansali NB. Paracetamol induced
drug reaction with eosinophilia and systemic symptoms
(Dress syndrome): a case report. Int J Pharm Sci Rev Res
2015;32:246-8.
22. Ward KE, Archambault R, Mersfelder TL. Severe adverse
skin reactions to nonsteroidal antiinflammatory drugs:
a review of the literature. Am J Health Syst Pharm
2010;67:206-13. Crossref
23. Borchers AT, Lee JL, Naguwa SM, Cheema GS, Gershwin
ME. Stevens-Johnson syndrome and toxic epidermal
necrolysis. Autoimmun Rev 2008;7:598-605. Crossref
24. Torres MJ, Mayorga C, Blanca M. Nonimmediate allergic
reactions induced by drugs: pathogenesis and diagnostic
tests. J Investig Allergol Clin Immunol 2009;19:80-90.
25. Mockenhaupt M, Kelly JP, Kaufman D, Stern RS; SCAR
Study Group. The risk of Stevens-Johnson syndrome and
toxic epidermal necrolysis associated with nonsteroidal
antiinflammatory drugs: a multinational perspective. J
Rheumatol 2003;30:2234-40.
26. Levi N, Bastuji-Garin S, Mockenhaupt M, et al. Medications
as risk factors of Stevens-Johnson syndrome and toxic
epidermal necrolysis in children: a pooled analysis.
Pediatrics 2009;123:e297-304. Crossref
27. Ferrandiz-Pulido C, Garcia-Patos V. A review of causes of
Stevens-Johnson syndrome and toxic epidermal necrolysis
in children. Arch Dis Child 2013;98:998-1003. Crossref
28. Roujeau JC, Bioulac-Sage P, Bourseau C, et al. Acute
generalized exanthematous pustulosis. Analysis of 63
cases. Arch Dermatol 1991;127:1333-8. Crossref
29. Britschgi M, Steiner UC, Schmid S, et al. T-cell involvement
in drug-induced acute generalized exanthematous
pustulosis. J Clin Invest 2001;107:1433-41. Crossref
30. Sidoroff A, Dunant A, Viboud C, et al. Risk factors for
acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol 2007;157:989-96. Crossref
31. Szczeklik A, Sanak M. The broken balance in aspirin
hypersensitivity. Eur J Pharmacol 2006;533:145-55. Crossref
32. Sánchez-Borges M. NSAID hypersensitivity (respiratory,
cutaneous, and generalized anaphylactic symptoms). Med
Clin North Am 2010;94:853-64, xiii. Crossref
33. Zembowicz A, Mastalerz L, Setkowicz M, Radziszewski W,
Szczeklik A. Histological spectrum of cutaneous reactions
to aspirin in chronic idiopathic urticaria. J Cutan Pathol
2004;31:323-9. Crossref
34. Kim SH, Sanak M, Park HS. Genetics of hypersensitivity
to aspirin and nonsteroidal anti-inflammatory drugs.
Immunol Allergy Clin North Am 2013;33:177-94. Crossref
35. Gómez F, Perkins JR, García-Martín E, Canto G, Cornejo-García JA. Genetic basis of hypersensitivity reactions to
nonsteroidal anti-inflammatory drugs. Curr Opin Allergy
Clin Immunol 2015;15:285-93. Crossref
36. Cheong HS, Park SM, Kim MO, et al. Genome-wide
methylation profile of nasal polyps: relation to aspirin
hypersensitivity in asthmatics. Allergy 2011;66:637-44. Crossref
37. Strauss J, Greeff O. Excipient-related adverse drug
reactions: a clinical approach. Curr Allergy Clin Immunol
2015;28:24-7.
38. The electronic medicines compendium. Available from:
https://www.medicines.org.uk/emc/medicine/10741.
Accessed 23 May 2017.
39. Search Drug Database. Drug Office, Department of Health,
The Government of the Hong Kong Special Administrative
Region. Available from: https://www.drugoffice.gov.hk/eps/do/en/consumer/search_drug_database.html.
Accessed 23 May 2017.
40. Knowles SR, Drucker AM, Weber EA, Shear NH.
Management options for patients with aspirin and
nonsteroidal antiinflammatory drug sensitivity. Ann
Pharmacother 2007;41:1191-200. Crossref
41. Naranjo CA, Busto U, Sellers EM, et al. A method for
estimating the probability of adverse drug reactions. Clin
Pharmacol Ther 1981;30:239-45. Crossref
42. Topal E, Celiksoy MH, Catal F, Gamze Sayan Y, Sancak
R. The value of the clinical history for the diagnosis
of immediate nonsteroidal anti-inflammatory drug
hypersensitivity and safe alternative drugs in children.
Allergy Asthma Proc 2016;37:57-63. Crossref
43. Wölbing F, Fischer J, Köberle M, Kaesler S, Biedermann T.
About the role and underlying mechanisms of cofactors in
anaphylaxis. Allergy 2013;68:1085-92. Crossref
44. Demoly P, Adkinson NF, Brockow K, et al. International
Consensus on drug allergy. Allergy 2014;69:420-37. Crossref
45. Misirlioglu ED, Toyran M, Capanoglu M, Kaya A, Civelek E,
Kocabas CN. Negative predictive value of drug provocation
tests in children. Pediatr Allergy Immunol 2014;25:685-90. Crossref
46. Aberer W, Bircher A, Romano A, et al. Drug provocation
testing in the diagnosis of drug hypersensitivity reactions:
general considerations. Allergy 2003;58:854-63. Crossref
47. Ho MH, Tung JY, Lee TL, Tsoi NS, Lau YL. Anaphylaxis to
paracetamol. J Paediatr Child Health 2008;44:746-7.
48. Zambonino MA, Torres MJ, Muñoz C, et al. Drug
provocation tests in the diagnosis of hypersensitivity
reactions to non-steroidal anti-inflammatory drugs in
children. Pediatr Allergy Immunol 2013;24:151-9. Crossref
49. de Paramo BJ, Gancedo SQ, Cuevas M, Camo IP,
Martin JA, Cosmes EL. Paracetamol (acetaminophen)
hypersensitivity. Ann Allergy Asthma Immunol 2000;85(6
Pt 1):508-11. Crossref
50. Ariza A, Fernandez TD, Doña I, et al. Basophil activation
after nonsteroidal anti-inflammatory drugs stimulation in
patients with immediate hypersensitivity reactions to these
drugs. Cytometry A 2014;85:400-7. Crossref
51. Pichler WJ, Tilch J. The lymphocyte transformation test in
the diagnosis of drug hypersensitivity. Allergy 2004;59:809-20. Crossref
52. Macy E, Bernstein JA, Castells MC, et al. Aspirin challenge
and desensitization for aspirin-exacerbated respiratory
disease: a practice paper. Ann Allergy Asthma Immunol
2007;98:172-4. Crossref
53. Kidon MI, Kang LW, Chin CW, et al. Early presentation with
angioedema and urticaria in cross-reactive hypersensitivity
to nonsteroidal antiinflammatory drugs among young,
Asian, atopic children. Pediatrics 2005;116:e675-80. Crossref
54. Corzo JL, Zambonino MA, Muñoz C, et al. Tolerance
to COX-2 inhibitors in children with hypersensitivity
to nonsteroidal anti-inflammatory drugs. Br J Dermatol 2014;170:725-9. Crossref
55. Nosbaum A, Braire-Bourrel M, Dubost R, et al. Prevention
of nonsteroidal inflammatory drug-induced urticaria
and/or angioedema. Ann Allergy Asthma Immunol
2013;110:263-6. Crossref
56. Dobovišek A, Fajmut A, Brumen M. Strategy for NSAID
administration to aspirin-intolerant asthmatics in
combination with PGE2 analogue: a theoretical approach.
Med Biol Eng Comput 2012;50:33-42. Crossref
57. Liew WK, Loh W, Chiang WC, Goh A, Chay OM, Iancovici
Kidon M. Pilot study of the use of Yin Qiao San in children
with conventional antipyretic hypersensitivity. Asia Pac
Allergy 2015;5:222-9. Crossref
58. Section on Clinical Pharmacology and Therapeutics;
Committee on Drugs, Sullivan JE, Farrar HC. Fever and
antipyretic use in children. Pediatrics 2011;127:580-7. Crossref
59. Henker R. Evidence-based practice: fever-related
interventions. Am J Crit Care 1999;8:481-7.