Hong Kong Med J 2022 Jun;28(3):204–14 | Epub 14 Jun 2022
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Outcomes of adolescents with acute lymphoblastic leukaemia
J Feng, PhD1,2; Frankie WT Cheng, MD, FHKAM (Paediatrics)3; Alan KS Chiang, PhD, FRCPCH3,4,5; Grace KS Lam, MB, BS, FHKAM (Paediatrics)3; Terry TW Chow, MB, BS, FHKAM (Paediatrics)3; SY Ha, MB, BS, FHKAM (Paediatrics)3,5; CW Luk, MB, BS, FHKAM (Paediatrics)3,6; CH Li, MB, ChB, FHKAM (Paediatrics)7; SC Ling, MB, BS, FHKAM (Paediatrics)8; PW Yau, MB, BS, FHKAM (Paediatrics)3,6; Karin KH Ho, MB, ChB, FHKAM (Paediatrics)7; Alex WK Leung, MB, ChB, FHKAM (Paediatrics)1,3,9; Natalie PH Chan, FHKAM (Pathology)10; Margaret HL Ng, MD, FHKAM (Pathology)10; CK Li, MD, FHKAM (Paediatrics)1,3,11
1 Department of Paediatrics, The Chinese University of Hong Kong, Hong
Kong
2 Department of Paediatrics, The First Affiliated Hospital of Wenzhou
Medical University, Wenzhou, PR China
3 Department of Paediatrics and Adolescent Medicine, Hong Kong
Children’s Hospital, Hong Kong
4 Department of Paediatrics and Adolescent Medicine, The University of
Hong Kong, Hong Kong
5 Department of Paediatrics and Adolescent Medicine, Queen Mary
Hospital, Hong Kong
6 Department of Paediatrics and Adolescent Medicine, Queen Elizabeth
Hospital, Hong Kong
7 Department of Paediatrics and Adolescent Medicine, Tuen Mun Hospital,
Hong Kong
8 Department of Paediatrics and Adolescent Medicine, Princess Margaret
Hospital, Hong Kong
9 Department of Paediatrics, Prince of Wales Hospital, Hong Kong
10 Department of Anatomical and Cellular Pathology, The Chinese
University of Hong Kong, Hong Kong
11 Hong Kong Hub of Paediatrics Excellence, The Chinese University of
Hong Kong, Hong Kong
Corresponding author: Prof CK Li (ckli@cuhk.edu.hk)
Abstract
Introduction: Compared with young children
who have acute lymphoblastic leukaemia (ALL),
adolescents with ALL have unfavourable disease
profiles and worse survival. However, limited data
are available regarding the characteristics and
outcomes of adolescents with ALL who underwent
treatment in clinical trials. The aim of this study
was to investigate the causes of treatment failure in
adolescents with ALL.
Methods: We retrospectively analysed the outcomes
of 711 children with ALL, aged 1-18 years, who
were enrolled in five clinical trials of paediatric ALL
treatment between 1993 and 2015.
Results: Among the 711 children with ALL, 530
were young children (1-9 years at diagnosis) and
181 were adolescents (including 136 younger
adolescents [10-14 years] and 45 older adolescents
[15-18 years]). Compared with young children who
had ALL, adolescents with ALL were less likely to
have favourable genetic features and more likely to
demonstrate poor early response to treatment. The
10-year overall survival and event-free survival rates
were significantly lower among adolescents than
among young children (77.9% vs 87.6%, P=0.0003;
69.7% vs 76.5%, P=0.0117). There were no significant
differences in the 10-year cumulative incidence of
relapse, but the 10-year cumulative incidence of
treatment-related death (TRD) was significantly
greater among adolescents (7.2%) than among young
children (2.3%; P=0.002). Multivariable analysis
showed that both younger and older adolescents
(vs young children) had worse survival and greater
incidence of TRD.
Conclusion: Adolescents with ALL had worse
survival because they experienced a greater
incidence of TRD. There is a need to investigate optimal treatment adjustments and novel targeted
agents to achieve better survival rates (without
excessive toxicity) among adolescents with ALL.
New knowledge added by this study
- Compared with young children who had acute lymphoblastic leukaemia (ALL), adolescents with ALL were more likely to have a T-cell immunophenotype and less likely to have favourable genetic features (high hyperdiploidy and ETV6-RUNX1).
- A greater proportion of adolescents with ALL had poor day 8 prednisone response and did not achieve complete remission.
- Adolescents with ALL had worse survival and a greater incidence of treatment-related death.
- There is a need to investigate optimal treatment adjustments and novel targeted agents to achieve better survival rates (without excessive toxicity) among adolescents who receive paediatric ALL treatment protocols.
- Novel targeted agents for patients with poor early response to ALL treatment may overcome treatment resistance and improve clinical outcomes.
Introduction
Despite dramatic improvement in the prognosis of
paediatric acute lymphoblastic leukaemia (ALL), the
age at diagnosis remains a major prognostic factor:
adolescents with ALL have worse outcomes than
their younger counterparts.1 2 3 4 This is partly related to
differences in disease biology, such that older children
with ALL more frequently have a T-cell phenotype
and less frequently have high hyperdiploidy or
ETV6-RUNX1 translocation.1 1 1 1 1 9 Therefore, older
children constitute a distinct subgroup for which an
optimal treatment strategy has not been determined.
Although intensive treatment protocols for
paediatric ALL reportedly improve outcomes among
adolescents,3 5 10 11 12 limited data are available from
East Asian countries regarding the characteristics
of adolescents with ALL who underwent treatment
in clinical trials.13 The National Cancer Institute
criteria, used for risk stratification in most
international ALL trials, define age ≥10 years as a
risk factor for B-cell precursor ALL1 2 3 4 5 10 11 12 14; however, most treatment-related toxicities occur with
significantly greater frequency in older adolescents
(aged ≥15 years).1 2 3 4 5 10 11 12 14 To our knowledge, there
is limited available information regarding the
differences in clinical characteristics and long-term
treatment outcomes between adolescents (younger
adolescents aged 10-14 years and older adolescents
aged 15-18 years) and young children (aged 1-9 years) who receive intensive paediatric treatment
protocols for ALL.13 15 Additionally, because ALL
is a comparatively uncommon disorder in older
adolescents, specific treatment outcome data for
such patients are limited. We aimed to study the
territory-wide outcome of adolescents with ALL
treated by uniform chemotherapy protocols in Hong
Kong, and tried to identify the treatment response
and toxicity profile in the adolescents, and also
the causes of treatment failure in particular older
adolescents who shared similar characteristics of
young adults.
Methods
Patients
In total, 711 patients (aged 1-18 years) newly
diagnosed with ALL were enrolled in consecutive
clinical trials during the period from 1993 to 2015;
these trials were HKALL 9316 (1993-1997, n=144),
HKALL 9717 (1997-2002, n=170), ALL IC-BFM 200218 (2003-2008, n=169), CCLG-ALL 200819 (2008-2015, n=221), and EsPhALL20 (2008-2014, n=7).
Risk classification and treatment
Detailed treatment stratification and therapy
protocols used in the five trials have been described
elsewhere. Briefly, stratification in the HKALL
93, HKALL 97, and ALL IC-BFM 2002 trials was
performed using the following information: initial
white blood cell count, central nervous system
(CNS) status, immunophenotype, age at diagnosis,
molecular-genetic abnormalities (t[9;22]/BCR-ABL1,
ETV6-RUNX1, t[1;19]/TCF3-PBX1, and KMT2A-rearranged),
and early response to chemotherapy
(day 8 prednisone response and post-induction
bone marrow status). Thus, patients were stratified
into three risk groups within the respective trials:
standard-risk, intermediate-risk, and high-risk. In
the CCLG-ALL 2008 trial, therapy stratification was
performed using flow cytometry and polymerase
chain reaction–based analyses of minimal residual
disease (MRD).19 Definitive risk assignment (for
provisional standard- or intermediate-risk cases
based on presenting features) was performed after
MRD evaluation during therapy. In the EsPhALL
trial, patients were stratified into good and poor
risk groups according to their early response to
induction therapy (day 8 prednisone response and
post-induction bone marrow status).
Statistical analysis
Characteristics were compared among age-groups
using the Chi squared test or Fisher’s exact test for
categorical variables; the Wilcoxon rank-sum test
was used for comparisons of continuous variables.
We used the following age-group definitions: young children were patients aged 1 to 9 years
and adolescents were patients aged 10 to 18 years;
younger adolescents were patients aged 10 to 14 years
and older adolescents were patients aged 15 to
18 years. Complete remission (CR) was defined as
<5% bone marrow lymphoblasts and the absence of
peripheral lymphoblasts or extramedullary disease.
Event-free survival (EFS) was defined as the length
of time from diagnosis to the last follow-up or first
event (relapse, secondary malignancy, or death from
any cause). Overall survival (OS) was defined as the
length of time from diagnosis to the last follow-up
or death from any cause. The probabilities of EFS
and OS were estimated by Kaplan–Meier analysis;
they were compared between groups using the
log-rank test. Time to relapse was defined as the
length of time from the end of remission induction
chemotherapy (for patients who achieved CR) to
relapse. The cumulative incidence of relapse was
estimated according to time period; death from any
cause before relapse was regarded as a competing
event. Time to treatment-related death (TRD)
was defined as the length of time from the date of
diagnosis until death from non-progressive disease. The cumulative incidence of TRD was estimated by
regarding leukaemia-related death and relapse as
competing risk factors. Gray’s methods were used
to assess the effects of age-group on the cumulative
incidences of relapse and TRD. Univariable and
multivariable Cox proportional hazard regression
models were used to identify predictors of survival;
univariable and multivariable competing risks
regression models were used to identify predictors
of TRD. Predictors with P values <0.1 in univariable
analyses were included in the corresponding
multivariable model. All tests were two-sided, and P
values <0.05 were considered statistically significant.
Stata Statistical Software (version 12.0; StataCorp,
College Station [TX], United States) was used for
all statistical analyses. The STROBE checklist was
followed to ensure standardised reporting.
Results
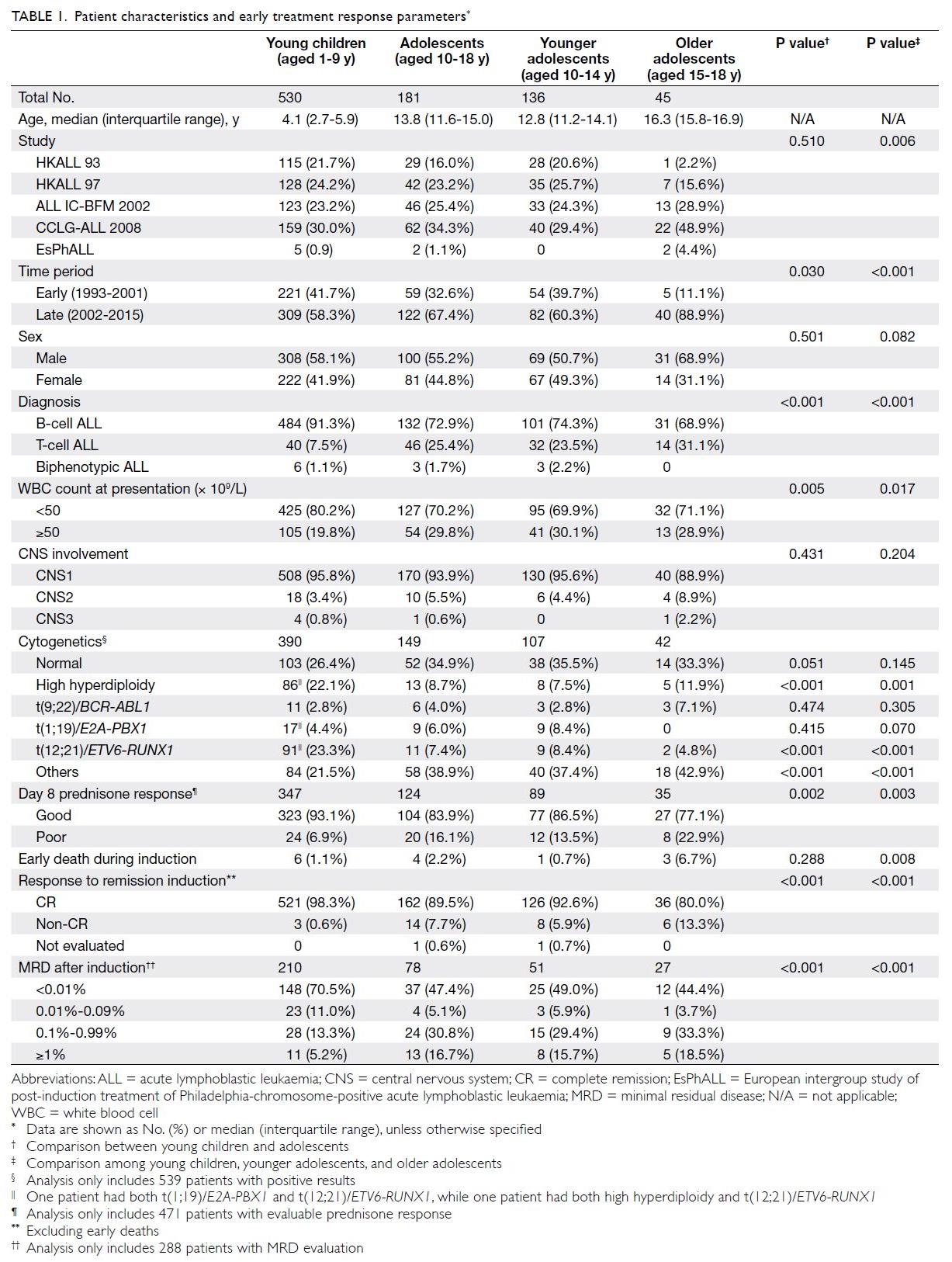
Patient characteristics
The characteristics of the 711 patients analysed in this study are shown in Table 1. There were 530 young
children, 136 younger adolescents, and 45 older
adolescents. Sex distribution did not differ between
young children and adolescents, but the proportion
of male patients tended to be higher among older
adolescents. The proportion of patients with white
blood cell count ≥50 × 109/L at presentation was
greater among adolescents than among young
children (29.8% vs 19.8%, P=0.005). The proportion
of patients with a B-cell immunophenotype was
greater among young children (91.3% vs 72.9%),
while the proportions of patients with a T-cell
immunophenotype were significantly greater among older and younger adolescents than among
young children (31.1% vs 23.5% vs 7.5%, P<0.001).
The incidences of CNS involvement at diagnosis
(CNS2/3 status) were 11.1%, 4.4%, and 4.2%
among older adolescents, younger adolescents,
and young children, respectively; these values did
not significantly differ (P=0.102). Concerning the
karyotypes of leukaemic cells, the proportion of
patients with high hyperdiploidy (≥51 chromosomes)
was significantly greater among young children than
among older or younger adolescents (P=0.001).
ETV6-RUNX1 fusion was also significantly more
common among young children (P<0.001).
In total, 471 patients underwent evaluations
of blast count in peripheral blood after 7 days of
prednisone therapy. The proportion of patients
with poor prednisone response (blast count >1.0 × 109/L after 7 days of prednisone therapy) was
greater among older adolescents than among
younger adolescents or young children (22.9% vs
13.5% vs 6.9%, P=0.003). Additionally, the CR rate
was significantly lower among older adolescents
than among younger adolescents or young children
(80.0% vs 92.6% vs 98.3%, P<0.001). The early death
rate during induction therapy was higher among
older adolescents than among younger adolescents
or young children (6.7% vs 0.7% vs 1.1%, P=0.008).
In total, 288 patients underwent MRD assessment
at the end of remission induction; the proportion
of patients with MRD ≥1% was greater among
adolescents than among young children (16.7% vs
5.2%), while the proportion of patients with MRD
<0.01% was lower among adolescents than among
young children (47.4% vs 70.5%, P<0.001). However,
MRD response did not differ between younger
adolescents and older adolescents.
Treatments and outcomes of 45 older
adolescents with lymphoblastic leukaemia
The treatments and outcomes of older adolescents
with ALL are shown in the online supplementary
Figure. Three patients died during induction (two
had TRD and one had leukaemia-related death).
Among the 36 older adolescents who achieved CR,
three patients underwent allogeneic hematopoietic
stem cell transplantation (HSCT) during CR1; one
died of transplant-related infection, one relapsed
(they achieved CR2 after salvage chemotherapy and
remained in continuous CR), and one remained in
continuous CR. The remaining 33 patients received
only chemotherapy; 28 remained in continuous CR,
one died of treatment-related infection, and five
relapsed. Among the patients who relapsed, one was
lost to follow-up, two died of progressive leukaemia,
and two received allogeneic HSCT during CR2; one
of the two transplant patients died of transplant-related
infection, while the other remained in
continuous CR.
Among the six patients who failed to achieve
CR after remission induction chemotherapy, two
died of progressive leukaemia, while four achieved
CR after salvage chemotherapy. Among the four
patients who achieved CR, three received allogeneic
HSCT during CR1 and remained in continuous CR;
the other patient relapsed and received allogeneic
HSCT after achievement of CR2, then died of
transplant-related infection. In summary, six of the
11 deaths among older adolescents were treatment-related;
the main cause of TRD was infection.
Overall outcome analysis
The median follow-up interval (for all groups)
was 12.78 years (interquartile range=6.73-19.09).
Young children had significantly better 10-year OS
and EFS rates, compared with adolescents (87.6%
[95% confidence interval (CI)=84.4%-90.2%] vs
77.9% [95% CI=71.0%-83.4%], P=0.0003; 76.5%
[95% CI=72.6%-79.9%] vs 69.7% [95% CI=62.3%-76.0%], P=0.0117; Fig 1a and b). Ten-year relapse
rates were similar between young children and adolescents: 20.6% (95% CI=17.3%-24.4%) for
young children vs 22.8% (95% CI=16.9%-30.4%) for
adolescents (P=0.479; Fig 1c). The 10-year incidence
of TRD was significantly greater among adolescents
(7.2% [95% CI=4.1%-12.4%]) than among young
children (2.3% [95% CI=1.2%-4.1%]) [P=0.002; Fig 1d]. Subgroup analysis revealed that OS and EFS rates, as well as cumulative incidences of relapse and
TRD, were similar between younger adolescents and
older adolescents (Fig 2).
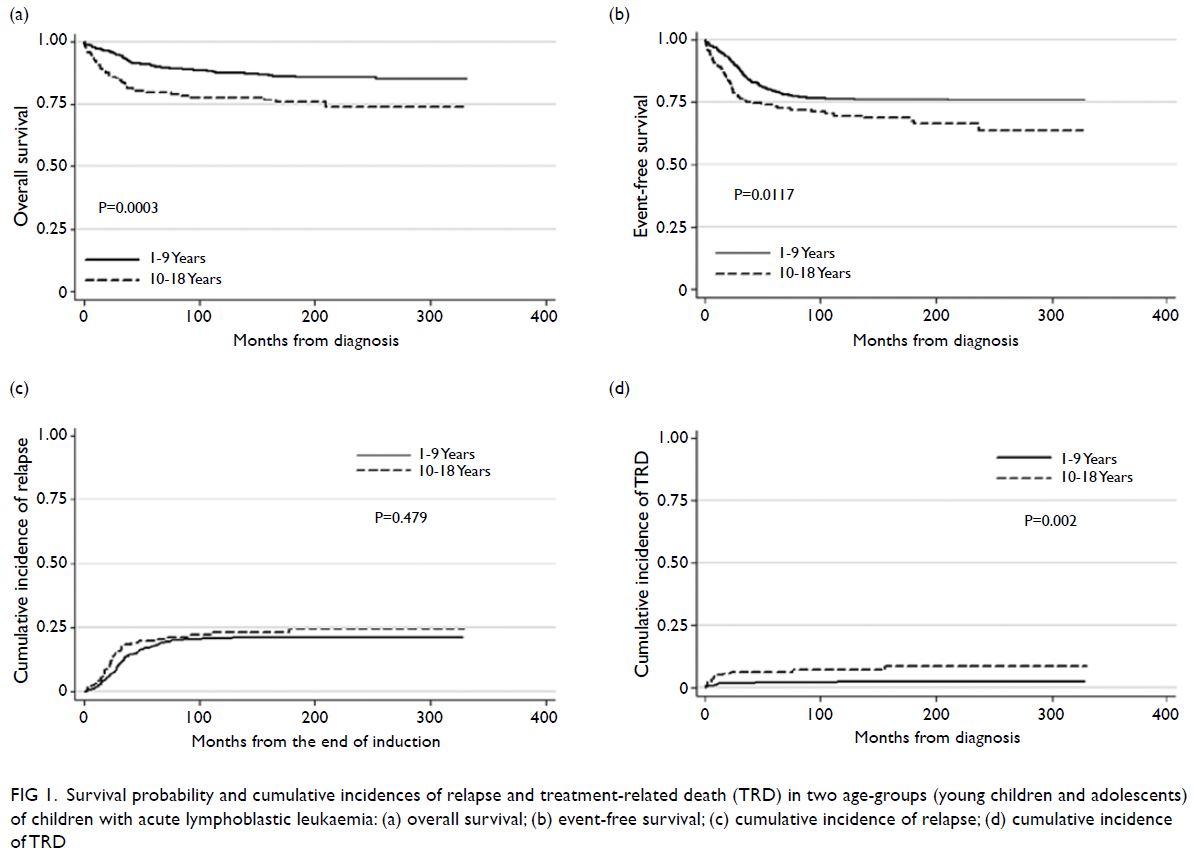
Figure 1. Survival probability and cumulative incidences of relapse and treatment-related death (TRD) in two age-groups (young children and adolescents) of children with acute lymphoblastic leukaemia: (a) overall survival; (b) event-free survival; (c) cumulative incidence of relapse; (d) cumulative incidence of TRD
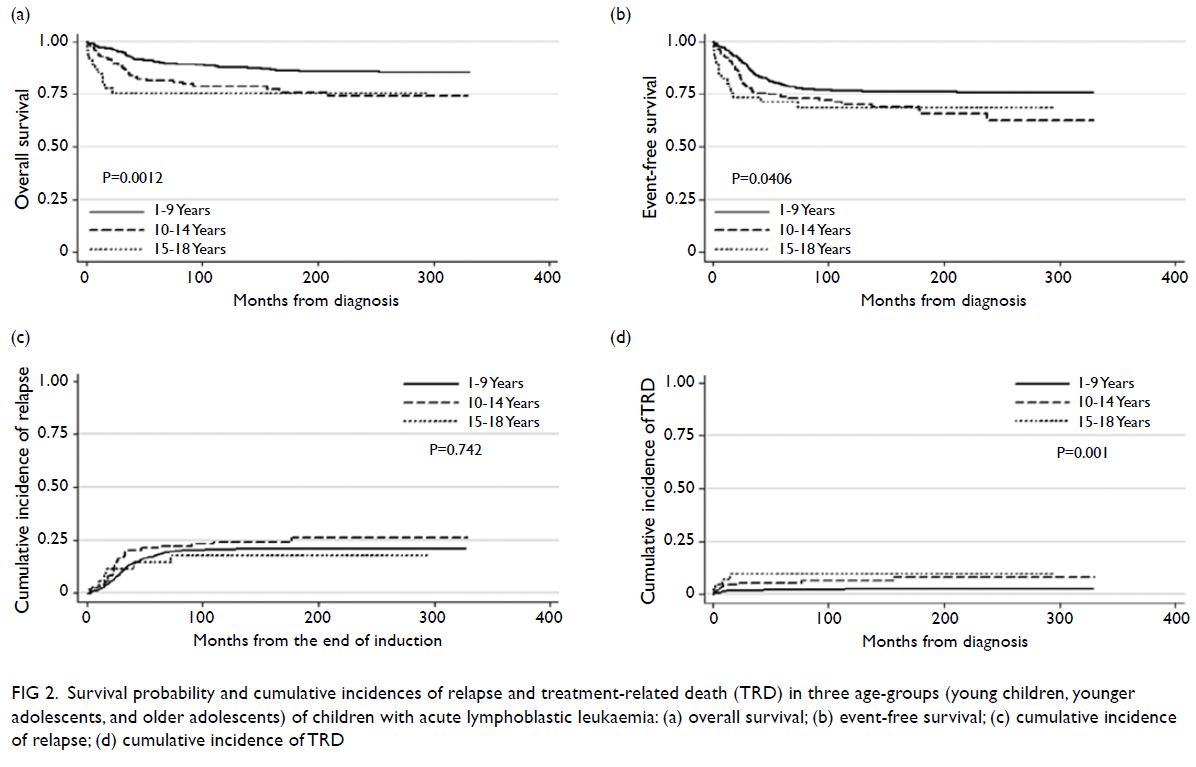
Figure 2. Survival probability and cumulative incidences of relapse and treatment-related death (TRD) in three age-groups (young children, younger adolescents, and older adolescents) of children with acute lymphoblastic leukaemia: (a) overall survival; (b) event-free survival; (c) cumulative incidence of relapse; (d) cumulative incidence of TRD
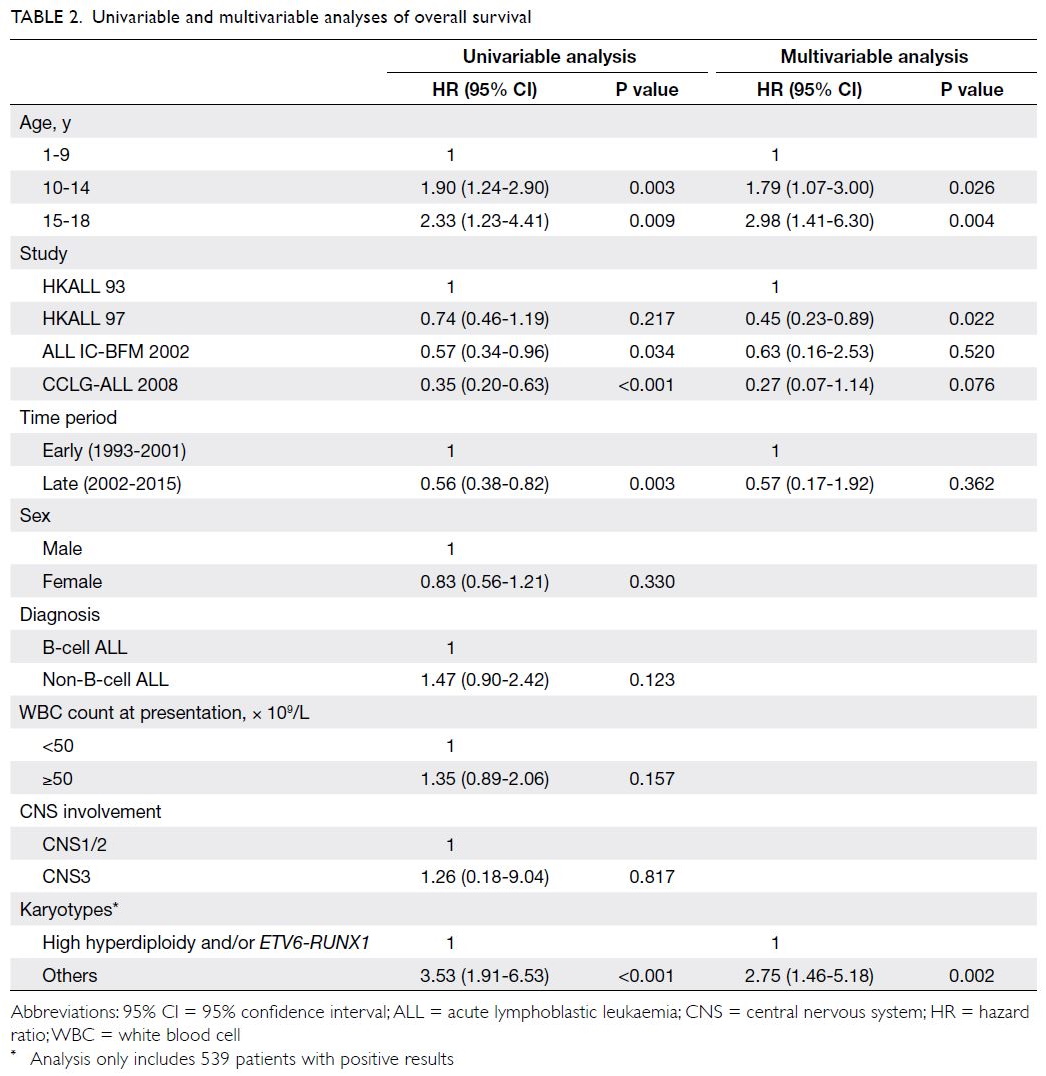
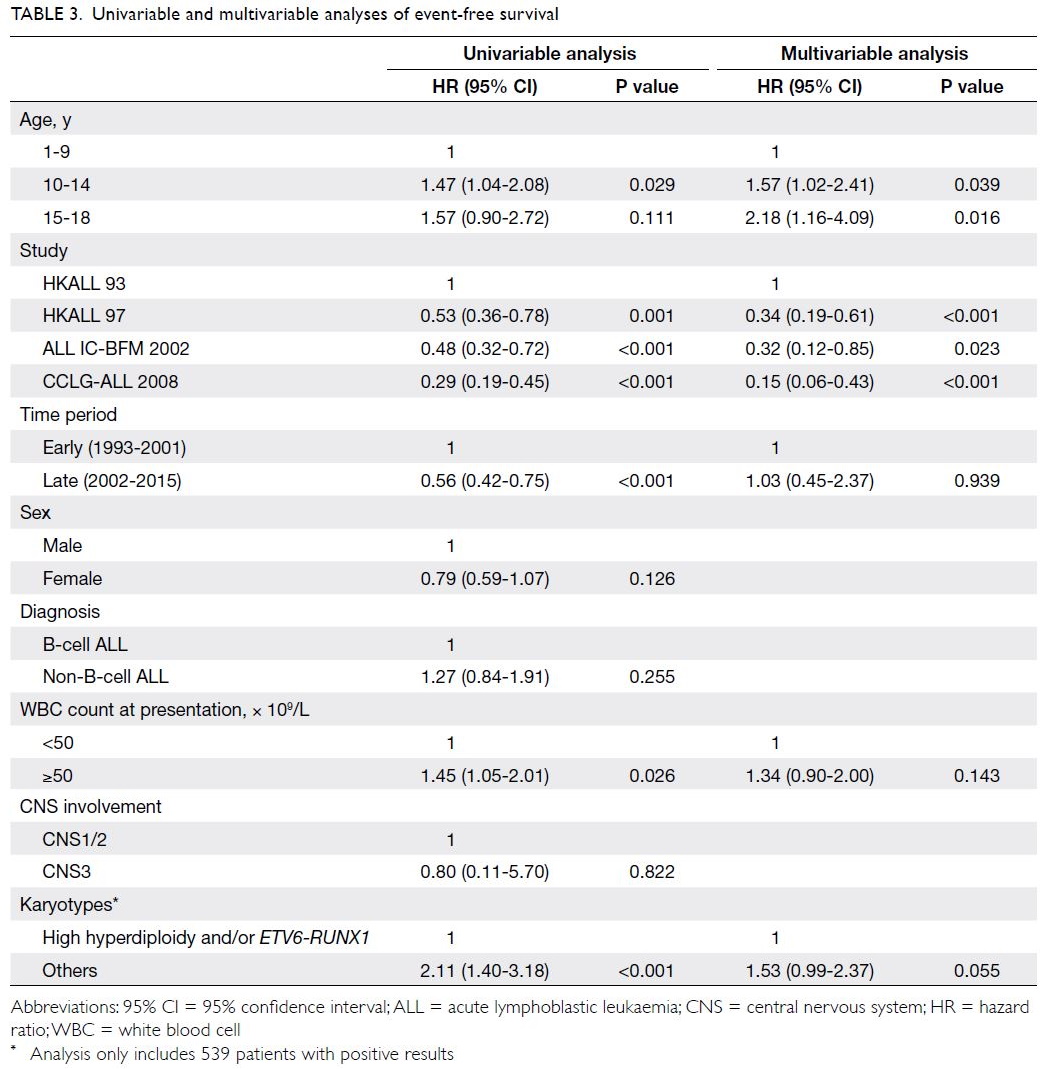
Predictors of OS and EFS are shown in
Tables 2 and 3, respectively. Univariable analysis
showed that both younger and older adolescent
age-groups (vs young children) were associated
with poor OS (P=0.003 and P=0.009). Additionally,
univariable analysis showed that more recent time
periods and treatment protocols (ALL IC-BFM
2002 and CCLG-ALL 2008), as well as favourable
cytogenetics (high hyperdiploidy and/or ETV6-RUNX1), were significantly associated with better
OS. After adjustments for parameters with P values <0.1 in univariable analysis, multivariable
Cox regression analysis revealed that both younger and older adolescent age-groups remained
independent predictors of OS (hazard ratio=1.79
[95% CI=1.07-3.00], P=0.026; hazard ratio=2.98
[95% CI=1.41-6.30], P=0.004). Favourable
cytogenetics also remained an independent predictor
of OS (P=0.002). Similarly, univariable analysis
showed that the younger adolescent age-group (vs
young children) was significantly associated with
poor EFS (P=0.029); the older adolescent age-group
(vs young children) tended to show an association
with poor EFS, although this was not statistically
significant (P=0.111). Upon inclusion of all
parameters with P values <0.1 in univariable analysis,
multivariable Cox regression analysis revealed that
both younger and older adolescent age-groups (vs
young children) were significantly associated with
poor EFS (hazard ratio=1.57 [95% CI=1.02-2.41],
P=0.039; hazard ratio=2.18 [95% CI=1.16-4.09],
P=0.016).
Predictors of the cumulative incidence of TRD
are shown in Table 4. Univariable analysis showed
that only younger and older adolescent age-groups
(vs young children) were significantly associated
with a greater incidence of TRD (hazard ratio=3.25
[95% CI=1.35-7.83], P=0.009; hazard ratio=4.50 [95%
CI=1.43-14.13], P=0.010). Furthermore, favourable cytogenetics (high hyperdiploidy and/or ETV6-RUNX1) tended to show an association with lower incidence of TRD, although this was not statistically
significant (P=0.088). After adjustments for
parameters with P values <0.1 in univariable analysis,
multivariable competing risks regression analysis
revealed that both younger and older adolescent
age-groups remained independent predictors of
a greater incidence of TRD [hazard ratio=3.16
(95% CI=1.11-9.01), P=0.031; hazard ratio=4.69
(95% CI=1.28-17.20), P=0.020].
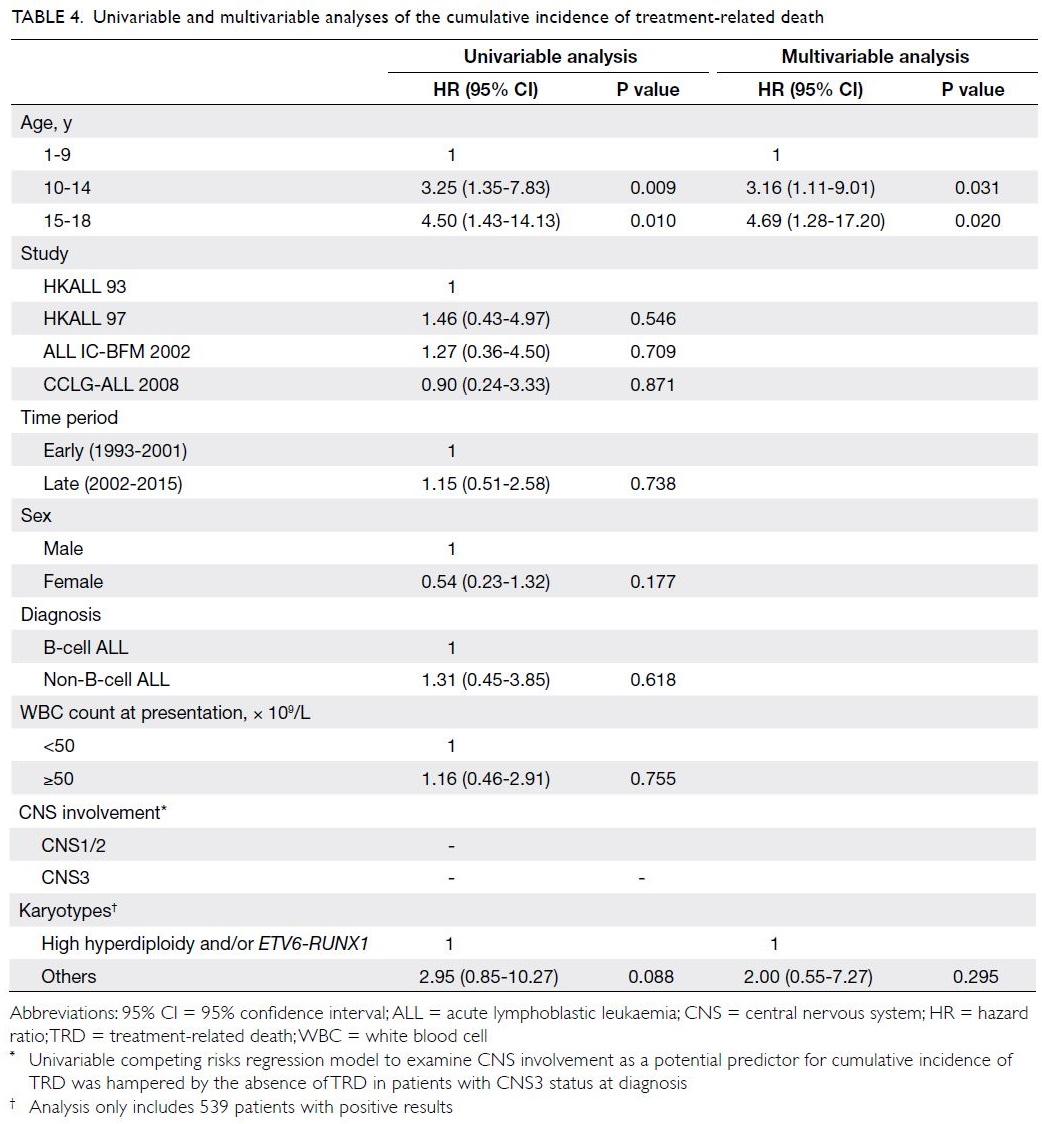
Table 4. Univariable and multivariable analyses of the cumulative incidence of treatment-related death
Discussion
In this retrospective study, we combined five clinical trials of paediatric ALL treatment in Hong Kong
to compare characteristics and outcomes among
young children, younger adolescents, and older
adolescents with ALL; we specifically focused on
the outcomes of older adolescents. Among the
overall cohort of patients with ALL in this study,
which covered a 20-year period and included 711
non-infant patients, 6.3% were older adolescents;
this proportion was comparable with the findings
in previous studies.1 4 8 21 22 Additionally, our results
are consistent with published literature in that adolescents with ALL were more likely to have a
T-cell immunophenotype and less likely to have
favourable genetic features (eg, high hyperdiploidy
or ETV6-RUNX1), compared with young children
who had ALL.1 4 5 6 7 8 9 13 These findings are consistent
with the results of previous studies conducted in
Western countries.1 4 5 6 7 8 9
Over the past two decades, several
comparative analyses have shown that adolescents
with ALL experience better outcomes when they
receive paediatric treatment protocols, rather than
adult treatment protocols.6 10 23 24 Adult protocols
for ALL (eg, hyperfractionated cyclophosphamide,
vincristine, doxorubicin, and dexamethasone) only
achieved 5-year OS rates of 40% to 60% in adolescents
and young adults with ALL.25 Although most adult treatment programmes for ALL have evolved from the
multi-agent approach used in paediatric protocols,
there are some notable differences in treatment
design. Paediatric ALL protocols generally use more
intensive dosing of several key therapeutic agents,
including corticosteroids, vincristine, asparaginase/PEG-asparaginase, and anti-metabolites (eg,
methotrexate and 6-mercaptopurine); they also use
more intensive and prolonged CNS prophylaxis with
intrathecal chemotherapy.25 26 27 In the present study,
the 10-year EFS (70.2% vs 68.6%) and OS (78.8%
vs 75.4%) rates for younger and older adolescents
confirm the favourable outcomes of paediatric ALL
protocols for adolescents aged ≤18 years.4 13 15 21 22 28 29 30
There are some important challenges involved
in the treatment of adolescents with intensive chemotherapy protocols; these include a greater
frequency of treatment-related complications (eg,
liver derangement and thrombosis) than in young
children who receive similar treatment. Drug
compliance is also challenging in adolescents; poor
adherence to long-term maintenance treatment may
lead to worse outcomes.31
Notably, the long-term OS and EFS rates
remained worse in adolescents with ALL than in
young children (aged 1-9 years) with ALL. Our
results indicate that this difference is not related
to an increased rate of relapse; it arises from an
increased risk of TRD. An age-related increase in
treatment-related toxicity has been reported in
almost all cohorts of patients with ALL who have received paediatric treatment protocols. Most
studies have shown that, compared with young
children, adolescents have greater risks of severe
adverse events.28 32 The use of paediatric intensive
combination chemotherapy is effective for preventing
relapse in adolescents with ALL, but these patients
may not tolerate the toxicity of intensive multi-agent
chemotherapy (eg, myeloablative allogeneic
HSCT). For example, among older adolescents in
the present study, the high incidence of TRD was
mainly attributed to two TRDs in 45 patients who
received remission induction chemotherapy, one
TRD in 33 patients who received post-induction
chemotherapy during CR1, and three TRDs in nine
patients who received allogeneic HSCT during CR1 or CR2. Further studies are needed to identify
optimal treatment adjustments that can improve
toxicity profiles among adolescents with ALL who
receive paediatric treatment protocols.
Consistent with previous findings,1 33 34 the
present study showed that poor early response to
treatment was more common in adolescents, a greater
proportion of whom had poor day 8 prednisone
response and did not achieve CR. Minimal residual
disease response after induction is an important
prognostic indicator of treatment failure. In our
more recent treatment protocols, MRD was included
in the disease monitoring. A greater proportion
of adolescents had MRD ≥1% after remission
induction, but the relapse rate was not greater in adolescents than in young children. Adolescents
received higher intensity consolidation, reinduction,
and continuation therapy; some received allogeneic
HSCT during CR1. The higher intensity of post-induction
treatment led to a lower relapse rate but
resulted in greater treatment-related mortality; thus,
the OS and EFS rates were worse in adolescents than
in young children. To improve survival outcomes
among adolescents with ALL, clinical trials have
been initiated with a focus on new agents that might
achieve better survival without excessive toxicity;
these agents include the proteasome inhibitor
bortezomib, as well as antibody- or cell-mediated
immunotherapy (eg, rituximab, inotuzumab,
blinatumomab, or tisagenlecleucel).35 36 37 38
This study had some limitations. First, it used
a retrospective design, which might have allowed
incomplete reporting bias and missing data. For
example, cytogenetic information at diagnosis was
missing for 172 (24.2%) of 711 patients because of
culture failure or poor bone marrow blast growth.
Individuals with missing data were excluded during
overall outcome analyses. However, our estimates
might have been biased because of this restricted
statistical analysis approach.39 Second, confounding
factors (eg, selection bias and enrolment bias) might
have been present. For example, the distributions of
high-risk ALL subgroups (eg, Ph-like ALL and early-T-precursor ALL) were not examined in our analysis
because of limited data. Therefore, caution is needed
when interpreting the results of this study.
In conclusion, our analysis of children with
ALL suggested that long-term EFS and OS rates
were favourable among adolescents who received
intensive paediatric treatment protocols. However,
ALL treatment outcomes were worse among
adolescents than among young children; further
optimisation is needed to reduce treatment-related
mortality. Novel targeted agents for patients with
poor early response to ALL treatment may overcome
treatment resistance, eradicate MRD, and improve
clinical outcomes.
Author contributions
Concept or design: CK Li.
Acquisition of data: FWT Cheng, AKS Chiang, GKS Lam, TTW Chow, SY Ha, CW Luk, CH Li, SC Ling, PW Yau, KKH Ho, AWK Leung.
Analysis or interpretation of data: J Feng, FWT Cheng, AWK Leung, NPH Chan, MHL Ng, CK Li.
Drafting of the manuscript: J Feng.
Critical revision of the manuscript for important intellectual content: FWT Cheng, AKS Chiang, GKS Lam, TTW Chow, SY Ha, CW Luk, CH Li, SC Ling, PW Yau, KKH Ho, AWK Leung, NPH Chan, MHL Ng, CK Li.
Acquisition of data: FWT Cheng, AKS Chiang, GKS Lam, TTW Chow, SY Ha, CW Luk, CH Li, SC Ling, PW Yau, KKH Ho, AWK Leung.
Analysis or interpretation of data: J Feng, FWT Cheng, AWK Leung, NPH Chan, MHL Ng, CK Li.
Drafting of the manuscript: J Feng.
Critical revision of the manuscript for important intellectual content: FWT Cheng, AKS Chiang, GKS Lam, TTW Chow, SY Ha, CW Luk, CH Li, SC Ling, PW Yau, KKH Ho, AWK Leung, NPH Chan, MHL Ng, CK Li.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
The authors thank Ms H Wong for contributing to the data collection.
Funding/support
The Children’s Cancer Foundation provided technical support for data management and funding for minimal residual
disease testing.
Ethics approval
This study was approved by The Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research
Ethics Committee (CRE2008.007T).
References
1. Pichler H, Reismüller B, Steiner M, et al. The inferior
prognosis of adolescents with acute lymphoblastic
leukaemia (ALL) is caused by a higher rate of treatmentrelated
mortality and not an increased relapse rate—a
population-based analysis of 25 years of the Austrian
ALL-BFM (Berlin-Frankfurt-Munster) Study Group. Br J
Haematol 2013;161:556-65. Crossref
2. Pole JD, Alibhai SM, Ethier MC, et al. Adolescents with
acute lymphoblastic leukemia treated at pediatric versus
adult hospitals. Ann Oncol 2013;24:801-6. Crossref
3. Boudestein K, Kamps WA, Veerman AJ, Pieters R. Different
outcome in older children with acute lymphoblastic
leukemia with different treatment protocols in the
Netherlands. Pediatr Blood Cancer 2012;58:17-22. Crossref
4. Pui CH, Pei D, Campana D, et al. Improved prognosis for
older adolescents with acute lymphoblastic leukemia. J
Clin Oncol 2011;29:386-91. Crossref
5. Boissel N, Baruchel A. Acute lymphoblastic leukemia in
adolescent and young adults: treat as adults or as children?
Blood 2018;132:351-61. Crossref
6. Boissel N, Auclerc MF, Lheritier V, et al. Should
adolescents with acute lymphoblastic leukemia be treated
as old children or young adults? Comparison of the French
FRALLE-93 and LALA-94 trials. J Clin Oncol 2003;21:774-80. Crossref
7. Plasschaert SL, Kamps WA, Vellenga E, de Vries EG,
de Bont ES. Prognosis in childhood and adult acute
lymphoblastic leukaemia: a question of maturation?
Cancer Treat Rev 2004;30:37-51. Crossref
8. Moricke A, Zimmermann M, Reiter A, et al. Prognostic
impact of age in children and adolescents with acute
lymphoblastic leukemia: data from the trials ALL-BFM 86,
90, and 95. Klin Padiatr 2005;217:310-20. Crossref
9. Usvasalo A, Raty R, Knuutila S, et al. Acute lymphoblastic
leukemia in adolescents and young adults in Finland.
Haematologica 2008;93:1161-8. Crossref
10. Stock W, La M, Sanford B, et al. What determines the
outcomes for adolescents and young adults with acute
lymphoblastic leukemia treated on cooperative group
protocols? A comparison of Children’s Cancer Group
and Cancer and Leukemia Group B studies. Blood
2008;112:1646-54. Crossref
11. Ribera JM, Oriol A, Sanz MA, et al. Comparison of the
results of the treatment of adolescents and young adults
with standard-risk acute lymphoblastic leukemia with
the Programa Espanol de Tratamiento en Hematologia
pediatric-based protocol ALL-96. J Clin Oncol
2008;26:1843-9. Crossref
12. Ramanujachar R, Richards S, Hann I, et al. Adolescents
with acute lymphoblastic leukaemia: outcome on UK
national paediatric (ALL97) and adult (UKALLXII/E2993)
trials. Pediatr Blood Cancer 2007;48:254-61. Crossref
13. Kato M, Manabe A, Koh K, et al. Treatment outcomes of
adolescent acute lymphoblastic leukemia treated on Tokyo
Children’s Cancer Study Group (TCCSG) clinical trials. Int
J Hematol 2014;100:180-7. Crossref
14. Chiaretti S, Vitale A, Cazzaniga G, et al. Clinico-biological
features of 5202 patients with acute lymphoblastic leukemia
enrolled in the Italian AIEOP and GIMEMA protocols and stratified in age cohorts. Haematologica 2013;98:1702-10. Crossref
15. Testi AM, Attarbaschi A, Valsecchi MG, et al. Outcome of
adolescent patients with acute lymphoblastic leukaemia
aged 10-14 years as compared with those aged 15-17 years:
long-term results of 1094 patients of the AIEOP-BFM ALL
2000 study. Eur J Cancer 2019;122:61-71. Crossref
16. Li CK, Chik KW, Chan GC, et al. Treatment of acute
lymphoblastic leukemia in Hong Kong children: HKALL
93 study. Hematol Oncol 2003;21:1-9. Crossref
17. Li CK, Chik KW, Ha SY, et al. Improved outcome of acute
lymphoblastic leukaemia treated by delayed intensification
in Hong Kong children: HKALL 97 study. Hong Kong Med
J 2006;12:33-9.
18. Stary J, Zimmermann M, Campbell M, et al. Intensive
chemotherapy for childhood acute lymphoblastic
leukemia: results of the randomized intercontinental trial
ALL IC-BFM 2002. J Clin Oncol 2014;32:174-84. Crossref
19. Cui L, Li ZG, Chai YH, et al. Outcome of children with
newly diagnosed acute lymphoblastic leukemia treated
with CCLG-ALL 2008: the first nation-wide prospective
multicenter study in China. Am J Hematol 2018;93:913-20. Crossref
20. Biondi A, Schrappe M, De Lorenzo P, et al. Imatinib after
induction for treatment of children and adolescents with
Philadelphia-chromosome-positive acute lymphoblastic
leukaemia (EsPhALL): a randomised, open-label,
intergroup study. Lancet Oncol 2012;13:936-45. Crossref
21. Barry E, DeAngelo DJ, Neuberg D, et al. Favorable outcome
for adolescents with acute lymphoblastic leukemia treated
on Dana-Farber Cancer Institute Acute Lymphoblastic
Leukemia Consortium Protocols. J Clin Oncol 2007;25:813-9. Crossref
22. Nachman JB, La MK, Hunger SP, et al. Young adults with
acute lymphoblastic leukemia have an excellent outcome
with chemotherapy alone and benefit from intensive
postinduction treatment: a report from the children’s
oncology group. J Clin Oncol 2009;27:5189-94. Crossref
23. Siegel SE, Stock W, Johnson RH, et al. Pediatric-inspired
treatment regimens for adolescents and young adults with
Philadelphia chromosome-negative acute lymphoblastic
leukemia: a review. JAMA Oncol 2018;4:725-34. Crossref
24. de Bont JM, Holt B, Dekker AW, van der Does-van den
Berg A, Sonneveld P, Pieters R. Significant difference
in outcome for adolescents with acute lymphoblastic
leukemia treated on pediatric vs adult protocols in the
Netherlands. Leukemia 2004;18:2032-5. Crossref
25. Siegel SE, Advani A, Seibel N, et al. Treatment of young
adults with Philadelphia-negative acute lymphoblastic
leukemia and lymphoblastic lymphoma: hyper-CVAD vs.
pediatric-inspired regimens. Am J Hematol 2018;93:1254-66. Crossref
26. Larsen EC, Devidas M, Chen S, et al. Dexamethasone and
high-dose methotrexate improve outcome for children
and young adults with high-risk B-acute lymphoblastic
leukemia: a report from Children’s Oncology Group Study
AALL0232. J Clin Oncol 2016;34:2380-8. Crossref
27. Carobolante F, Chiaretti S, Skert C, Bassan R. Practical
guidance for the management of acute lymphoblastic
leukemia in the adolescent and young adult population.
Ther Adv Hematol 2020;11:2040620720903531. Crossref
28. Hough R, Rowntree C, Goulden N, et al. Efficacy and
toxicity of a paediatric protocol in teenagers and young
adults with Philadelphia chromosome negative acute
lymphoblastic leukaemia: results from UKALL 2003. Br J
Haematol 2016;172:439-51. Crossref
29. Pieters R, de Groot-Kruseman H, Van der Velden V, et al.
Successful therapy reduction and intensification for
childhood acute lymphoblastic leukemia based on minimal
residual disease monitoring: Study ALL10 from the Dutch
Childhood Oncology Group. J Clin Oncol 2016;34:2591-601. Crossref
30. DeAngelo DJ, Stevenson KE, Dahlberg SE, et al. Long-term
outcome of a pediatric-inspired regimen used for adults
aged 18-50 years with newly diagnosed acute lymphoblastic
leukemia. Leukemia 2015;29:526-34. Crossref
31. Schmiegelow K, Heyman M, Gustafsson G, et al. The
degree of myelosuppression during maintenance therapy
of adolescents with B-lineage intermediate risk acute
lymphoblastic leukemia predicts risk of relapse. Leukemia
2010;24:715-20. Crossref
32. Gupta A, Matloub Y, Damania R, O’Riordan M, Ahuja SP.
Increased toxicity among adolescents and young adults
treated for acute lymphoblastic leukemia at US Children’s
Hospitals [abstract]. Blood 2017;130(Suppl 1):222.
33. Conter V, Bartram CR, Valsecchi MG, et al. Molecular
response to treatment redefines all prognostic factors
in children and adolescents with B-cell precursor acute
lymphoblastic leukemia: results in 3184 patients of the
AIEOP-BFM ALL 2000 study. Blood 2010;115:3206-14. Crossref
34. Moricke A, Zimmermann M, Valsecchi MG, et al. Dexamethasone vs prednisone in induction treatment of
pediatric ALL: results of the randomized trial AIEOP-BFM
ALL 2000. Blood 2016;127:2101-12. Crossref
35. Kantarjian H, Stein A, Gokbuget N, et al. Blinatumomab
versus chemotherapy for advanced acute lymphoblastic
leukemia. N Engl J Med 2017;376:836-47. Crossref
36. Lo Nigro L, Pulvirenti G, Cannata E, Bonaccorso P,
Andriano N, Russo G. “Feasible and effective administration
of Bortezomib with Rituximab in children with relapsed/resistant B-cell precursor acute lymphoblastic leukemia
(BCP-ALL): a step toward the first line”. Pediatr Hematol
Oncol 2019;36:438-44. Crossref
37. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel
in children and young adults with B-cell lymphoblastic
leukemia. N Engl J Med 2018;378:439-48. Crossref
38. Kantarjian HM, DeAngelo DJ, Stelljes M, et al.
Inotuzumab ozogamicin versus standard therapy for acute
lymphoblastic leukemia. N Engl J Med 2016;375:740-53. Crossref
39. Mallinckrodt CH, Sanger TM, Dube S, et al. Assessing and interpreting treatment effects in longitudinal clinical trials with missing data. Biol Psychiatry 2003;53:754-60. Crossref