Contrasting evidence for corticosteroid treatment for coronavirus-induced cytokine storm
Hong Kong Med J 2020 Jun;26(3):269–71 | Epub 5 Jun 2020
Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
LETTER TO THE EDITOR
Contrasting evidence for corticosteroid
treatment for coronavirus-induced
cytokine storm
Karen KY Leung, MB, BS, MRCPCH1; KL Hon, MB, BS, MD1; SY Qian, MD2; Frankie WT Cheng, MB, ChB, MD1
1 Department of Paediatrics and Adolescent Medicine, The Hong Kong
Children’s Hospital, Hong Kong
2 Pediatric Intensive Care Unit, Beijing Children’s Hospital, Capital Medical
University, National Center for Children’s Health, China
Corresponding author: Dr KL Hon (ehon@hotmail.com)
To the Editor—Two recent articles concerning
corticosteroid usage in the coronavirus disease
2019 (COVID-19) pandemic provide opposing
evidence and run the risk of muddying the waters
on this controversial yet important topic.1 2 On
the one hand, Russell et al1 tabulated a number of
mainly observational clinical studies cautioning
more harm than benefit with corticosteroid usage.
On the other hand, Shang et al,2 acknowledging that
existing evidence is inconclusive at best, referenced
recommendations by Chinese physicians with
frontline clinical experiences of COVID-19 who
advocate short courses of corticosteroids at low-to-moderate
doses for more severe disease.
In clinical settings, physicians tend to use
corticosteroids only for treating critically ill patients. Therefore, selection bias and confounders
in observational studies might contribute to any
observed increased mortality in patient groups
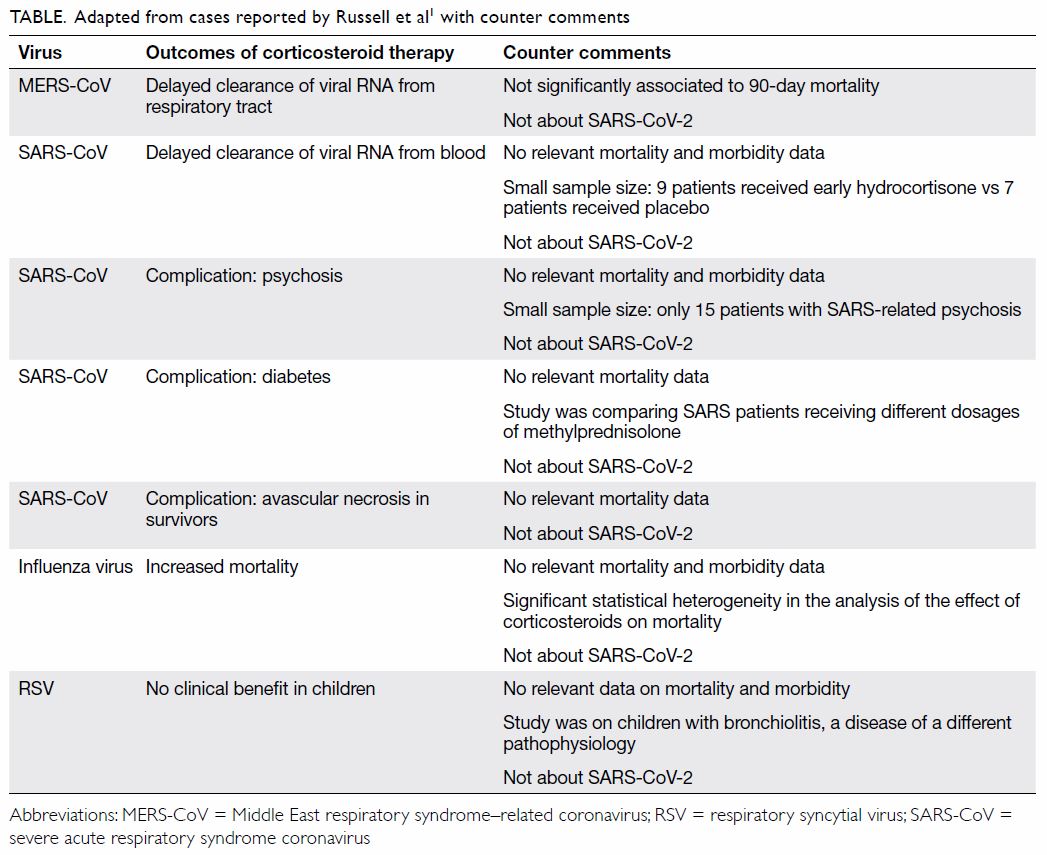
treated with corticosteroids. The papers cited by
Russell et al1 omit to address coronavirus mortality,
and the strength of the evidence presented does not
support the certainty of the authors’ conclusions
(Table).
Similar to respiratory viral diseases such as the
seasonal influenza, two categories of people seem
susceptible to die from COVID-19: older adults,
especially those with chronic disease or other co-morbidities,
and seemingly healthy adults with
exacerbated autoinflammatory syndrome termed
the cytokine storm syndromes.3 4 5 On the contrary,
children and infants seem to survive epidemics of coronavirus infections with very mild disease.6
We acknowledge the potential risks associated
with high-dose corticosteroids in treating
COVID-19 pneumonia, and agree that corticosteroid
usage should be avoided if there are other efficacious
anti-inflammatory immunomodulating medications
against the cytokine storm, such as intravenous
immunoglobulin, interleukin-1 inhibitors,
interleukin-6 inhibitors, and Janus kinase inhibitors.4
However, on the basis of recommendations by
frontline Chinese physicians and local clinical
experience during the severe acute respiratory
syndrome epidemic, a short course of corticosteroids
at low-to-moderate dose is probably justifiable for
critically ill patients with hyperinflammation.7 8
Chinese researchers are running a prospective
randomised controlled trial to review the efficacy
and safety of corticosteroids.9 Until further evidence
becomes available, whether to use corticosteroids or
not remains controversial.
Author contributions
All authors contributed to the concept of the study, acquisition
and analysis of the data, drafting of the manuscript, and critical revision of the manuscript for important intellectual
content. All authors had full access to the data, contributed to
the study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Funding/support
This letter received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
1. Russell CD, Millar JE, Baillie JK. Clinical evidence does
not support corticosteroid treatment for 2019-nCoV lung
injury. Lancet 2020;395:473-5. Crossref
2. Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of
corticosteroids for 2019-nCoV pneumonia. Lancet
2020;395:683-4. Crossref
3. Novel Coronavirus Pneumonia Emergency Response
Epidemiology Team. The epidemiological characteristics of
an outbreak of 2019 novel coronavirus diseases (COVID-19) in China [in Chinese]. Zhonghua Liu Xing Bing Xue Za
Zhi 2020;41:145-51.
4. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall
RS, Manson JJ, et al. COVID-19: consider cytokine
storm syndromes and immunosuppression. Lancet
2020;395:1033-4. Crossref
5. Ng PC, Lam CW, Li AM, Wong CK, Cheng FW, Leung TF,
et al. Inflammatory cytokine profile in children with severe
acute respiratory syndrome. Pediatrics 2004;113:e7-14. Crossref
6. Hon KL, Leung CW, Cheng WT, Chan PK, Chu WC,
Kwan YW, et al. Clinical presentations and outcome of
severe acute respiratory syndrome in children. Lancet
2003;361:1701-3. Crossref
7. Zhao JP, Hu Y, Du RH, Chen ZS, Jin Y, Zhou M, et al.
Expert consensus on the use of corticosteroid in patients
with 2019-nCoV pneumonia [in Chinese]. Zhonghua Jie
He He Hu Xi Za Zhi 2020;43:183-4.
8. Yam LY, Lau AC, Lai FY, Shung E, Chan J, Wong V, et
al. Corticosteroid treatment of severe acute respiratory
syndrome in Hong Kong. J Infect 2007;54:28-39. Crossref
9. US National Library of Medicine, US Government. Efficacy
and Safety of Corticosteroids in COVID-19. Available
from: https://clinicaltrials.gov/ct2/show/NCT04273321.
Accessed 24 Mar 2020.