Hong Kong Med J 2022;28(6):466–74 | Epub 5 Dec 2022
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE CME
Systematic review and meta-analysis of ketamine-associated uropathy
Erica OT Chan, MRCS, MB, ChB1; Vinson WS Chan, MB, ChB2,3,4; Tori ST Tang1; Vanessa Cheung1; Martin CS Wong, FHKCFP, FHKAM (Family Medicine)5; CH Yee, FRCSEd (Urol), FHKAM (Surgery)1; CF Ng, FRCSEd (Urol), FHKAM (Surgery)1; Jeremy YC Teoh, FRCSEd (Urol), FHKAM (Surgery)1
1 Department of Surgery, The Chinese University of Hong Kong, Hong Kong
2 Division of Surgery and Interventional Sciences, University College London, London, United Kingdom
3 Royal Derby Hospital, University Hospitals of Derby and Burton NHS Foundation Trust, Derby, United Kingdom
4 Leeds Institute of Medical Research, University of Leeds, Leeds, United Kingdom
5 The Jockey Club School of Public Health and Primary Care, The Chinese University of Hong Kong, Hong Kong
Corresponding author: Prof Jeremy YC Teoh (jeremyteoh@surgery.cuhk.edu.hk)
Abstract
Introduction: This systematic review and meta-analysis
focused on the literature regarding
ketamine-associated uropathy to summarise its
clinical manifestations, the results of urological
assessments, and current management.
Methods: A literature search was conducted using
keywords and MeSH terms related to ketamine
abuse, urinary tracts, and urological examinations.
Databases including Embase, MEDLINE, and the
Cochrane Central Register of Controlled Trials were
searched up to 26 June 2020.
Results: In total, 1365 articles were retrieved; 45
articles (4921 patients) were included in the analysis
of patient demographics, clinical manifestations,
examination results, and treatments. Frequency was
the most common manifestation (pooled prevalence
77.1%, 95% confidence interval [CI]=56.9%-92.2%),
followed by urgency (69.9%, 95% CI=48.8%-87.3%)
and suprapubic pain (60.4%, 95% CI=35.3%-82.9%). Upper urinary tract involvement was less
common; the pooled prevalence of hydronephrosis
was 30.2% (95% CI=22.0%-39.2%). Further workup
revealed a pooled functional bladder capacity
of 95.23 mL (95% CI=63.57-126.88 mL), pooled
voided volume of 113.31 mL (95% CI=59.44-167.19 mL), and pooled maximum urine flow rate of
8.69 mL/s (95% CI=5.54-11.83 mL/s). Cystoscopic
examinations and bladder biopsy revealed frequent
urothelial denudation, inflammatory changes, and
inflammatory cell infiltration. Treatments included
oral medications for symptomatic relief, intravesical
therapy, and surgery (eg, hydrodistension and bladder reconstruction), but ketamine abstinence
was necessary for improvement.
Conclusion: Ketamine-associated uropathy
frequently involves frequency, urgency, and
suprapubic pain; upper urinary tract involvement is
less common. Affected patients showed reductions in
bladder capacity and urine flow rate. Endoscopic and
histological analyses often revealed cystitis. Despite
variations in treatment, ketamine abstinence is
important for all patients with ketamine-associated
uropathy.
Introduction
First synthesised in the 1960s as an antagonist of the N-methyl-D-aspartate receptor, ketamine is a short-acting
anaesthetic agent which can also serve as an
analgesic for chronic pain management.1 Ketamine
was used recreationally beginning in the 1970s and
subsequently became popular among young people.2
In Hong Kong, ketamine was the most commonly
abused psychotropic substance from 2009 to 2014;
for example, 5280 individuals reportedly abused
ketamine in 2009.3 Data from the Central Registry
of Drug Abuse indicate that ketamine abuse persists
in Hong Kong, although its rate of abuse has decreased in the past several years. For example,
405 individuals reportedly abused ketamine in 2019,
which comprised 7.3% of all drug users in Hong
Kong that year.3
Ketamine abuse is known to affect the urinary
system, and lower urinary tract symptoms (LUTS)
are the most common manifestations. In 2007, the
clinical feature of ketamine-associated cystitis was
proposed by Shahani et al4 in their report of nine
patients with a history of ketamine abuse who had
LUTS; those patients also presented with bladder
wall thickening, reduced bladder capacity, and
cystoscopic findings indicative of cystitis. In 2008, Chu et al5 described 59 patients with a history of
ketamine abuse who had LUTS. Their findings
indicated that although ketamine abuse consistently
affected the lower urinary tract, it also affected the
upper urinary tract in some patients, as indicated
by the presence of hydronephrosis, renal papillary
necrosis, and an elevated level of creatinine. Because
ketamine-associated uropathy (KAU) is a relatively
new clinical entity, and its incidence is influenced
by the time- and location-dependent popularity of
ketamine, relevant literature remains limited. Thus,
it is challenging to optimise management for young
patients who present with uropathy and a history of
ketamine abuse.
Here, we performed a systematic review and
meta-analysis of KAU to summarise its key clinical
manifestations, the results of urological assessments,
and current management approaches. This review is
expected to provide insights concerning the optimal
management of KAU.
Methods
This systematic review and meta-analysis of published literature regarding KAU was conducted
in accordance with the Preferred Reporting Items for
Systematic Reviews and Meta-Analysis (PRISMA)
statement.6
Literature search
A literature search was conducted using
combinations of keywords and Medical Subject
Headings (MeSH) terms related to ‘ketamine use’,
‘ketamine addict’, ‘urinary tracts’, ‘urinary organs’, and
‘urological examinations’. Databases included in the
search were Embase, MEDLINE, and the Cochrane
Central Register of Controlled Trials. The search was
limited to human studies published in English up to
26 June 2020, excluding conference abstracts. The
reference lists of the included articles were searched
to identify additional relevant literature. The search
strategy is shown in the online Appendix 1.
Screening process and selection criteria
All articles retrieved from the literature search were
independently screened by at least two independent
reviewers. Case series, case-cohort studies, non-randomised
studies, and randomised controlled
trials related to ketamine abuse and uropathy
were included. Articles were excluded if they met
any of the following criteria: they were editorials,
commentaries, reviews, or case reports; they
described non-human studies; the full text was not
available in English; no full text was available; and/or
they described the use of ketamine for anaesthesia.
Disagreements during the screening process were
resolved by a third senior reviewer.
Data extraction
The following data were extracted from eligible studies
using a standardised form: patient demographics,
clinical features, examination results (eg,
urodynamics, radiological workup, and endoscopic
workup), and the treatment administered. Patient
demographics included age, sex, and cumulative
exposure to ketamine. Clinical features included
presenting symptoms and the results of urinalysis
and renal function tests. Urodynamic results
included the voided volume, maximum urine flow
rate (Qmax), and detrusor overactivity. Radiological
features included hydronephrosis, ureteral stricture,
and descriptive findings (eg, bladder wall thickening).
Endoscopic workup included cystoscopic findings
and bladder biopsy results. Questionnaire scores
were also recorded. Finally, treatments and the
corresponding responses were recorded.
Data analysis and statistical analysis
Quantitative and qualitative assessments were
conducted in this systematic review and meta-analysis.
Quantitative assessments included prevalences of urological symptoms, scores of
questionnaires that reflected urological symptoms,
and results of urodynamics workups. Pooled data
were analysed by OpenMeta[Analyst] when results
were available for more than two studies with >100
patients. The studies included in each pooled analysis
were individually reviewed to ensure that there was
no patient overlap among the studies. Random
effects models and double arcsine transformation
were used to pool the results. Cochran’s Q test
was used to identify heterogeneity, and P<0.10 was
considered indicative of significant heterogeneity.
I2 statistics were used to measure variations across
studies, and I2>50% was considered indicative of
significant heterogeneity. Qualitative assessments
included narrative descriptions of cystoscopic
findings, bladder biopsy results, and treatments
administered.
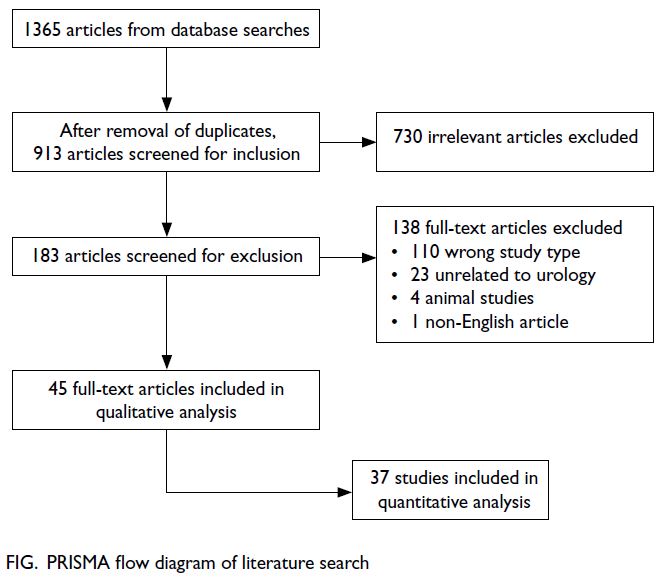
Results
In total, 1365 articles were identified by database
searches, whereas no articles were identified by
manual searches. After the removal of duplicates,
913 articles remained; 730 articles were removed
during the initial screening, and 138 articles were
removed during full-text screening. Forty-five
articles were included in the qualitative analysis, and
37 articles were included in the quantitative analysis.
The PRISMA flow diagram is shown in the Figure.
Demographics
Our analysis included 4890 patients (3518 men and
1372 women) with a mean age of 25.46 ± 6.03 years
and mean ketamine exposure duration of 54.99 ± 43.54 months.
Clinical manifestations
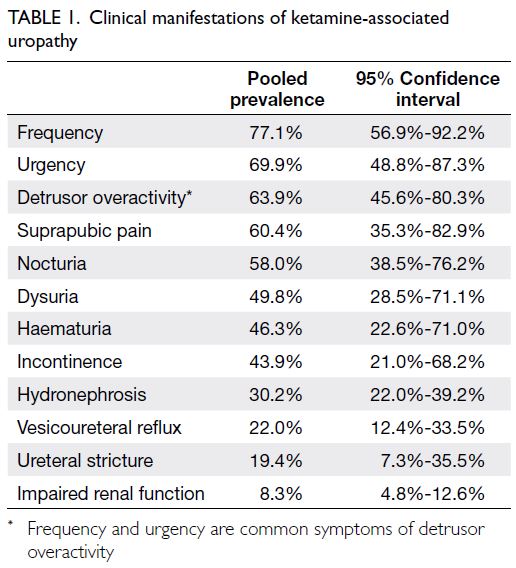
Our meta-analysis concerning clinical manifestations
of KAU included 37 studies with 4314 patients. All
reported manifestations are summarised in Table 1 and online Appendix 2. Frequency was the most
common manifestation (pooled prevalence 77.1%,
95% confidence interval [CI]=56.9%-92.2%), followed
by urgency (69.9%, 95% CI=48.8%-87.3%), bladder or
suprapubic pain (60.4%, 95% CI=35.3%-82.9%), and
nocturia (58.0%, 95% CI=38.5%-76.2%). Additionally,
substantial numbers of patients presented with
dysuria, haematuria, and/or incontinence. Upper
urinary tract involvement was less common. The
pooled prevalence of hydronephrosis was 30.2%
(95% CI=22.0%-39.2%), whereas approximately
20% of patients presented with ureteral strictures
and 10% of patients presented with impaired renal
function.
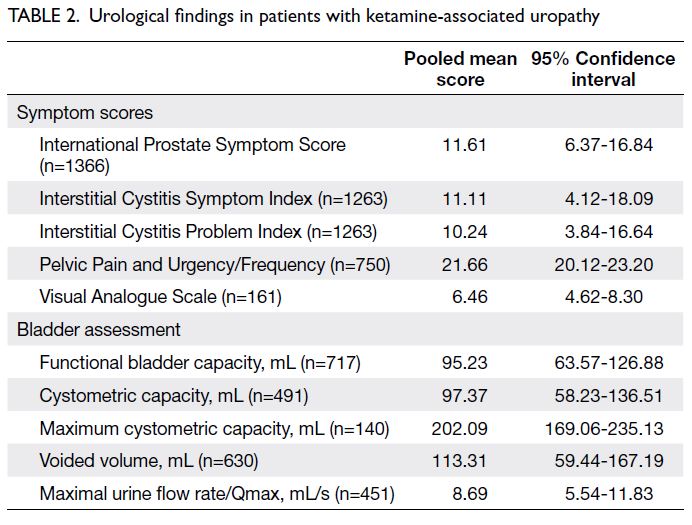
Symptom scores
Various questionnaires and scoring systems were used among studies to assess the severities of patient
symptoms. The International Prostate Symptom
Score (IPSS), Interstitial Cystitis Symptom Index
(ICSI), Interstitial Cystitis Problem Index (ICPI),
Pelvic Pain and Urgency/Frequency (PUF) total
score, and Visual Analogue Scale (VAS) were most
commonly used. Our meta-analysis of symptom
scores included 13 studies (2135 patients); the results are summarised in Table 2 and online Appendix 3.
Five studies with 1366 patients were focused on
IPSS; the pooled score was 11.61 (95% CI=6.37-16.84). Five studies (1263 patients) were focused
on ICSI and ICPI; the pooled ICSI score was 11.11
(95% CI=4.12-18.09) and the pooled ICPI score was
10.24 (95% CI=3.84-16.64). The pooled PUF score
was 21.66 (95% CI=20.12-23.20), and the pooled
VAS score was 6.46 (95% CI=4.62-8.30).
Bladder assessments
Bladder assessments in the reviewed articles
included cystoscopy, urodynamic studies,
videocystometry, bladder imaging, and bladder
biopsy. The results are shown in Table 2 and online Appendix 4. Fifteen studies (1221 patients) included
data regarding bladder capacity, including functional
bladder capacity, cystometric capacity, or maximum
cystometric capacity. The pooled functional bladder
capacity was 95.23 mL (95% CI=63.57-126.88 mL).
The pooled and maximum cystometric capacities
were 97.37 mL (95% CI=58.23-136.51 mL) and
202.09 mL (95% CI=169.06-235.13 mL), respectively.
Urodynamic studies revealed a pooled mean
voided volume of 113.31 mL (95% CI=59.44-167.19 mL) and a pooled mean Qmax of 8.69 mL/s
(95% CI=5.54-11.83 mL/s). The pooled prevalence
of detrusor overactivity was 63.9% (95% CI=45.6%-80.3%). Videocystometry or bladder imaging (eg,
ultrasound or computed tomography) showed
vesicoureteral reflux with a pooled prevalence of
22.0% (95% CI=12.4%-33.5%).
In total, 14 studies included cystoscopy results
and bladder biopsy findings. Common findings on cystoscopy included epithelial inflammation
in the bladder mucosa, erythematous patches,
neovascularisation, and ulcerations. Petechial
haemorrhage was observed in patients with severe
disease. Bladder biopsy results were consistent
with the above inflammatory changes. Urothelial
denudation and focal reactive changes were
evident. The lamina propria exhibited granulation
tissue, vascular congestion, and oedema, along
with infiltrating inflammatory cells including
lymphocytes, eosinophils, and mast cells. Perivesical
fibrosis was present in some patients. Chu et al5
found such inflammatory changes in 71% of their
patients.
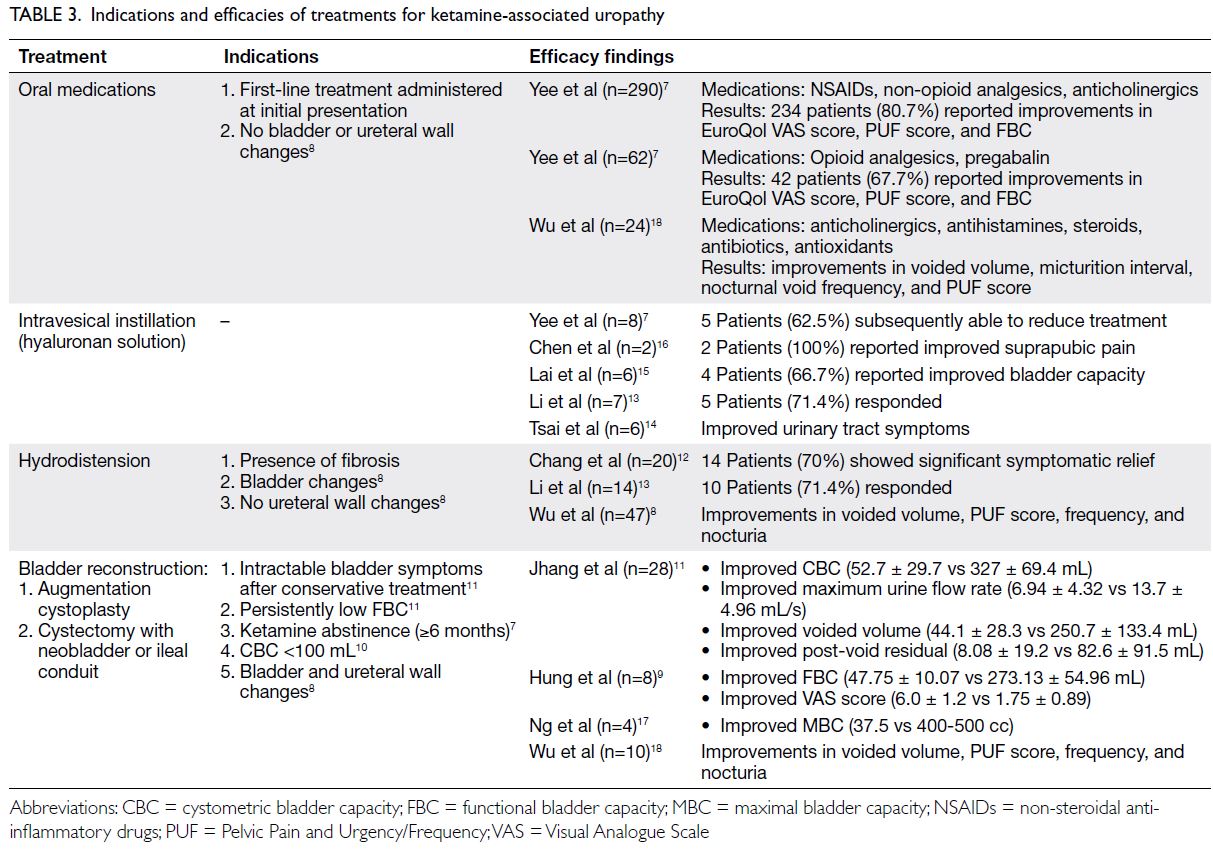
Treatments
Treatments for KAU considerably varied among
studies, and there were no trends consistent among all
studies. In total, 19 articles described the treatment
of KAU, which included oral, intravesical, and
surgical treatments. Yee et al7 and Wu et al8 adopted
a tiered treatment approach, which began with the
least invasive oral treatment and was escalated to the
most invasive surgical treatment if other treatments
failed. However, ketamine abstinence was regarded
as a key aspect of patient management in 11 of the
19 articles. Various treatments with their potential
indications and efficacies are summarised in
Table 3.7 8 9 10 11 12 13 14 15 16 17 18
Oral treatment
Oral treatment approaches involved non-steroidal
anti-inflammatory drugs, opioid or non-opioid
analgesics, anticholinergics, pregabalin, steroids,
antibiotics, antihistamines, antioxidants, and/or
pentosane polysulphate sodium. Yee et al7 and Wu
et al18 reported that oral treatments led to positive
outcomes; most patients showed improvements
in symptoms, functional bladder capacity, voided
volume, PUF scores, and EuroQol VAS scores. In a
case series of nine patients, Shahani et al4 observed
that oral pentosane polysulphate sodium produced
symptomatic relief when combined with ketamine
abstinence. However, 11 patients in another study
exhibited poor responses to treatment involving non-steroidal
anti-inflammatory drugs, anticholinergics,
and antibiotics.14
Intravesical treatment
In total, seven studies reviewed intravesical instillation of hyaluronan solutions (eg, sodium hyaluronate and
hyaluronic acid). In six of these studies (34 patients),
30 patients showed improvements in symptoms,
including suprapubic pain (2 patients) and bladder
capacity (4 patients).7 13 14 15 16 19 In the remaining study
(2 patients), intravesical treatment did not lead to
symptomatic improvement.12
Surgical treatment
Surgical treatments for patients with KAU included
hydrodistension and bladder reconstruction. In most
studies, surgical treatments were performed after
patients had not responded to oral treatment. Three
studies described the efficacy of hydrodistension,
which involves the use of fluid to stretch the bladder
in an anaesthetised patient. Wu et al8 reported that
hydrodistension led to improvements in voided
volume, PUF score, frequency, and nocturia in 35
of 47 patients (74.5%). Chang et al12 and Li et al13
reported that 70.0% (14/20) and 71.4% (10/14) of
patients, respectively, exhibited symptomatic relief
after hydrodistension. In a few studies, botulinum
toxin type A injection was conducted along with
hydrodistension. Whereas Zeng et al20 reported
that symptoms were generally improved with such
treatment, Sihra et al21 found that only four of
29 patients experienced subjective symptomatic relief.
Another surgical approach comprises
bladder reconstruction, which can be achieved by
augmentation cystoplasty or by cystectomy and
subsequent construction of a neobladder or an
ileal conduit.21 Augmentation cystoplasty increases
the bladder capacity by anastomosing a segment of elastic gastrointestinal tract to the diseased fibrotic
bladder wall. In the cystectomy method, retention of
the bladder neck is combined with the construction
of a neobladder to store urine or an ileal conduit to
divert urine. Among five studies concerning these
reconstruction methods, all demonstrated efficacy
in the treatment of advanced ketamine cystitis (KC; a
key feature of KAU).9 10 11 17 22 Lee et al10 reported that 26
of 26 patients showed postoperative improvements
in bladder capacity, voided volume, and residual
volume; similarly, Hung et al9 reported that all eight
patients showed postoperative improvements in
bladder capacity, renal function, and pain perception.
Furthermore, concomitant ureteral reimplantation
can be performed in patients with vesicoureteral
reflux severity of grade ≥3.10 Importantly, surgical
treatments were only effective when patients avoided
further ketamine abuse.10 11 17 22
Discussion
Clinical presentation of ketamine-associated
uropathy
Ketamine-associated uropathy is a clinical entity
that has emerged with the increasing popularity of ketamine as an illicit drug among young people. In
37 studies with >4000 patients, the most common
manifestations—observed in approximately 60%
of patients diagnosed with KAU—were frequency,
nocturia, urgency, and bladder pain. Other common
urinary symptoms, with prevalences of 40% to 50%,
included dysuria, haematuria, and incontinence.
Upper urinary tract involvement (eg, hydronephrosis
and/or ureteral stricture) was observed in fewer than
one-third of patients; renal function was impaired
in <10% of patients. Further examinations revealed
functional and cystometric bladder capacities of
<100 mL, as well as voiding dysfunction. The bladder
assessment findings were consistent with the clinical
manifestations among affected patients. Symptom
severity may be associated with the chronicity and
dose of ketamine abuse.23 In the United Kingdom, a
survey of 3806 individuals—51.1% with a long-term
history of ketamine abuse and 33.8% with a history
of ketamine abuse in the previous 1 year—revealed
that the dose and frequency of ketamine abuse were
associated with the extent of urinary symptoms.23 In
Taiwan, Tsai et al14 observed that urinary symptoms
began 1 month after the initiation of ketamine abuse
and were considerably worse after 1 year of abuse.
Potential pathophysiology of ketamine-associated
uropathy
Despite the establishment of an association
between ketamine abuse and urinary tract damage,
the underlying pathophysiology remains unclear.
Multiple hypotheses have been proposed to explain
the mechanisms that underlie the onset of urinary
tract damage. Chu et al5 described four potential
mechanisms: direct toxin damage to the urinary tract
interstitium, microvascular injury to the urinary
tract, autoimmune reaction to ketamine exposure
in the urothelial epithelium and submucosa,
and (less likely) unrecognised bacteriuria. The
initial mechanism ultimately induces chronic
inflammatory changes in the bladder, which lead
to fibrosis, poor compliance, and contracture. The
extent of bladder fibrosis may serve as an indicator of
disease severity.24 The occurrence of fibrotic changes
is consistent with the common manifestations
of frequency, nocturia, and pain associated with
reduced bladder capacity; the occurrence of such
changes is also consistent with the reduced bladder
capacity observed during further urological workup.
Wu et al8 attributed the onset of LUTS and reduced
bladder capacity among individuals abusing
ketamine to a dysfunctional bladder epithelium and a
defective glycosaminoglycan layer. The results of our
review indicated that glomerulation, erythematous
congestion, neovascularisation, and ulceration were
common findings in cystoscopic examination of the
urothelial epithelium. Bladder biopsy examinations
revealed that urothelial denudation was common; infiltrating eosinophils and mast cells were observed
in the lamina propria. Accordingly, KAU may
involve hypersensitivity or an allergic reaction; this
hypothesis is supported by the work of Jhang et al,25
who found that the level of serum immunoglobulin E
was higher in patients with KAU than in the control
group. Although the exact pathophysiological
mechanism remains unclear, the urothelial changes
induced by ketamine and its metabolites suggest
the onset of an inflammatory process that results in
cystitis.8
Clinical diagnosis of ketamine-associated
uropathy
Ketamine cystitis, a key feature of KAU, is closely
associated with LUTS. Notably, KC is similar to
interstitial cystitis (IC) in terms of clinical and
histological findings. Despite these similarities, it is
not difficult to distinguish KC and IC. The presence
of cystitis in a patient with a history of ketamine
abuse is strongly suggestive of KC. However, it
is challenging to determine whether a patient is
engaged in ketamine abuse because many patients
do not voluntarily provide such information. The
onset of cystitis in a patient aged ≤30 years is a
potential clue because IC / bladder pain syndrome
usually occurs in patients aged ≥40 years. Because of
differences in pathophysiology, Yek et al26 suggested
that the course of disease progression differed
between KC and IC. For example, KC causes bladder
contraction that leads to progressive obstructive
uropathy and the onset of hydronephrosis / hydroureter. This process explains the potential for
upper urinary tract involvement in patients with
KAU. In contrast, IC is a chronic bladder condition;
its severity generally is not equivalent to the severity
of KC.24 In a comparison of clinical characteristics
between 16 patients with IC and 13 patients with
KC, Wu et al18 found that patients with KC had more
severe bladder dysfunction, along with reduced
bladder capacity, greater bladder wall thickness,
and increased bladder mass volume. Patients with
KC also reported greater subjective discomfort (ie,
a lower VAS score), compared with patients who had
IC.
The similarities between KC and IC should
be considered during the workup of suspected
KAU. The main consideration involves the use of
questionnaires in assessing symptoms and disease
severity. Among the articles included in this review,
the IPSS, ICSI, ICPI, and PUF were commonly used
to assess IC; they generally helped to identify patients
who required further examination.27 Clemons et al28
suggested that the ICSI and ICPI were suitable for
the assessment of LUTS in patients with IC, but
these questionnaires may not be appropriate for
use during initial diagnosis. Accordingly, Clemons
et al28 proposed that 7 of 15 and 5 of 14 should be regarded as the respective scores for the ICSI
and ICPI. In our review, the pooled ICSI and ICPI
scores were 11.11 and 10.24, respectively. In 2012,
the PUF score was validated by Ng et al29 for the
assessment of LUTS associated with the use of street
ketamine—the score was correlated with disease
severity, as indicated by patient symptoms and the
results of other examinations. In that study, Ng et al29
proposed using a PUF score of 17 as a threshold to
indicate serious urological consequences. In our
review, the pooled PUF score was 21.66. The use of
questionnaires during the workup of suspected KAU
is a subjective assessment of patient symptoms that
may provide insights concerning disease severity
and quality of life. Although there is no consensus
or standardised questionnaire specific to KAU, these
non-invasive questionnaires are useful for initial
patient assessment and can help guide subsequent
management.
In addition to the use of subjective
questionnaires, the workup of suspected KAU
commonly consists of radiological, urodynamic,
and endoscopic assessments. Despite the absence
of standard diagnostic criteria, suspicion of KAU
may be based on the presence of urinary tract
symptoms combined with a history of ketamine
abuse. Multiple aspects should be considered
during the workup of suspected KAU. The main
goal is the exclusion of other causes that explain the
symptoms, including urinary tract infections and
urolithiasis. Therefore, standard initial examinations
comprise urinalysis, urine culture, and X-ray
imaging. Anatomical involvement can be assessed by
computed tomography urography to identify upper
urinary tract involvement (eg, ureteral stricture,
vesicoureteral reflux, and hydronephrosis) and
confirm lower urinary tract involvement (eg, diffuse
bladder wall thickening, mucosal enhancement,
and inflammatory changes in perivesical tissue).30
Urinary system functionality can be evaluated
by urodynamics studies that investigate voided
volume, Qmax, detrusor compliance, and detrusor
overactivity. Cystoscopy and biopsy enable detailed
bladder assessments that clarify cystitis severity
and should be considered during a later stage of
workup if indicated. However, caution is needed
when considering the use of cystoscopy. Routine
cystoscopy is not recommended17 because it may
discourage young patients from undergoing further
medical examinations, with long-term impacts on
their health.
Management of ketamine-associated
uropathy
Although there is no specific regimen or algorithm
for the treatment of KAU, ketamine abstinence is
consistently effective, regardless of KAU severity.
The effects of ketamine abstinence on alleviating and controlling symptoms of KAU are well known.31 32
In a study of 66 individuals with a history of
ketamine abuse, Mak et al33 observed significantly
improved maximal voided volume after ≥1 year of
abstinence (387 mL), compared with patients who
had <3 months of abstinence (243 mL). Yee et al24
found that hydronephrosis spontaneously resolved
in patients who abstained from ketamine abuse. At
initial presentation, medications such as analgesics,
anti-inflammatory drugs, and antihistamines were
commonly used for symptomatic control.26 Further
management options involve intravesical instillation
of pentosan polysulphate and hyaluronic acid to
protect the bladder lining and repair the damaged
glycosaminoglycan layer in the urothelium.19 There
is considerable variation in patient responses
to the above oral and intravesical treatments.
Surgery is indicated when conservative treatments
fail. Bladder reconstruction surgery generally
leads to improvements in symptoms and bladder
functionality,32 but it carries more risks than
other treatment options. The available treatment
options comprise a spectrum of effectiveness
and invasiveness. The most invasive bladder
reconstruction surgery is associated with greater
effectiveness. Yee et al7 proposed a standardised
treatment protocol that comprised the following
four-tier approach, where patients receive
increasingly invasive treatment if less invasive
treatments are ineffective: (1) anti-inflammatory or
anticholinergic drugs, (2) opioid or pregabalin, (3)
intravesical hyaluronic acid, and (4) hydrodistension
and bladder reconstruction surgery (including
augmentation cystoplasty and cystectomy with
an ileal conduit or neobladder). Wu et al8 adopted
a similar tiered approach that involved assigning
patients to three clinical staging groups according
to their radiological and urodynamic findings. Stage
I patients received lifestyle modification guidance
and appropriate medications, stage II patients
underwent hydrodistension, and stage III patients
underwent bladder reconstruction surgery if other
treatments were ineffective for 3 months. This tiered
approach favourably balances the invasiveness
and effectiveness among the available treatment
modalities.
Limitations
There were some limitations in this systematic
review and meta-analysis. First, all included studies
were retrospective; there is a need for prospective
studies of patients with KAU. Second, there was
heterogeneity among the included studies, primarily
because of variations in workup and treatment;
standardisation would be beneficial for future
management of patients with KAU. Third, treatment
efficacy could not be fully assessed because baseline
characteristics were not described in a consistent manner in the included studies. Fourth, because
the incidence of KAU depends on the locality-dependent
popularity of ketamine, most studies
were from Asian countries/regions (eg, Taiwan
and Hong Kong). Nevertheless, this comprehensive
review provides an important summary concerning
the limited available information about KAU.
Conclusion
Patients with KAU most commonly present with
frequency, urgency, suprapubic pain, and nocturia;
the upper urinary tract is occasionally involved.
The occurrence of urinary symptoms in young
patients with a history of ketamine abuse should
lead to suspicion of KAU. Validated symptom
scores are useful in patient-based subjective
assessment of symptom severity and treatment
progress, whereas radiological and urodynamic
examinations objectively define the extent of urinary
tract involvement and the functional impairment
that results from KAU. In terms of management,
ketamine abstinence is essential and a tiered
treatment approach is preferred, beginning with
the least invasive medications and progressing to
surgery if conservative treatments are ineffective.
Author contributions
Concept or design: EOT Chan, VWS Chan, MCS Wong, JYC
Teoh.
Acquisition of data: EOT Chan, VWS Chan, TST Tang, V Cheung.
Analysis or interpretation of data: EOT Chan, VWS Chan, JYC Teoh.
Drafting of the manuscript: EOT Chan.
Critical revision of the manuscript for important intellectual content: MCS Wong, CH Yee, CF Ng, JYC Teoh.
Acquisition of data: EOT Chan, VWS Chan, TST Tang, V Cheung.
Analysis or interpretation of data: EOT Chan, VWS Chan, JYC Teoh.
Drafting of the manuscript: EOT Chan.
Critical revision of the manuscript for important intellectual content: MCS Wong, CH Yee, CF Ng, JYC Teoh.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
As editors of the journal, MCS Wong, CF Ng, and JYC Teoh were not involved in the peer review process. Other authors
have disclosed no conflicts of interest.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
Ethics approval is not required for this study which is a review on published research and not involving patient data
collection and retrieval of patient data.
References
1. Noppers I, Niesters M, Aarts L, Smith T, Sarton E, Dahan A. Ketamine for the treatment of chronic non-cancer pain. Expert Opin Pharmacother 2010;11:2417-29. Crossref
2. Morgan CJ, Curran HV, Independent Scientific Committee on Drugs. Ketamine use: a review. Addiction 2012;107:27-
38. Crossref
3. Narcotics Division, Security Bureau, Hong Kong SAR
Government. Central Registry of Drug Abuse Sixty-ninth
Report. 2019. Available from: https://www.nd.gov.hk/en/crda_69th_report.html. Accessed 11 Nov 2020.
4. Shahani R, Streutker C, Dickson B, Stewart RJ. Ketamine-associated
ulcerative cystitis: a new clinical entity. Urology
2007;69:810-2. Crossref
5. Chu PS, Ma WK, Wong SC, et al. The destruction of the
lower urinary tract by ketamine abuse: a new syndrome?
BJU Int 2008;102:1616-22. Crossref
6. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA
Group. Preferred reporting items for systematic reviews
and meta-analyses: the PRISMA statement. Int J Surg
2010;8:336-41. Crossref
7. Yee CH, Lai PT, Lee WM, Tam YH, Ng CF. Clinical
outcome of a prospective case series of patients with
ketamine cystitis who underwent standardized treatment
protocol. Urology 2015;86:236-43. Crossref
8. Wu P, Wang Q, Huang Z, Wang J, Wu Q, Lin T. Clinical
staging of ketamine-associated urinary dysfunction: a
strategy for assessment and treatment. World J Urol
2016;34:1329-36. Crossref
9. Hung CH, Hsieh SW, Chen SK, Lin CM. Augmentation
enterocystoplasty for patients with ketamine-induced
cystitis: an 8-year experience and a review of series. Urol
Sci 2019;30:232-7. Crossref
10. Lee YK, Jhang JF, Kuo HC. Clinical outcome of augmentation
enterocystoplasty for patients with ketamine-induced
cystitis. Pain Physician 2017;20:E431-6. Crossref
11. Jhang JF, Birder LA, Chancellor MB, Kuo HC. Patient
characteristics for different therapeutic strategies in
the management ketamine cystitis. Neurourol Urodyn
2017;36:687-91. Crossref
12. Chang T, Lin CC, Lin AT, Fan YH, Chen KK. Ketamine-induced
uropathy: a new clinical entity causing lower
urinary tract symptoms. Low Urin Tract Symptoms
2012;4:19-24. Crossref
13. Li CC, Wu ST, Cha TL, Sun GH, Yu DS, Meng E. A survey
for ketamine abuse and its relation to the lower urinary
tract symptoms in Taiwan. Sci Rep 2019;9:7240. Crossref
14. Tsai TH, Cha TL, Lin CM, et al. Ketamine-associated bladder dysfunction. Int J Urol 2009;16:826-9. Crossref
15. Lai Y, Wu S, Ni L, et al. Ketamine-associated urinary tract
dysfunction: an underrecognized clinical entity. Urol Int
2012;89:93-6. Crossref
16. Chen CH, Lee MH, Chen YC, Lin MF. Ketamine-snorting
associated cystitis. J Formos Med Assoc 2011;110:787-91. Crossref
17. Ng CF, Chiu PK, Li ML, et al. Clinical outcomes of
augmentation cystoplasty in patients suffering from
ketamine-related bladder contractures. Int Urol Nephrol
2013;45:1245-51. Crossref
18. Wu SY, Jhang JF, Jiang YH, Kuo HC. Increased bladder wall
thickness is associated with severe symptoms and reduced
bladder capacity in patients with bladder pain syndrome.
Urol Sci 2016;27:263-8. Crossref
19. Meng E, Tsao CW, Tang SH, et al. Intravesical hyaluronic
acid treatment for ketamine-associated cystitis:
preliminary results. Urol Sci 2015;26:176-9. Crossref
20. Zeng J, Lai H, Zheng D, et al. Effective treatment of
ketamine-associated cystitis with botulinum toxin type
a injection combined with bladder hydrodistention. J Int
Med Res 2017;45:792-7. Crossref
21. Sihra N, Ockrim J, Wood D. The effects of recreational
ketamine cystitis on urinary tract reconstruction—a
surgical challenge. BJU Int 2018;121:458-65. Crossref
22. Chu PS, Kwok SC, Lam KM, et al. ‘Street ketamine’–associated bladder dysfunction: a report of ten cases. Hong
Kong Med J 2007;13:311-3.
23. Winstock AR, Mitcheson L, Gillatt DA, Cottrell AM. The
prevalence and natural history of urinary symptoms among
recreational ketamine users. BJU Int 2012;110:1762-6. Crossref
24. Yee CH, Teoh JY, Lai PT, et al. The risk of upper urinary
tract involvement in patients with ketamine-associated
uropathy. Int Neurourol J 2017;21:128-32. Crossref
25. Jhang JF, Hsu YH, Jiang YH, Kuo HC. Elevated serum IgE
may be associated with development of ketamine cystitis. J
Urol 2014;192:1249-56. Crossref
26. Yek J, Sundaram P, Aydin H, Kuo T, Ng LG. The clinical
presentation and diagnosis of ketamine-associated
urinary tract dysfunction in Singapore. Singapore Med J
2015;56:660-4. Crossref
27. Kushner L, Moldwin RM. Efficiency of questionnaires used to screen for interstitial cystitis. J Urol 2006;176:587-92. Crossref
28. Clemons JL, Arya LA, Myers DL. Diagnosing interstitial
cystitis in women with chronic pelvic pain. Obstet Gynecol
2002;100:337-41. Crossref
29. Ng CM, Ma WK, To KC, Yiu MK. The Chinese version
of the pelvic pain and urgency/frequency symptom scale:
a useful assessment tool for street-ketamine abusers
with lower urinary tract symptoms. Hong Kong Med J
2012;18:123-30.
30. Mason K, Cottrell AM, Corrigan AG, Gillatt DA,
Mitchelmore AE. Ketamine-associated lower urinary tract
destruction: a new radiological challenge. Clin Radiol
2010;65:795-800. Crossref
31. Wood D, Cottrell A, Baker SC, et al. Recreational ketamine:
from pleasure to pain. BJU Int 2011;107:1881-4. Crossref
32. Chung SD, Wang CC, Kuo HC. Augmentation
enterocystoplasty is effective in relieving refractory
ketamine-related bladder pain. Neurourol Urodyn
2014;33:1207-11. Crossref
33. Mak SK, Chan MT, Bower WF, et al. Lower urinary tract changes in young adults using ketamine. J Urol
2011;186:610-4. Crossref