Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE CME
2020 Hong Kong College of Obstetricians and
Gynaecologists guideline on investigations of premenopausal women with abnormal uterine bleeding
Jacqueline HS Lee, FHKCOG, FHKAM (Obstetrics and Gynaecology)1; Edith OL Cheng, FHKCOG, FHKAM (Obstetrics and Gynaecology)2; KM Choi, FHKCOG, FHKAM (Obstetrics and Gynaecology)3; SF Ngu, FHKCOG, FHKAM (Obstetrics and Gynaecology)4; Rachel YK Cheung, FHKCOG, FHKAM (Obstetrics and Gynaecology)1,5 for the Hong Kong College of Obstetricians and Gynaecologists
1 Department of Obstetrics and Gynaecology, The Chinese University of Hong Kong, Hong Kong
2 Department of Obstetrics and Gynaecology, United Christian Hospital, Hong Kong
3 Department of Obstetrics and Gynaecology, Pamela Youde Nethersole Eastern Hospital, Hong Kong
4 Department of Obstetrics and Gynaecology, The University of Hong Kong, Hong Kong
5 Chairman, HKCOG Guideline Sub-committee
Corresponding author: Dr Rachel YK Cheung (rachelcheung@cuhk.edu.hk)
Abstract
Abnormal uterine bleeding in premenopausal
women is a common gynaecological symptom and
composes of abnormality in the frequency, duration,
regularity, and flow volume of menstruation. It could
constitute the presentation of various gynaecological
malignancies. An appropriate history and physical
examination are mandatory to ascertain the diagnosis.
Depending on the clinical condition, a complete
blood picture, thyroid function test, clotting profile,
chlamydia test, cervical smear, and pregnancy test
can be performed. Ultrasound should be performed
in cases with a pelvic mass, unsatisfactory physical
examination, persistent symptoms, or no response
to medical treatment. In women aged ≥40 years,
an out-patient endometrial biopsy with Pipelle
should be performed. In women aged <40 years
with risk factors for endometrial cancer, persistent
symptoms, or no response to medical treatment, an
endometrial biopsy should be performed to rule out
endometrial cancer. Hysteroscopy or saline infusion sonohysterography is more sensitive than ultrasound
for diagnosing endometrial pathology. Details of the
above recommendations are presented.
Introduction
Abnormal uterine bleeding (AUB) is a common
problem in gynaecological practice and represents a
major proportion of out-patient attendance. A postal
survey in the United Kingdom found that AUB and
its subgroup, heavy menstrual bleeding, affected
15% to 25% of women aged 18 to 54 years.1 In Hong
Kong, the prevalence of AUB is not available, but
the following reference provides some information.
According to the 2014 Hong Kong College of
Obstetricians and Gynaecologists Territory Wide
Audit, the numbers of hospital admissions with
the diagnoses of ‘menorrhagia’ and ‘dysfunctional
uterine bleeding’ were 4080 and 3806, respectively.2
There were 6455 diagnostic hysteroscopies and
3075 hysteroscopic procedures conducted.2 Of all
operative hysteroscopic procedures performed,
the numbers of polypectomies and myomectomies
were 2468 (80%) and 380 (12%), respectively.2
Although many patients with AUB might not require
admission, the case volume mentioned in the above
report may reflect the scope of the problem locally.
As patterns of investigation became diversified, a guideline on AUB was considered
necessary. Premenopausal women are targeted by
this guideline. Postmenopausal bleeding is caused by
a different disease spectrum and is not included in
this guideline.
Definitions and initial
investigations of abnormal uterine
bleeding
The International Federation of Gynecology and
Obstetrics classifies AUB in the reproductive
years into chronic versus acute non-gestational
AUB.3 Chronic non-gestational AUB is defined as
bleeding from the uterine corpus that is abnormal
in frequency, duration, regularity, and/or volume
(Table 1) and has been present for the majority of
the preceding 6 months. Acute AUB is defined as
an episode of heavy bleeding that, in the clinician’s
opinion, is of sufficient quantity to require immediate
intervention to minimise or prevent further blood
loss. Menorrhagia is heavy cyclical menstrual blood
loss over several consecutive cycles without any intermenstrual or postcoital bleeding (ie, without
cycle disturbance). The National Institute for Health
and Care Excellence (NICE) defines heavy menstrual
bleeding as excessive menstrual loss that interferes
with a woman’s physical, social, emotional, and/or
material quality of life.4 Intermenstrual bleeding,
premenstrual and postmenstrual spotting, and
perimenopausal bleeding can be considered as
dysfunctional uterine bleeding after exclusion of
organic causes.
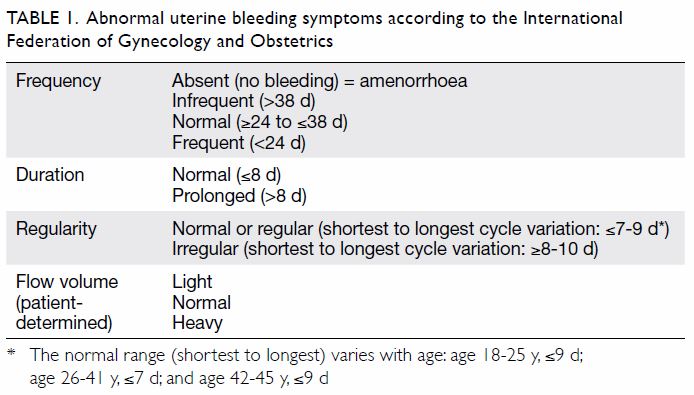
Table 1. Abnormal uterine bleeding symptoms according to the International Federation of Gynecology and Obstetrics
Obtaining an accurate menstrual history is
mandatory to guide the clinician’s diagnosis and
understand the impact on a woman’s quality of life
(online supplementary Appendix 1). On general
examination, any pallor or thyroid gland enlargement
should be noted. If there are features suggestive of
thyroid dysfunction or coagulopathy from history
or physical examination, a thyroid function test or
coagulopathy screening can be ordered accordingly.
History suggestive of coagulopathy includes heavy
menstrual bleeding since menarche, a family history
of coagulopathy, easy bruising, bleeding of the gums,
and epistaxis. However, routine thyroid function tests
or coagulopathy screening are not recommended
in all patients with menorrhagia. Speculum or
bimanual examination could elucidate the causes for
abnormal bleeding, such as cervical polyps, cervical
carcinoma, uterine fibroids, adenomyosis, or ovarian
tumours (online supplementary Appendix 1). The
following investigations can be arranged depending
on the clinical situation: (1) complete blood count to
look for anaemia; (2) pregnancy test; (3) ultrasound
scan, especially if physical examination suggests a
pelvic mass; (4) endometrial assessment; (5) cervical
smear if due; and (6) chlamydia screening in cases of
postcoital or intermenstrual bleeding.
Endometrial assessment
There are five main methods of endometrial
assessment: ultrasound scanning, magnetic
resonance imaging (MRI), endometrial biopsy or
aspirate, hysteroscopy, and dilatation and curettage
(D&C) under various modes of anaesthesia.
Ultrasound scanning
Ultrasound scanning, particularly the transvaginal
route, is used to assess endometrial thickness,
endometrial and myometrial consistency, and
abnormalities of the endometrial lining (eg,
submucosal fibroids or polyps). However, most
studies have investigated the endometrial thickness
of postmenopausal women. Smith-Bindman et al5
observed that the average endometrial thicknesses
were 4 mm, 10 mm, 14 mm, and 20 mm in normal
postmenopausal women, those with endometrial
polyps, those with endometrial hyperplasia, and
those with endometrial carcinoma, respectively. However, the prediction of endometrial pathology
based on ultrasound results in premenopausal
women was not reliable because of the great overlap
between the normal range and that of women with
endometrial pathology.
The NICE guideline4 recommends that in
patients with examination suggestive of fibroids,
a pelvic ultrasound should be performed.
Depending on the size of the uterus, transvaginal
or transabdominal ultrasonography could be
performed. Transvaginal ultrasonography produces
better image quality because of its higher frequency,
which allows greater image resolution at the expense
of decreased depth of penetration. In patients
in whom physical examination is impossible or
unsatisfactory, or symptoms persist despite medical
treatment, an ultrasound should also be arranged.
Pelvic ultrasound can be useful for detecting gross
endometrial or myometrial pathology such as fibroids
and adenomyosis. However, pelvic ultrasonography
does not replace an endometrial biopsy.
In cases where vaginal access is difficult or impossible, such as in adolescents and virgin girls,
transrectal ultrasonography should be offered. This
technique has been shown to provide better image
quality compared with the transabdominal route
without causing significant discomfort to patients.6
Saline infusion sonohysterography (SIS)
involves the instillation of 5 to 15 mL of normal
saline into the uterine cavity and may allow better
detection of endometrial polyps and submucosal
fibroids. A 2017 meta-analysis concluded that two-dimensional
SIS is highly sensitive for detection of
endometrial polyps and submucosal uterine fibroids,
with pooled sensitivity values of 93% and 94% and
specificity of 81% and 81%, respectively.7 Clinicians
may consider SIS in cases where further evaluation
of endometrial lesions is required.
Magnetic resonance imaging
Magnetic resonance imaging has been shown to
be more sensitive than transvaginal ultrasound
(TVS) for the identification of fibroids, especially
the growth of submucosal fibroids into the uterine
cavity.8 Magnetic resonance imaging is slightly more
sensitive than TVS for diagnosing adenomyosis
(sensitivity: 77% vs 72%).9 10 However, the chance of
identifying important additional findings by MRI
over ultrasound has to be weighed against the waiting
time and cost of MRI. Magnetic resonance imaging
should not be the routine for all cases of AUB. In
cases where vaginal access is difficult or impossible,
or when it is difficult to differentiate between fibroids
and adenomyosis, there is a role for MRI.
Endometrial biopsy
The main purpose of obtaining an endometrial
biopsy or endometrial aspirate is to exclude
endometrial pathology like hyperplasia, disordered
endometrium, or malignancies. Most endometrial
biopsies can be performed in out-patient or office
clinics and have the advantages of being simple,
quick, safe, and convenient and avoiding the
need for anaesthesia. Furthermore, the device is
disposable, and the procedure is much less costly
than conventional D&C.
The Pipelle is the most common out-patient
endometrial assessment device used in the United
Kingdom and Hong Kong (Fig). Other devices
includes Novak (a silastic cannula with a beveled
lateral opening), Tis-u-Trap (a plastic curette with
suction), the Vabra aspirator (a cannula connected
to a vacuum pump), Endorette (a plastic cannula
with multiple openings), Tao Brush (a sheath brush
device), Cytospat (a polypropylene cannula with a
rhomboid head), Accurette (a quadrilateral-shaped
curette with four cutting edges), Explora (a plastic
curette with a Randall-type cutting edge), and
Z-sampler (a flexible polypropylene device). A meta-analysis
including 60 articles found that Pipelle
performs as well as D&C and as well as or better
than other endometrial sampling devices in terms of
sampling adequacy and sensitivity. Pipelle seems to
be better than the other options in terms of pain/discomfort and costs.11 The sample adequacy rate
was consistently high for Pipelle, mostly >85%,
compared with 98% for D&C.11 Pipelle’s specimen
adequacy rate and concordance rate to histology
on hysterectomy were similar to those of D&C.
Pipelle biopsy is reliable for excluding endometrial
carcinoma: previous studies showed that Pipelle
detected 98% of endometrial carcinomas.12
The Vabra device can sample a larger
proportion of the endometrium (42%) compared
with Pipelle (4%).13 However, other studies did not
find a better specimen adequacy rate of the Vabra
device over Pipelle14 15 (Table 2 11 12 16 17 18).
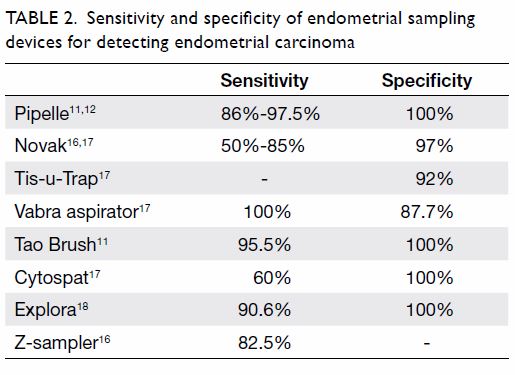
Table 2. Sensitivity and specificity of endometrial sampling devices for detecting endometrial carcinoma
Endometrial cancer is thought to be uncommon
in women aged <40 years, and this matches the
reported local experience.19 In 2017, the Hong Kong
Cancer Registry report showed that out of a total of
1076 new cases of cancer of the endometrial corpus,
only 47 cases (4.4%) occurred in women aged
<40 years.19 Although endometrial cancer is
uncommon in women aged <40 years, its incidence
is increasing. In 2017, the age-specific incidences for
endometrial carcinoma in various age-groups were
4, 9, 18, 66, and 64 per 100 000 women at ages 30, 35,
40, 50, and 55 years, respectively, with the incidence
peaking at ages 50 to 54 years.19 In 2007, the figures
were 2 and 6 per 100 000 women at ages 30 and 35,
respectively.
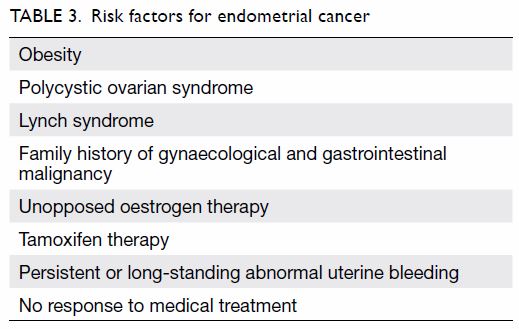
At what age should the gynaecologist perform endometrial biopsy? It had been suggested that
routine endometrial biopsy is not necessary for
AUB in women aged <40 years. However, in view
of the increasing incidence of endometrial cancers
among younger women, an endometrial assessment
is warranted for women aged <40 years who present
with AUB and also have other high-risk features
(Table 3). Instead of arbitrarily choosing an age at
which endometrial biopsy should or should not be
done, the woman’s risk of endometrial carcinoma
should be assessed. When they present with AUB,
women at high risk of endometrial cancer need
endometrial biopsy regardless of age. Therefore, Hong
Kong College of Obstetricians and Gynaecologists
recommends endometrial biopsy in all women
with AUB aged ≥40 years and in women with risk
factors for endometrial carcinoma irrespective of
age. Patients with persistent symptoms or in whom
medical treatments have failed should also undergo
endometrial biopsy.
Hysteroscopy
Hysteroscopy allows direct visualisation of the
whole endometrial cavity, lower segment, and
cervical canal. Hysteroscopy can detect small
polyps or submucosal fibroids and provide an
opportunity for endometrial biopsy without the
need for general anaesthesia. The NICE guideline
recommends out-patient hysteroscopy for
women with uterine cavity abnormalities or when
endometrial pathology is suspected because it is
more accurate than pelvic ultrasound.4 A Hong
Kong study showed that out-patient hysteroscopy
was successful in 92% of patients.20 Prospective
studies have shown that diagnostic hysteroscopy
had significantly better diagnostic performance
than SIS and TVS.16 The sensitivity and specificity
for any uterine abnormality of SIS and TVS were
92% and 89% versus 60% and 56%, respectively.
The sensitivity and specificity for diagnostic
hysteroscopy were 97% and 92%, respectively.21
The patients’ acceptability was high, and the failure rate was low, with failure mainly occurring due to
pain during the procedure, distorted uterine cavity,
and tight cervical os, especially in postmenopausal
and nulliparous patients. The last problem can be
partially overcome by using a hysteroscope of smaller
diameter (minihysteroscopy). A ‘no touch’ approach
with vaginoscopy has been shown to be quicker,
less painful, and more successful than standard
hysteroscopy and can be considered for out-patient
hysteroscopy.22
A randomised controlled trial23 comparing
TVS, out-patient hysteroscopy, and endometrial
biopsy with in-patient hysteroscopy and D&C showed
that a combination of transvaginal scan, Pipelle
endometrial biopsy, and out-patient hysteroscopy
had similar efficacy to in-patient hysteroscopy and
D&C for the investigation of AUB. Transvaginal scan
and endometrial biopsy can therefore be considered
as the first-line investigation, followed by out-patient
hysteroscopy.24
Some authors have suggested that a normal
cavity on hysteroscopy obviates the need for an
endometrial biopsy. However, normal hysteroscopy
findings are not conclusive of the absence of
premalignant or malignant lesions and do not
eliminate the need for endometrial sampling, as they
do not substitute for benign histological examination
findings.25
Dilatation and curettage
Dilatation and curettage, and the endometrial
histology obtained by that method, were previously
considered as the ‘gold standard’ in AUB
management. However, multiple studies showed that
D&C is not superior to endometrial assessment with
Pipelle or other out-patient endometrial assessment
devices, and D&C requires general anaesthesia.11
Dilation and curettage only should no longer be
the gold standard in endometrial pathological
assessment, but D&C with concurrent hysteroscopy
may be useful when intrauterine lesions are
suspected, as it allows direct visual assessment of
the endometrial cavity. For patients in whom out-patient
hysteroscopy or endometrial biopsy is not
possible, in-patient hysteroscopy and D&C under
general anaesthesia should be offered, but D&C
does not have therapeutic value in AUB except for
temporarily stopping heavy menstrual bleeding.
Summary of recommendations
1. The chance of endometrial carcinoma in women
aged <40 years is low. However, endometrial
assessment is warranted if there are risk factors
for endometrial carcinoma, if symptoms are
persistent/long-standing, or symptoms fail to
respond to medical treatment (Grade B).
2. Pelvic ultrasound (preferably TVS) and endometrial sampling with Pipelle are the preferred first-line methods of assessing AUB. Hysteroscopy is indicated if uterine cavity abnormalities are suspected (Grade B).
3. Out-patient hysteroscopy is safe and reliable and should be the preferred setting for diagnostic hysteroscopy (Grade A).
4. Routine first-line D&C should be discouraged. Dilation and curettage should be reserved for women requiring general anaesthesia for other indications (Grade A).
2. Pelvic ultrasound (preferably TVS) and endometrial sampling with Pipelle are the preferred first-line methods of assessing AUB. Hysteroscopy is indicated if uterine cavity abnormalities are suspected (Grade B).
3. Out-patient hysteroscopy is safe and reliable and should be the preferred setting for diagnostic hysteroscopy (Grade A).
4. Routine first-line D&C should be discouraged. Dilation and curettage should be reserved for women requiring general anaesthesia for other indications (Grade A).
A summary of the recommendations are
shown in the online supplementary Appendix 2.
Author contributions
Concept or design: All authors.
Acquisition of data: JHS Lee, EOL Cheng, KM Choi, SF Ngu.
Analysis or interpretation of data: JHS Lee, EOL Cheng, KM Choi, SF Ngu.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: JHS Lee, EOL Cheng, KM Choi, SF Ngu.
Analysis or interpretation of data: JHS Lee, EOL Cheng, KM Choi, SF Ngu.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the
study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
This guideline was produced by the Hong Kong College of
Obstetricians and Gynaecologists as an educational aid and
reference for obstetricians and gynaecologists practising in
Hong Kong. The guideline does not define a standard of care,
nor is it intended to dictate an exclusive course of management.
It presents recognised clinical methods and techniques for
consideration by practitioners for incorporation into their
practice. It is acknowledged that clinical management may
vary and must always be responsive to the needs of individual
patients, resources, and limitations unique to the institution
or type of practice. Particular attention is drawn to areas
of clinical uncertainty in which further research may be
indicated.
Declaration
The content of this guideline has been published in the
Hong Kong College of Obstetricians and Gynaecologists
Guidelines Number 5, revised July 2020 (http://www.hkcog.org.hk/hkcog/Download/Guideline_on_investigations_of_premenopausal_women_with_abnormal_uterine_bleeding.pdf). This is a revised version of the 2001 Hong Kong College of
Obstetricians and Gynaecologists guideline on investigations
of premenopausal women with abnormal uterine bleeding
(http://www.hkcog.org.hk/hkcog/Download/Abnormal%20uterine%20bleeding_2001.pdf).
Funding/support
This medical practice paper received no specific grant from
any funding agency in the public, commercial, or not-for-profit
sectors.
References
1. Shapley M, Jordan K, Croft PR. An epidemiological survey
of symptoms of menstrual loss in the community. Br J Gen
Pract 2004;54:359-63.
2. Hong Kong College of Obstetricians & Gynaecologists.
Territory-wide audit in obstetrics & gynaecology.
2014. Available from: http://www.hkcog.org.hk/hkcog/Download/Territory-wide_Audit_in_Obstetrics_Gynaecology_2014.pdf. Accessed 1 May 2020.
3. Munro MG, Critchley HO, Fraser IS, FIGO Menstrual
Disorders Committee. The two FIGO systems for normal
and abnormal uterine bleeding symptoms and classification
of causes of abnormal uterine bleeding in the reproductive
years: 2018 revisions. Int J Gynaecol Obstet 2018;143:393-408. Crossref
4. National Institute for Health and Care Excellence. Heavy
menstrual bleeding: assessment and management NICE
guideline [NG88]. 2018. Available from: https://www.nice.org.uk/guidance/ng88. Accessed 25 Feb 2020.
5. Smith-Bindman R, Kerlikowska K, Feldstein V, et al.
Endovaginal ultrasound to exclude endometrial cancer and
other endometrial abnormalities. JAMA 1998;280:1510-7. Crossref
6. Timor-Tritsch IE, Monteagudo A, Rebarber A, Goldstein SR,
Tsymbal T. Transrectal scanning: an alternative when
transvaginal scanning is not feasible. Ultrasound Obstet
Gynecol 2003;21:473-9. Crossref
7. Bittencourt CA, Dos Santos Simões R, Bernardo WM,
et al. Accuracy of saline contrast sonohysterography
in detection of endometrial polyps and submucosal
leiomyomas in women of reproductive age with abnormal
uterine bleeding: systematic review and meta-analysis.
Ultrasound Obstet Gynecol 2017;50:32-9. Crossref
8. Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F.
Evaluation of the uterine cavity with magnetic resonance
imaging, transvaginal sonography, hysterosonographic
examination, and diagnostic hysteroscopy. Fertil Steril
2001;76:350-7. Crossref
9. Bazot M, Daraï E. Role of transvaginal sonography and
magnetic resonance imaging in the diagnosis of uterine
adenomyosis. Fertil Steril 2018;109:389-97. Crossref
10. Champaneria R, Abedin P, Daniels J, Balogun M, Khan KS.
Ultrasound scan and magnetic resonance imaging for the
diagnosis of adenomyosis: systematic review comparing
test accuracy. Acta Obstet Gynecol Scand 2010;89:1374-84. Crossref
11. Narice BF, Delaney B, Dickson JM. Endometrial sampling
in low-risk patients with abnormal uterine bleeding: a
systematic review and meta-synthesis. BMC Fam Pract
2018 30;19:135. Crossref
12. Stovall G, Photopulos GJ, Poston WM, Ling FW, Sandles LG.
Pipelle endometrial sampling in patients with known
endometrial carcinoma. Obstet Gynecol 1991;77:954-6.
13. Rodriguez GC, Yaqub N, King ME. A comparison of
the Pipelle device and Vabra aspirator as measured by
endometrial denudation in hysterectomy specimens:
the Pipelle samples significantly less of the endometrial
surface than the Vabra aspirator. Am J Obstet Gynecol
1993;168:55-9. Crossref
14. Eddowes HA, Read MD, Codling BW. Pipelle: a more
acceptable technique for outpatient endometrial biopsy. Br
J Obstet Gynaecol 1990;97:961-2. Crossref
15. Naim NM, Mahdy ZA, Ahmad S, Razi ZR. The Vabra
aspirator versus the Pipelle device for outpatient endometrial sampling. Aust N Z J Obstet Gynaecol
2007;47:132-6. Crossref
16. Larson DM, Krawisz BR, Johnson KK, Broste SK.
Comparison of the Z-sampler and Novak endometrial
biopsy instruments for in-office diagnosis of endometrial
cancer. Gynecol Oncol 1994;54:64-7. Crossref
17. Antoni J, Folch E, Costa J, et al. Comparison of Cytospat
and Pipelle endometrial biopsy instruments. Eur J Obstet
Gynecol Reprod Biol 1997;72:57-61. Crossref
18. Kufahl J, Pedersen I, Sindberg Eriksen P, et al. Transvaginal
ultrasound, endometrial cytology sampled by Gynoscann
and histology obtained by Uterine Explora Curette
compared to the histology of the uterine specimen. A
prospective study in pre- and postmenopausal women
undergoing elective hysterectomy. Acta Obstet Gynecol
Scand 1997;76:790-6. Crossref
19. Hong Kong Cancer Registry, Hospital Authority, Hong
Kong SAR Government. Cancer incidence and mortality
report in Hong Kong. 2016-2017 Available from: www3.ha.org.hk/cancereg, Assessed 25 Feb 2020.
20. Lo KW, Yuen PM. The role of outpatient diagnostic
hysteroscopy in identifying anatomic pathology and
histopathology in the endometrial cavity. J Am Assoc Gynecol Laparosc 2000;7:381-5. Crossref
21. Grimbizis GF, Tsolakidis D, Mikos T, et al. A prospective
comparison of transvaginal ultrasound, saline infusion
sonohysterography, and diagnostic hysteroscopy in
the evaluation of endometrial pathology. Fertil Steril
2010;94:2720-5. Crossref
22. Smith PP, Kolhe S, O’Connor S, Clark TJ. Vaginoscopy
against standard treatment: a randomised controlled trial.
BJOG 2019;126:891-9. Crossref
23. Tahir MM, Bigrigg MA, Browning JJ, Brookes ST, Smith PA.
A randomised controlled trial comparing transvaginal
ultrasound, outpatient hysteroscopy and endometrial
biopsy with inpatient hysteroscopy and curettage. Br J
Obstet Gynaecol 1999;106:1259-64. Crossref
24. Bain C, Parkin DE, Cooper KG. Is outpatient diagnostic
hysteroscopy more useful than endometrial biopsy
alone for the investigation of abnormal uterine bleeding
in unselected premenopausal women? A randomised
comparison. BJOG 2002;109:805-11. Crossref
25. Bakour SH, Dwarakanath LS, Khan KS, Newton JR. The
diagnostic accuracy of outpatient miniature hysteroscopy
in predicting premalignant and malignant endometrial
lesions. Gynaecol Endosc 1999;8:143-8. Crossref