Hong
Kong Med J 2019 Apr;25(2):120–6 | Epub 28 Mar 2019
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Efficacy and safety of perioperative tranexamic acid in
elderly patients undergoing trochanteric fracture surgery: a randomised
controlled trial
F Chen, MD; Z Jiang, MD; M Li, MD; X Zhu, MD
Department of Anesthesiology, The First Affiliated
Hospital of Nanchang University, Jiangxi, China
Corresponding author: Dr X Zhu (13907915506@163.com)
Abstract
Introduction: Trochanteric
fractures result in a high frequency of considerable blood loss, a high
incidence of blood transfusions, and a high risk of perioperative
morbidity and mortality in elderly patients. This study aimed to
evaluate the efficacy and safety of a three-dose regimen of tranexamic
acid on blood loss and transfusion rate in elderly patients with
trochanteric fractures.
Methods: Eligible patients with
trochanteric fractures surgically treated by dynamic hip screw and
proximal anti-rotating intramedullary nail between March 2016 and
October 2017 were enrolled in the study. Patients were randomly assigned
to receive 15 mg/kg intravenous tranexamic acid dissolved in 100 mL of
saline (TXA group) or 100 mL of saline solution (placebo group) over 10
minutes before, during, and after surgery. Perioperative blood loss,
obvious blood loss, and hidden blood loss in the two groups were
calculated separately. Vascular events and patient mortality over 6
months’ follow-up were noted.
Results: In total, 176 patients
were included. Compared with the placebo group (n=88), patients in the
TXA group (n=88) had less blood loss: perioperative blood loss was 205.5
mL (P<0.001), obvious blood loss was 125 mL (P<0.001), and hidden
blood loss was 76.5 mL (P<0.001); reduced incidence of blood
transfusion (17% vs 35%, P=0.007); and shorter hospital stays (median
[interquartile range], 7 [6-8] vs 8.5 [7.5-9] days, P<0.001).
Conclusion: Tranexamic acid
significantly lowered perioperative blood loss and blood transfusion
rate without an increased risk of venous thromboembolism or mortality in
elderly patients with trochanteric fractures treated with dynamic hip
screw or proximal anti-rotating intramedullary nail.
New knowledge added by this study
- A three-dose regimen of tranexamic acid in elderly patients with trochanteric fractures treated with dynamic hip screw or proximal anti-rotating intramedullary nail significantly reduced perioperative blood loss and the transfusion rate.
- The three-dose regimen of tranexamic acid did not increase the risk of venous thromboembolism or mortality in patients.
- This study will help surgeons to reduce blood loss and transfusion during surgery in patients with trochanteric fractures.
Introduction
The increasing number of elderly people with
osteoporosis is bringing about an increased incidence of trochanteric
fractures, which include intertrochanteric fractures and subtrochanteric
fractures. This common type of fractures frequently results in
considerable blood loss,1 which
exposes patients to postoperative anaemia and reduced functional recovery.2 Further, large amounts of blood
loss usually result in blood transfusion and a high risk of perioperative
morbidity and mortality.3 Blood
transfusion increases the incidence of adverse reactions related to
allogeneic blood transfusion, such as infectious diseases, haemolytic
reaction, cardiovascular dysfunction, postoperative infection,4 5 6 7 and elevated
hospitalisation costs.8 9 Postoperative mortality rates have been reported as
high as 10% at 30 days and 30% at 1 year.10
Therefore, reducing perioperative blood loss concomitant to trochanteric
fractures in elderly patients would help to decrease the rate of
complications and improve surgical outcomes.
Tranexamic acid (TXA), a synthetic derivative of
the amino acid lysine, is an antifibrinolytic drug that competitively
blocks the plasminogen-binding site, inhibits plasminogen activation, and
interferes with fibrinolysis.11
Currently, TXA is widely used in clinical surgery. Numerous studies have
indicated that intravenous TXA reduces blood loss and transfusion rates
without increasing thrombotic events in joint arthroplasty.12 13 14 Similar results have been found in surgical patients
undergoing treatment for trauma, including decreased mortality due to
haemorrhage.15 However, studies
have also shown that TXA, as compared with placebo, not only offers no
significant benefit regarding transfusion rate, estimated blood loss, and
incidence of deep venous thrombosis in patients undergoing open reduction
and internal fixation with acetabular fracture,16
but also increases the formation of deep vein thrombosis after hip
fracture in elderly patients.17
Thus, the efficacy and safety of TXA in the perioperative period of hip
fracture in elderly patients remain controversial.
In the present study, we evaluated the effects of
TXA administration in elderly patients undergoing surgery for trochanteric
fracture. Specifically, we aimed to evaluate whether a three-dose regimen
of TXA decreases perioperative blood loss and the incidence of allogenic
blood transfusion without increasing the risk of venous thromboembolism
and mortality.
Methods
Patients and methods
To clarify the effects of TXA in surgical treatment
of trochanteric fractures in elderly patients, a placebo-controlled
double-blind randomised clinical trial was performed in our hospital in
accordance with the provisions of the Declaration of Helsinki, as revised
in 2013.18
Elderly patients with trochanteric fractures who
were treated with dynamic hip screw (DHS) and proximal anti-rotating
intramedullary nail (PFNA) in our hospital between March 2016 and October
2017 were included in this study. Patients eligible for inclusion had
American Society of Anesthesiologists (ASA) scores of II (mild systemic
disease that results in no functional limitation) or III (serious systemic
disease that results in functional impairment), were aged ≥65 years, and
were treated with either DHS or PFNA within 48 hours after injury.
Exclusion criteria were as follows: (1) allergy to TXA or
low-molecular-weight heparin; (2) severe dysfunction of heart, lung,
liver, kidney, or coagulation; (3) provoked deep venous thrombosis or
pulmonary embolism within 30 days or myocardial infarction,
cerebrovascular accident, or stent placement within 6 months; (4)
anticoagulant therapy such as antiplatelet drugs or warfarin before
surgery; (5) multiple fractures; and (6) blood transfusion before surgery.
All patients underwent preoperative medical
optimisation by a hospitalist medicine team. All patients received spinal
or general anaesthesia without regional blockade or local injection.
Patients were assigned at random to receive TXA treatment (TXA group) or
placebo control (placebo group). Patients in the TXA group received three
doses of 15 mg/kg intravenous TXA dissolved in 100 mL of saline. Each of
the doses was administered over 10 minutes: the first dose was used within
10 minutes just before incision, the second continuously pumped throughout
the entire surgery, and the third was used at 3 hours after surgery
(three-dose regimen).19 In the
placebo group, 100 mL of saline solution was administered following the
same three-dose regimen. During the surgery, crystalloid maintenance
fluids were administered at a rate of 1.5 mL/kg per hour. Blood losses
were replaced with Ringer’s lactate in a 3:1 ratio, colloidal solution (or
5% albumin) in a 1:1 ratio, or a combination (crystalloid/colloidal ratio,
2:1) until the haemoglobin (Hb) concentration fell below the transfusion
trigger point. Changes of 20% in baseline heart rate or blood pressure due
to hypovolaemia were managed with boluses of 10 mL/kg crystalloid solution
or 500 mL of colloidal solution (5% albumin).
Perioperative transfusion was based on the Chinese
“Measures for the management of clinical blood use in medical
institutions” guideline.20
Intra-operative allogenic blood transfusion was administered for all
patients who had Hb levels <7.0 g/dL and those with Hb levels <10
g/dL also suspected to have myocardial ischaemia or haemorrhagic shock.
Postoperative blood transfusion was based on arterial blood gas analysis
and routine blood examination. When a patient receives a blood
transfusion, an arterial blood gas analysis should be performed after each
infusion of 1 unit of red blood cells to reassess whether to continue the
transfusion.
All patients received deep venous thrombosis
prophylaxis including sequential compression devices throughout
hospitalisation and prophylactic low-molecular-weight heparin for 30 days
after surgery beginning on postoperative day (POD) 1 unless therapeutic
anticoagulation was contra-indicated because of pre-existing co-morbidities
or postoperative complications.
Sample size
The perioperative transfusion rate of trochanteric
fractures was 34% at our institution. Assuming that a similar rate of
transfusion would be observed in the control subjects, our sample size was
calculated to be able to detect a difference of 34% with respect to 10% (a
risk reduction of approximately 1/3 of the baseline rate) between the TXA
and placebo groups. We then calculated that a sample of 88 participants
per study group would provide 90% power to detect such a difference (α =
0.05, two-sided test).
Randomisation and blinding
Participants were randomised into either the TXA
group or the placebo group using a computerised dynamic allocation
programme and stratification according to sex (male or female), age
(<75 or ≥75 years), and type of surgery (DHS or PFNA) by means of a
central telephone system with a computer-generated randomisation list to
ensure that subject allocation remained balanced throughout the entire
subject accrual phase. All operations were performed by the same
orthopaedic surgeons, who determined the type of surgery. All
investigational drugs were administered by a nurse during preoperative
preparation and then delivered to the operating room in packaging simply
labelled as “study drug”. To ensure that subjects, physicians, and data
collectors were blinded, the patient caregivers, investigators collecting
the data, safety monitoring board, and members of the adjudication
committee remained unaware of the study group assignments.
Outcomes
Patient characteristics including sex, age, body
mass index, ASA score, preoperative Hb concentration, proportions of
preoperative hypertension and diabetes, surgery type, time from injury to
surgery, surgical duration, and length of hospital stay were recorded.
All patient outcomes were assessed by the
independent adjudication committee. The primary outcomes included
perioperative blood loss and proportion of patients receiving blood
transfusion from the beginning of surgery to discharge. Secondary outcomes
including obvious blood loss; hidden blood loss; postoperative Hb
concentration of POD 1, POD 2, and POD 3; number of units of transfusion
during hospitalisation; and incidence of adverse events at 6-month
follow-up (including thromboembolic events, wound complications, and
mortality) were identified. Investigation for thromboembolic events,
defined as symptomatic deep venous thrombosis, pulmonary embolism,
myocardial infarction, and cerebrovascular accident diagnosed by duplex
ultrasound, was only performed in patients with acute symptoms. Diagnosis
of pulmonary embolism was performed using contrast chest computed
tomography. Myocardial infarction was diagnosed using electrocardiography
and cardiac enzymes. Confirmation of cerebrovascular accident was done by
brain computed tomography or magnetic resonance imaging. Wound
complications were defined to include haematoma and deep or superficial
infection. The patients’ medical history was asked before surgery. If the
patient had any symptoms associated with thromboembolic events, tests were
given to him/her.
Calculation methods
Patient blood volume was calculated using the
formula of Nadler et al,21 as
follows: blood volume = (k1 × height3 [m]) + (k2 × weight [kg])
+ k3, where k1 = 0.3669, k2 = 0.03219, and k3 = 0.6041 for men and k1 =
0.3561, k2 = 0.03308, and k3 = 0.1833 for women.
The Gross equation was used to calculate the total
red blood cell volume loss,22 as
follows: total red blood cell volume loss = patient blood volume ×
(preoperative haematocrit – postoperative haematocrit), where preoperative
haematocrit is the haematocrit on the morning of the day of surgery, and
postoperative haematocrit is the haematocrit on POD 2. Haematocrit was
chosen for investigation because it is directly correlated with blood
volume.
Theoretical blood loss refers to the total red
blood cell volume loss / preoperative haematocrit. Perioperative blood
loss refers to hidden blood loss + obvious blood loss (surgical blood loss
+ postoperative drainage), or theoretical blood loss + blood transfusion
volume. Hidden blood loss refers to the amount of theoretical blood loss
and blood transfusion volume, minus obvious blood loss.
Statistical methods
Data were analysed using SPSS (Windows version
24.0; IBM Corp, Armonk [NY], United States). Descriptive data assumed to
follow normal distributions were expressed as mean ± standard deviation,
and comparisons of descriptive data for completely random distributions
were conducted with two independent-samples t tests. The
measurement data of skewed distributions were represented by median
(interquartile range; IQR) and compared with non-parametric Wilcoxon rank
sum tests between two independent samples. Categorical data were checked
by Chi squared tests. Baseline covariates were evaluated to ensure
consistency between groups. All statistical tests were two-sided, and the
threshold of statistical significance was set at α = 0.05.
Results
A total of 176 patients were included in this study
(88 patients in each group). All patients were followed up for 6 months.
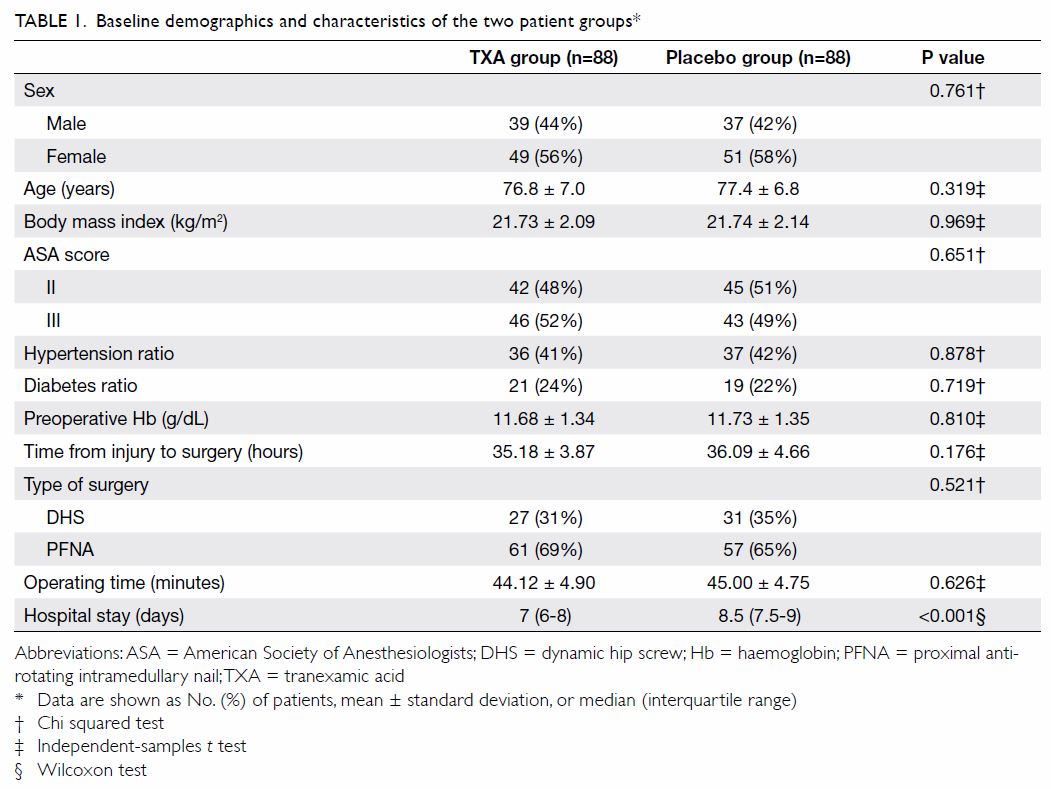
There were no statistically significant differences between the TXA and
placebo groups in terms of patients’ sex, age, body mass index, ASA
scores, proportion of preoperative hypertension and diabetes, or
preoperative Hb concentration. The TXA group had shorter median (IQR)
hospital stays than the placebo group (7 [6-8] vs 8.5 [7.5-9] days;
P<0.001). Furthermore, a similar number of patients underwent DHS and
PFNA in each group, and no differences were detected in terms of time to
surgery or operating time between the two groups (Table 1).
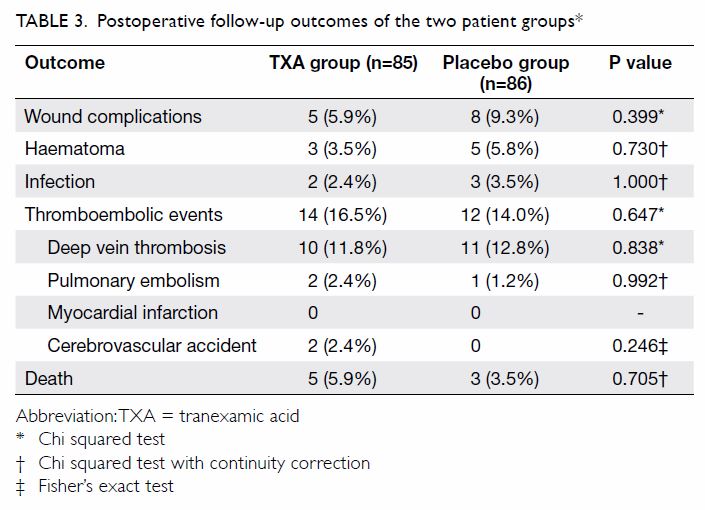
The postoperative Hb levels at POD 1, POD 2, and
POD 3 were higher in the TXA group than in the placebo group, there were
statistically significant differences on POD 1 (10.9 vs 10.3 g/dL,
P=0.004) and POD 2 (9.8 vs 9.3 g/dL, P=0.028), but no statistically
significant differences on POD 3 (9.5 vs 9.1 g/dL, P=0.057) [Table
2].
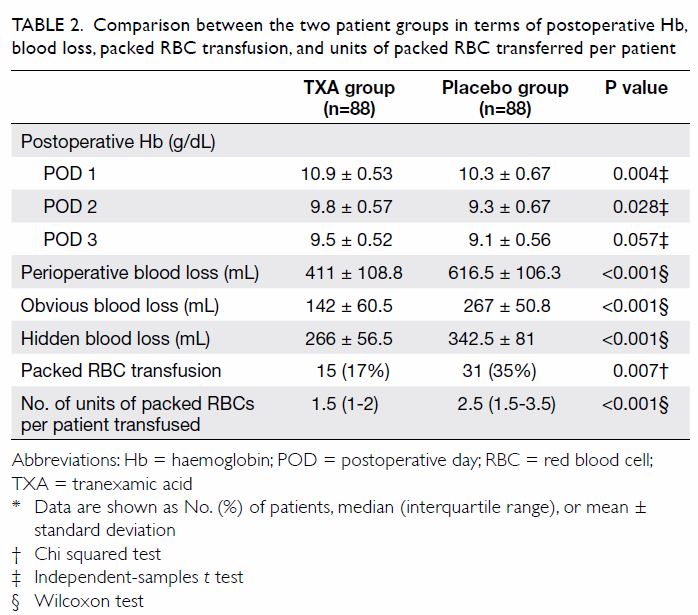
Table 2. Comparison between the two patient groups in terms of postoperative Hb, blood loss, packed RBC transfusion, and units of packed RBC transferred per patient
Mean perioperative blood loss in the TXA group was
205.5 mL lower than that in the placebo group (411 vs 616.5 mL,
P<0.01). Obvious blood loss was 125 mL lower in the TXA group than in
the placebo group (142 vs 267 mL, P<0.01), and hidden blood loss was
76.5 mL lower in the TXA group than in the placebo group (266 vs 342.5 mL,
P<0.01) [Table 2].
Fewer patients in the TXA group than the placebo
group received allogenic blood transfusions (17% vs 35%, P=0.007).
Furthermore, patients with TXA tended to require less total blood product
(median 1.5 units packed red blood cells/patient; IQR, 1-2 units) than
those in the placebo group did (median 2.5 units packed red blood
cells/patient; IQR, 1.5-3.5 units; P<0.01) [Table 2].
After surgery, three patients were lost to
follow-up in the TXA group and two were lost to follow-up in the placebo
group. In the TXA group, five (5.9%) patients had wound complications,
including three (3.5%) with haematoma and two (2.4%) with infection. The
incidence of thromboembolic events was 16.5% in the TXA group, including
10 (11.8%) patients with deep venous thrombosis and two (2.4%) each with
pulmonary embolism and cerebrovascular accident. Five patients died: the
mortality rate in the TXA group was 5.9%. In the placebo group, wound
complications occurred in eight (9.3%) cases, including five (5.8%) with
haematoma and three (3.5%) with infection. We observed thromboembolic
events in 12 (14.0%) cases, including 11 (12.8%) cases with deep venous
thrombosis and one (1.2%) with pulmonary embolism. Three patients died:
the mortality rate in the placebo group was 3.5%. There were no
statistically significant differences between the two groups in wound
complications, thromboembolic events, or death [Table 3].
Discussion
Trochanteric fractures caused by osteoporosis have
become common. Trochanteric fractures account for a large number of
hospital days, much blood loss, and high mortality.23 With a mortality rate of up to 30% in the year after
injury, these patients are among the most frail that orthopaedic surgeons
treat.24 Although TXA is known to
be an effective and safe agent for reducing surgical blood loss,25 26 27 with improved perioperative care for patients
undergoing hip arthroplasty,28
there are limited data regarding its use in trochanteric fracture surgery.17 Therefore, in the present study,
we sought to determine whether intravenous TXA administration would
improve perioperative blood management without increasing levels of
adverse complications.
The mean rate of transfusion (26.1%) and mean
estimated blood loss (567 mL) in the current study are similar to those in
previous reports about surgical treatment of trochanteric fractures (DHS
or PFNA).29 30 Older patients have lower preoperative Hb values, and
older age and intramedullary nail osteosynthesis both increase the risk of
erythrocyte transfusion.17 This
study design accounted for this major confounder through stratification of
randomisation by age.
The dosage and timing of TXA administration in our
study were selected in accordance with Maniar et al,19 which indicated that the three-dose regimen produces
maximum effective reduction of drainage loss and total blood loss. The
present findings suggest that the three-dose regimen could significantly
reduce blood loss and decrease the rate of transfusion without increasing
the risk of the postoperative complications of thromboembolic events and
mortality. We will further study the effects of different TXA
administration regimens on perioperative blood loss and blood transfusion
rate in elderly patients with hip fracture.
Blood conservation is particularly important in
patients with trochanteric fracture to prevent complications related to
acute postoperative anaemia. The three-dose regimen of TXA has been
reported to decrease blood loss in patients undergoing hip fracture
surgery.31 32 In the present study, a significant reduction in the
incidence of transfusion (17% vs 35%), perioperative blood loss, obvious
blood loss, and hidden blood loss were found (Table 2) with TXA administration in trochanteric
fracture surgery. These results are consistent with those of previous
studies.33 34 Gausden et al35 and Schiavone et al36 reported significant reductions in transfusion rates
with TXA use versus placebo. In the TXA in Hip Fracture Surgery study,
Zufferey et al17 also reported the
same trend with a 30% relative reduction in transfusion rates with TXA
administration. Thus, our findings suggest that TXA has clear benefits in
DHS or PFNA of trochanteric fractures.
Despite the wide use of TXA, there has been concern
regarding its association with increased incidence of venous thrombosis
and mortality.32 Zufferey et al17 and Schiavone et al36 reported a three-fold increase in vascular events
(deep venous thrombosis, pulmonary embolism, cerebrovascular accident, and
myocardial infarction) with intravenous TXA administration in hip fracture
surgery, but this was not statistically significant. A number of
metaanalyses have found no increase in thromboembolic complications but
were unable to draw conclusions regarding the safety of TXA because of
potential bias.26 27 A recent population-based study conducted by Poeran
et al37 involving 872 416 patients
showed no increase in thromboembolic events. In our study, no
statistically significant differences were found between the TXA and
placebo groups for incidence of wound complications, thromboembolic
events, or mortality after 6 months’ follow-up (Table 3). Our results were in accordance with the
systematic reviews of Farrow et al38
and Zhang et al,33 which indicated
that TXA did not increase the risk of wound complications, thromboembolic
events, or mortality.
In our study, the length of hospital stay in the
TXA group was less than that in the placebo group. The reasons may be
that, first, TXA administration can reduce perioperative blood loss and
then prevent postoperative anaemia in patients; second, TXA may decrease
the risk associated with transfusion by reducing the rate of transfusion.
Minimising blood loss and red blood cell transfusion can enable early
activity, enhance patient rehabilitation, and facilitate early hospital
discharge.39
This study had some limitations. Although this was
a randomised controlled trial, the surgical procedure was decided by the
surgeon, which may have affected the outcome. In addition, the proper
usage method of TXA is still unclear. Furthermore, the optimal dosing and
timing of TXA administration is still controversial. Further study with a
larger sample and a multicentre trial would be helpful to verify the
present results.
Conclusion
The results of this study suggest that TXA can
reduce perioperative blood loss and decrease the risk associated with
transfusion by reducing the rate of transfusion without increasing the
incidence of complications of thromboembolic events or mortality in
patients with trochanteric fractures. Thus, off-label use of TXA can be
recommended for trochanteric fracture surgery. The blood conservational
effects of TXA are well established and appear to be safe and effective.
In the future, we will conduct a study to clarify the reasonable dosage
and timing of TXA in patients with different types of hip fractures.
Author contributions
All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Concept or design of study: X Zhu.
Acquisition of data: Z Jiang, M Li.
Analysis or interpretation of data: F Chen.
Drafting of the manuscript: F Chen.
Critical revision for important intellectual content: F Chen.
Acquisition of data: Z Jiang, M Li.
Analysis or interpretation of data: F Chen.
Drafting of the manuscript: F Chen.
Critical revision for important intellectual content: F Chen.
Conflicts of interest
The authors have no conflicts of interests to
disclose.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
Approval was obtained from our institution’s
internal ethics committee (No. 2016-01-025). Written informed consent was
obtained from all patients or a legally authorised representative.
References
1. Foss NB, Kehlet H. Hidden blood loss
after surgery for hip fracture. J Bone Joint Surg Br 2006;88:1053-9. Crossref
2. Lawrence VA, Silverstein JH, Cornell JE,
Pederson T, Noveck H, Carson JL. Higher Hb level is associated with better
early functional recovery after hip fracture repair. Transfusion
2003;43:1717-22. Crossref
3. Maxwell L, White S. Anaesthetic
management of patients with hip fractures: an update. Contin Educ Anaesth
Crit Care Pain 2013;13:179-83. Crossref
4. Vamvakas EC, Blajchman MA.
Transfusion-related mortality: the ongoing risks of allogeneic blood
transfusion and the available strategies for their prevention. Blood
2009;113:3406-17. Crossref
5. Newman ET, Watters TS, Lewis JS, et al.
Impact of perioperative allogeneic and autologous blood transfusion on
acute wound infection following total knee and total hip arthroplasty. J
Bone Joint Surg Am 2014;96:279-84. Crossref
6. Carson JL, Altman DG, Duff A, et al.
Risk of bacterial infection associated with allogeneic blood transfusion
among patients undergoing hip fracture repair. Transfusion
1999;39:694-700. Crossref
7. Allain JP, Stramer SL, Carneiro-Proietti
AB, et al. Transfusion-transmitted infectious diseases. Biologicals
2009;37:71-7. Crossref
8. Klein HG, Spahn DR, Carson JL. Red blood
cell transfusion in clinical practice. Lancet 2007;370:415-26. Crossref
9. Carson JL, Terrin ML, Noveck H, et al.
Liberal or restrictive transfusion in high-risk patients after hip
surgery. New Engl J Med 2011;365:2453-62. Crossref
10. National Clinical Guideline Centre.
NICE clinical guidelines No. 124: the management of hip fracture in
adults. London, United Kingdom: Royal College of Physicians; 2011: 68-81.
11. Astedt B, Liedholm P, Wingerup L. The
effect of tranexamic acid on the fibrinolytic activity of vein walls. Ann
Chir Gynaecol 1978;67:203-5.
12. Chen Y, Chen Z, Cui S, Li Z, Yuan Z.
Topical versus systemic tranexamic acid after total knee and hip
arthroplasty: a meta-analysis of randomized controlled trials. Medicine
(Baltimore) 2016;95:e4656. Crossref
13. Ueno M, Sonohata M, Fukumori N, Kawano
S, Kitajima M, Mawatari M. Comparison between topical and intravenous
administration of tranexamic acid in primary total hip arthroplasty. J
Orthop Sci 2016;21:44-7. Crossref
14. North WT, Mehran N, Davis JJ,
Silverton CD, Weir RM, Laker MW. Topical vs intravenous tranexamic acid in
primary total hip arthroplasty: a double-blind, randomized controlled
trial. J Arthroplasty 2016;31:1022-6. Crossref
15. CRASH-2 Trial Collaborators, Shakur H,
Roberts I, et al. Effects of tranexamic acid on death, vascular occlusive
events, and blood transfusion in trauma patients with significant
haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet
2010;376:23-32. Crossref
16. Lack WD, Crist BD, Seymour RB, Harvin
W, Karunakar MA, TXA Study Group II. Effect of tranexamic acid on
transfusion: a randomized clinical trial in acetabular fracture surgery. J
Orthop Trauma 2017;31:526-30. Crossref
17. Zufferey PJ, Miquet M, Quenet S, et
al. Tranexamic acid in hip fracture surgery: a randomized controlled
trial. Br J Anaesth 2010;104:23-30. Crossref
18. World Medical Association. World
Medical Association Declaration of Helsinki: ethical principles for
medical research involving human subjects. JAMA 2013;310:2191-4. Crossref
19. Maniar RN, Kumar G, Singhi T, Nayak
RM, Maniar PR. Most effective regimen of tranexamic acid in knee
arthroplasty: a prospective randomized controlled study in 240 patients.
Clin Orthop Relat Res 2012;470:2605-12. Crossref
20. Ministry of Public Health of China.
Measures for the management of clinical blood use in medical institutions.
China Med Pharm 2012;02:6-8.
21. Nadler SB, Hidalgo JH, Bloch T.
Prediction of blood volume in normal human adults. Surgery 1962;51:224-32.
22. Gross JB. Estimating allowable blood
loss: corrected for dilution. Anesthesiology 1983;58:277-80. Crossref
23. Dhanwal DK, Dennison EM, Harvey NC,
Cooper C. Epidemiology of hip fracture: worldwide geographic variation.
Indian J Orthop 2011;45:15-22. Crossref
24. Moran CG, Wenn RT, Sikand M, Taylor
AM. Early mortality after hip fracture: is delay before surgery important?
J Bone Joint Surg Am 2005;87:483-9. Crossref
25. McCormack PL. Tranexamic acid: a
review of its use in the treatment of hyperfibrinolysis. Drugs
2012;72:585-617. Crossref
26. Ker K, Edwards P, Perel P, Shakur H,
Roberts I. Effect of tranexamic acid on surgical bleeding: systematic
review and cumulative meta-analysis. BMJ 2012;344:e3054. Crossref
27. Huang F, Wu D, Ma G, Yin Z, Wang Q.
The use of tranexamic acid to reduce blood loss and transfusion in major
orthopedic surgery: a meta-analysis. J Surg Res 2014;186:318-27. Crossref
28. Watts CD, Pagnano MW. Minimising blood
loss and transfusion in contemporary hip and knee arthroplasty. J Bone
Joint Surg Br 2012;94(11 Suppl A):8-10. Crossref
29. Lei JL, Zhang B, Cong Y, et al.
Tranexamic acid reduces hidden blood loss in the treatment of
intertrochanteric fractures with PFNA: a single-center randomized
controlled trial. J Orthop Surg Res 2017;12:124. Crossref
30. Tian S, Shen Z, Liu Y, Zhang Y, Peng
A. The effect of tranexamic acid on hidden bleeding in older
intertrochanteric fracture patients treated with PFNA. Injury
2018;49:680-4. Crossref
31. Lee C, Freeman R, Edmondson M, Rogers
BA. The efficacy of tranexamic acid in hip hemiarthroplasty surgery: an
observational cohort study. Injury 2015;46:1978-82. Crossref
32. Tengberg PT, Foss NB, Palm H,
Kallemose T, Troelsen A. Tranexamic acid reduces blood loss in patients
with extracapsular fractures of the hip: results of a randomised
controlled trial. Bone Joint J 2016;98B:747-53. Crossref
33. Zhang P, He J, Fang Y, Chen P, Liang
Y, Wang J. Efficacy and safety of intravenous tranexamic acid
administration in patients undergoing hip fracture surgery for hemostasis:
A meta-analysis. Medicine (Baltimore) 2017;96:e6940. Crossref
34. Wingerter SA, Keith AD, Schoenecker
PL, Baca GR, Clohisy JC. Does tranexamic acid reduce blood loss and
transfusion requirements associated with the periacetabular osteotomy?
Clin Orthop Relat Res 2015;473:2639-43. Crossref
35. Gausden EB, Garner MR, Warner SJ, et
al. Tranexamic acid in hip fracture patients: a protocol for a randomised,
placebo controlled trial on the efficacy of tranexamic acid in reducing
blood loss in hip fracture patients. BMJ Open 2016;6:e010676. Crossref
36. Schiavone A, Bisaccia M, Inkov I, et
al. Tranexamic acid in pertrochanteric femoral fracture: is it a safe drug
or not? Folia Med (Plovdiv) 2018;60:67-78. Crossref
37. Poeran J, Rasul R, Suzuki S, et al.
Tranexamic acid use and postoperative outcomes in patients undergoing
total hip or knee arthroplasty in the United States: retrospective
analysis of effectiveness and safety. BMJ 2014;349:g4829. Crossref
38. Farrow LS, Smith TO, Ashcroft GP,
Myint PK. A systematic review of tranexamic acid in hip fracture surgery.
Br J Clin Pharmacol 2016;82:1458-70. Crossref
39. Willems JM, de Craen AJ, Nelissen RG,
van Luijt PA, Westendorp RG, Blauw GJ. Haemoglobin predicts length of
hospital stay after hip fracture surgery in older patients. Maturitas
2012;72:225-8. Crossref