Hong Kong Med J 2024 Apr;30(2):139–46 | Epub 25 Mar 2024
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Comparison of clinical characteristics between
ACOSOG Z0011–eligible cohort and sentinel lymph node–positive breast cancer patients in Hong Kong
Vivian Man, FCSHK, FRCSEd; Ava Kwong, FCSHK, FRCS
Department of Surgery, Queen Mary Hospital, Hong Kong SAR, China
Corresponding author: Prof Ava Kwong (avakwong@hku.hk)
Abstract
Introduction: The American College of Surgeons
Oncology Group (ACOSOG) Z0011 trial resulted
in de-escalation of axillary surgery among early-stage
breast cancer patients with low-volume
sentinel lymph node (SLN) disease undergoing
breast-conserving surgery and radiation therapy.
Nevertheless, the mastectomy rate in the Chinese
population remains high. This study compared the
clinical characteristics of the ACOSOG Z0011–eligible cohort with SLN-positive breast cancer
patients in Hong Kong.
Methods: This retrospective analysis of a
prospectively maintained database at a university-affiliated
breast cancer centre in Hong Kong was
performed from June 2014 to May 2019. The
database included all patients with clinical tumour
(T) stage T1 or T2 invasive breast carcinoma, no
palpable adenopathy, one or two positive SLNs on
histological examination, and no prior neoadjuvant
systemic treatment. Comparisons were made
between the mastectomy and breast-conserving
treatment groups in our cohort, along with the
sentinel-alone arm in the ACOSOG Z0011 trial.
Results: One hundred and seventy-one patients met
the inclusion criteria: 112 underwent mastectomy and 59 underwent breast-conserving treatment.
Our mastectomy group had higher prevalences
of T2 tumours (P<0.001), lymphovascular
invasion (P<0.001), and SLN macrometastases
(P=0.004) compared with the ACOSOG Z0011
cohort. However, in our patient population, mean
pathological size slightly differed between the
mastectomy and breast-conserving treatment groups
(2.2 cm vs 1.8 cm; P=0.005). Other histopathological
features were similar.
Conclusion: This study demonstrated that
clinicopathological features were comparable
between SLN-positive breast cancer patients
undergoing mastectomy and those undergoing
breast-conserving treatment. Low-risk SLN-positive
mastectomy patients may safely avoid completion
axillary lymph node dissection.
New knowledge added by this study
- Despite the high rate of mastectomy in Hong Kong, a small proportion of node-positive breast cancer patients met the American College of Surgeons Oncology Group (ACOSOG) Z0011 eligibility criteria to forgo axillary lymph node dissection.
- Sentinel lymph node–positive breast cancer patients undergoing mastectomy displayed clinicopathological features similar to those undergoing breast-conserving treatment in Hong Kong.
- By expanding the AMAROS trial (After Mapping of the Axilla: Radiotherapy Or Surgery?) eligibility to include ACOSOG Z0011–ineligible mastectomy patients, more patients could avoid axillary lymph node dissection with adjuvant radiotherapy, potentially reducing morbidity.
- Further studies are necessary to explore when adjuvant axillary radiotherapy is indicated among mastectomy patients with low axillary nodal burden.
Introduction
The evolution of optimal axillary management for
breast cancer patients has led to emphasis on the
de-escalation of axillary surgery and minimisation
of surgical morbidity. Favourable results from
the American College of Surgeons Oncology Group (ACOSOG) Z0011 phase 3 randomised
clinical trials have redefined the indications for
completion axillary lymph node dissection (ALND)
in patients with positive sentinel lymph nodes
(SLNs). Early-stage breast cancer patients who
undergo upfront breast-conserving surgery and have one or two positive SLNs can safely forgo
ALND while maintaining good overall survival and
disease-free survival.1 2 Consequently, the ASCO
(American Society of Clinical Oncology)3 and the
NCCN (National Comprehensive Cancer Network)4
have revised their clinical practice guidelines to
recommend against completion ALND in this
subset of patients. Although this guidance has led
to a significant decline in the rate of completion
ALND among ACOSOG Z0011–eligible patients,5 6 7
a similar reduction was observed among patients
undergoing mastectomy.8 9 This reduction was
particularly pronounced among patients with SLN
micrometastases.8 Further evidence was obtained
in the phase 3 IBCSG (International Breast Cancer
Study Group) 23-01 randomised controlled trials,
where approximately 10% of patients with SLN
micrometastases underwent mastectomy; subgroup
analysis demonstrated that disease-free survival
among patients without axillary dissection was non-inferior
to those with axillary dissection after 10
years of follow-up.10 11 Similarly, in the AMAROS
trial (After Mapping of the Axilla: Radiotherapy
Or Surgery?), 17% of patients with tumour (T)
staging T1 to T2 primary breast cancer underwent
mastectomy.12 Axillary radiotherapy led to an oncological outcome comparable to completion ALND but was associated with a lower rate of lymphoedema.
In Hong Kong, factors such as the relatively
small breast sizes among Chinese women13 and more
conservative cultural attitudes13 14 have contributed to
a higher rate of mastectomy. The decision to perform
mastectomy has prevented a substantial number of
breast cancer patients from meeting the ACOSOG
Z0011 criteria. Our previous study evaluated the
applicability of ACOSOG Z0011 criteria in Hong
Kong.15 Patients with clinical nodal (N) staging N0
breast cancer and one or more positive SLNs were
stratified into eligible and ineligible groups according
to the ACOSOG Z0011 criteria, with 93% of patients
in the ineligible group underwent mastectomy.15
Importantly, only 24% of patients in that study met
the ACOSOG Z0011 criteria and could potentially
avoid ALND.15 Therefore, it is important to identify a
low-risk subset of SLN-positive mastectomy patients
who could benefit from this non-ALND approach.
This retrospective study was conducted to compare
the clinical characteristics of SLN-positive breast
cancer patients in Hong Kong with the ACOSOG
Z0011–eligible cohort.
Methods
Patient recruitment
This retrospective analysis of a prospectively
maintained database was conducted at Queen Mary
Hospital, a university-affiliated tertiary breast cancer
centre in Hong Kong, from June 2014 to May 2019.
Potentially eligible patients in the database were
identified by an independent research assistant
according to whether they met the ACOSOG Z0011
criteria, irrespective of breast surgery type. Patients
were excluded if they had positive non-SLNs or
positive SLNs only detected by immunohistochemical
staining. Relevant data were extracted in July 2020
and missing information was verified using the
Clinical Management System, a central computer
system for medical records across public hospitals
in Hong Kong. Recruited patients were divided into
two groups, namely, the mastectomy group and the
breast-conserving treatment (ie, ACOSOG Z0011–eligible) group.
Clinical management and pathological
assessment
All breast cancer patients underwent mammography
and ultrasound of the breasts and axillae for clinical
tumour and nodal staging. Sentinel lymph node
biopsy (SLNB), offered to patients with clinically
node-negative disease, was performed with a dual
tracer of radioisotope and patent blue dye. Sentinel
lymph nodes were defined as lymph nodes with
ex vivo gamma probe counts exceeding 10% of the highest ex vivo reading or lymph nodes that
displayed blue staining. Non-SLNs were defined as
suspicious nodes that were neither hot (high gamma
probe counts) nor blue-stained during SLNB,
or nodes that were removed during completion
ALND. During the study period, intraoperative
frozen sections of SLNs or suspicious non-SLNs
were routinely collected; these were analysed
by standard haematoxylin and eosin staining.
Immunohistochemistry was performed in cases
of suspected nodal metastasis. Completion ALND
was conducted if frozen or paraffin sections showed
evidence of nodal metastasis. All final pathological
results were reviewed in multidisciplinary meetings.
The pathologies of SLNs were considered normal or
containing one of the following: macrometastases
(>2 mm), micrometastases (>0.2 to ≤2 mm), or
isolated tumour cells (≤0.2 mm). For patients
undergoing breast-conserving surgery, ‘no ink on
tumour’ was regarded as an adequate resection
margin16; alternatively, a second operation was
performed to ensure a clear resection margin.
Adjuvant treatment was administered by breast
oncology specialists according to decisions made in
multidisciplinary meetings.
Statistical analysis
Patient demographic characteristics and tumour
characteristics were retrieved from database records;
percentages were calculated. Missing information
was evaluated and managed by pairwise deletion.
Comparisons were made between the mastectomy
and breast-conserving treatment groups in our
cohort, along with the sentinel-alone arm in the
ACOSOG Z0011 trial (n=436, in intention to treat).1 2
Analyses followed the per-protocol approach and
calculations were performed with SPSS software
(Windows version 24.0; IBM Corp, Armonk [NY],
United States). Comparisons between cohorts were
conducted with Student’s t test or the Chi squared
test, as appropriate. Human epidermal growth
factor receptor 2 (HER2) status was not assessed
in the ACOSOG Z0011 study; therefore, HER2
statuses were only compared within our cohort. The
Memorial Sloan Kettering Cancer Center (MSKCC)
breast cancer nomogram,17 a well-validated
prediction tool to assess the likelihood of non–sentinel node metastases18 19 20 (including external
validation in the Chinese population19 20), was used
to calculate probability through an online calculator
that considered nine variables; comparisons were
made between the breast-conserving treatment and
mastectomy groups. P values <0.05 were considered
statistically significant.
Results
In our centre, the ACOSOG Z0011 criteria have been used to manage patients undergoing breast-conserving
surgery since June 2019. From June 2014
to May 2019, 1249 breast cancer patients underwent
SLNB in our institution; 171 patients (13.7%) met
the study inclusion criteria of clinical T1 or T2
invasive breast cancer and one or two positive SLNs.
One hundred and twelve patients (65.5%) underwent
mastectomy and 59 patients (34.5%) underwent
breast-conserving treatment. The median follow-up
period was 58 months (range, 25-84).
Our mastectomy group versus the sentinel-alone
arm in the ACOSOG Z0011 trial
Patient demographic characteristics and tumour
characteristics of our mastectomy group and the
sentinel-alone arm in the ACOSOG Z0011 trial
are presented in Table 1. Invasive ductal carcinoma
was more common in our patient population than
in the ACOSOG Z0011 group. A higher prevalence
of clinical T2 breast cancers (~50%) was observed in
our mastectomy group (P<0.001). There were also
significantly more patients with lymphovascular
invasion in our cohort than in the sentinel-alone arm
in the ACOSOG Z0011 trial (P<0.001). Although
nearly half of the original ACOSOG Z0011 cohort
had micrometastatic SLNs, approximately 70%
of mastectomy patients had macrometastatic
SLNs (P=0.004). These findings suggested that the
clinicopathological profile was more aggressive in
patients requiring mastectomy.
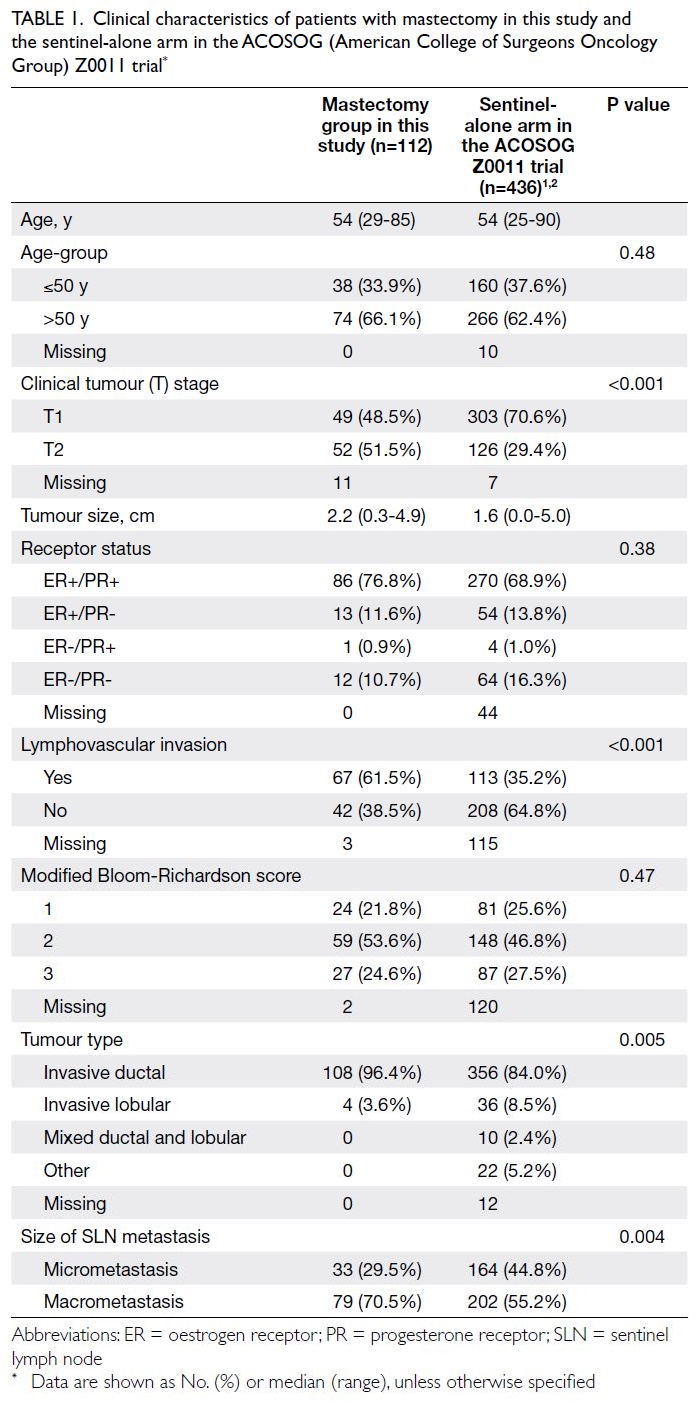
Table 1. Clinical characteristics of patients with mastectomy in this study and the sentinel-alone arm in the ACOSOG (American College of Surgeons Oncology Group) Z0011 trial
Our mastectomy group versus our breast-conserving
treatment group
In our patient cohort, the mastectomy group
exhibited many clinicopathological characteristics
similar to the breast-conserving treatment group
(Table 2). There were no statistically significant
differences in terms of age, tumour grade,
lymphovascular invasion status, oestrogen receptor/progesterone receptor status, or HER2 status. The
mastectomy group had relatively larger tumours
than the breast-conserving treatment group (mean:
2.2 cm vs 1.8 cm; P=0.005). Although the difference
was not statistically significant, the mastectomy
group tended to have larger proportions of patients
with two metastatic SLNs (24.1% vs 13.6%; P=0.1)
and SLN macrometastases (70.5% vs 57.6%; P=0.11)
than the breast-conserving treatment group.
Furthermore, the MSKCC probability for additional
metastatic non-SLNs was slightly higher in the
mastectomy group than in the breast-conserving
treatment group (37.1% vs 31.4%; P=0.03) [Table 2].
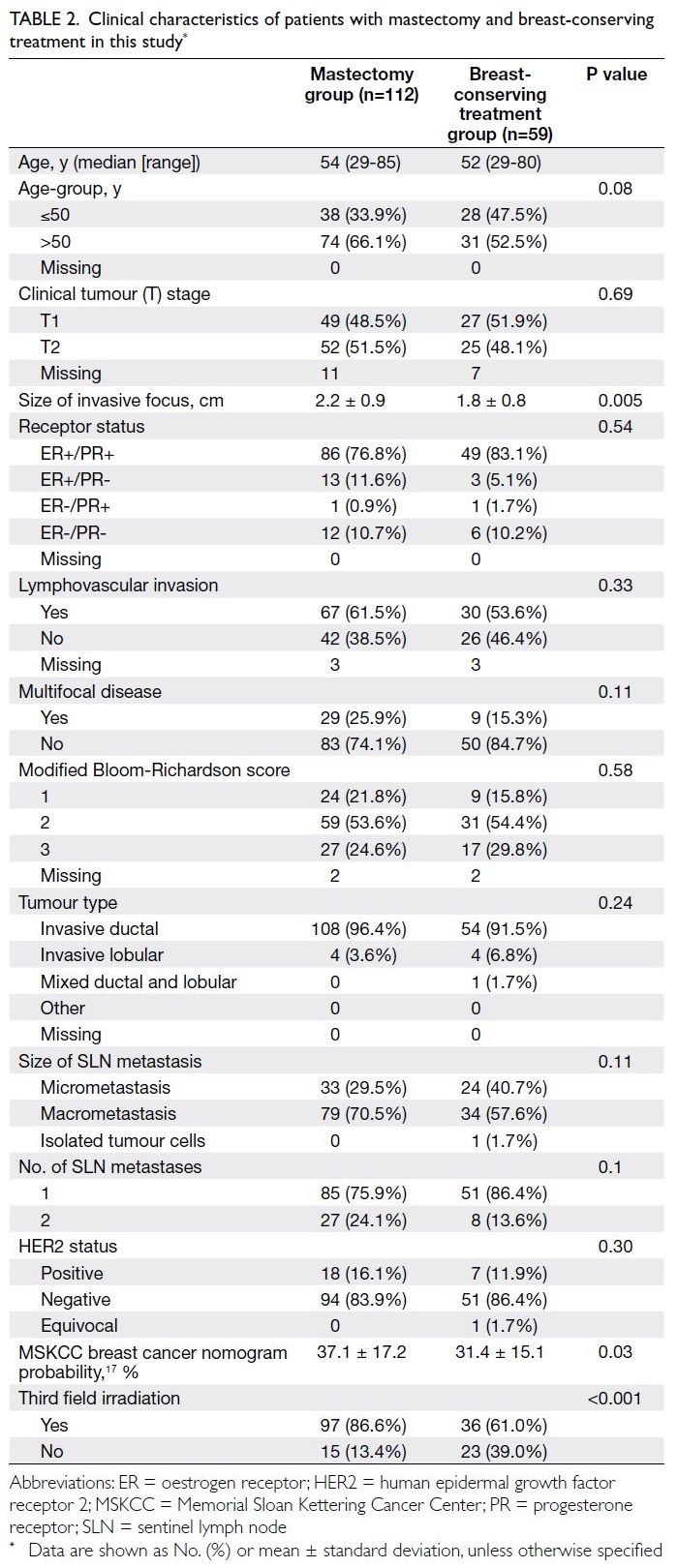
Table 2. Clinical characteristics of patients with mastectomy and breast-conserving treatment in this study
Ninety-seven patients (86.6%) in the
mastectomy group and 45 patients (76.3%) in the
breast-conserving treatment group underwent
completion ALND. Among patients who underwent
mastectomy and completion ALND, 26 patients (26.8%) had additional non-SLN metastases (range,
1-18). In contrast, eight patients (17.8%) in the
breast-conserving treatment group had additional
non-SLN metastases (range, 1-8). There was no statistically significant difference in the rate of
non-SLN metastases between the two treatment
arms (P=0.24). Twenty-nine patients (17.9%)
underwent SLNB alone; 15 of these patients were
in the mastectomy group. Most patients with SLNB
alone had micrometastatic SLNs (89.7%) and one
patient had isolated tumour cells. None of the
patients with SLNB alone experienced recurrence.
Adjuvant treatment
In the mastectomy group, 97 patients (86.6%)
underwent post-mastectomy irradiation targeting
the chest wall and third field regional nodes. Third
field regional nodes refer to level III axillary and
supraclavicular lymph node regions. None of these
patients developed chest wall or axillary recurrence
during the follow-up period. Among the 15 patients
who did not undergo post-mastectomy irradiation,
eight (53.3%) had micrometastatic SLNs and six
(40.0%) had macrometastatic SLNs. There were two
recurrences (13.3%). First, a 38-year-old patient with
one macrometastatic SLN developed ipsilateral chest
wall recurrence 4 years after the index operation;
this recurrence was managed by a second operation.
Second, a patient with two macrometastatic SLNs
refused adjuvant systemic treatment and died of
breast cancer‐related distant metastases. One
hundred and ten patients in the mastectomy group
(98.2%) received adjuvant systemic treatment: 10
patients (8.9%) required chemotherapy only, 22
patients (19.6%) required hormonal treatment
only, and 78 patients (69.6%) required both of these
treatments. Seven patients (6.3%) in the mastectomy
group developed distant recurrence, and there were
three (2.7%) breast cancer–related deaths.
In the breast-conserving treatment group, 58
of the 59 patients underwent adjuvant whole-breast
irradiation; 61.0% of these patients underwent
additional third field nodal irradiation. Fifty-eight
patients (98.3%) in the breast-conserving treatment
group received adjuvant systemic treatment
involving hormonal therapy and/or chemotherapy.
Three patients (5.1%) had distant recurrence; among
them, one (1.7%) died at 39 months after the initial
diagnosis. One patient experienced ipsilateral breast
recurrence at 30 months and underwent completion
mastectomy.
Discussion
The favourable oncological results of the ACOSOG
Z0011 trial1 2 have challenged the conventional
approach of performing completion ALND in
patients with SLN metastases. Patients with one
or two SLN metastases who underwent breast-conserving
surgery, whole-breast irradiation, and
adjuvant systemic treatment could safely forgo
completion ALND. This paradigm shift has led to substantial de-escalation of axillary surgery
worldwide.5 A meta-analysis by Schmidt-Hansen
et al,21 which involved 2020 patients and findings
from the IBCSG 23-0110 11 and the AATRM (Agència
d’Avaluació de Tecnologia i Recerca Mèdiques)
048/13/200022 trials, concluded that SLNB alone
was sufficient for locoregional control in early breast
cancer, without adverse effects on survival.
Limitations of the ACOSOG Z0011 study
Despite widespread adoption of the ACOSOG Z0011
criteria, the study has been criticised in several ways.
The low locoregional relapse rate of 1.5% indicates
that the study was underpowered.23 Furthermore,
significant deviation in the radiotherapy protocol,
such that 18.9% of patients received ‘high tangents’
radiotherapy, has raised questions concerning the
oncological safety of SLNB alone in patients without
third field nodal irradiation.24 Combined with the
insufficient numbers of mastectomy patients in
the IBCSG 23-01,10 11 AMAROS,12 and AATRM
048/13/200022 trials, it has been unclear whether
this non-ALND approach can be extrapolated to
SLN-positive breast cancer patients who undergo
mastectomy with or without radiotherapy.
Aggressive tumour characteristics among mastectomy patients and local or regional failure rate
In this study, we compared the clinicopathological
characteristics among our mastectomy group, our
breast-conserving treatment group, and the sentinel-alone
arm in the original ACOSOG Z0011 study.
Unsurprisingly, our mastectomy group exhibited
more aggressive tumour characteristics than the
sentinel-alone arm in the Western population;
specifically, it had a larger tumour size, more frequent
lymphovascular invasion, and a greater proportion
of patients with SLN macrometastases. These
differences in clinicopathological features have also
been reported in Western populations. For example,
Hennigs et al8 analysed a large German cohort that
included 4093 SLN-positive mastectomy patients.
Compared with the entire study cohort of 166 074
patients, T2 tumour and lymphovascular invasion
were more commonly found in patients requiring
mastectomy. Additionally, the study by Milgrom et al25
included 535 early-stage breast cancer patients with
a positive SLNB and no ALND. In their mastectomy
group, patients had significantly larger tumours and
more frequently displayed multifocal/multicentric
disease. However, these adverse pathological features
among mastectomy patients did not justify a more
aggressive axillary approach. Similarly, the low rates
of local and regional failure observed in our cohort
were consistent with previous reports, suggesting
that axillary-specific treatment can be considered in this group of patients with low-volume SLN
disease.25 26 27 28 Debate persists regarding the
comparatively large proportions of patients with
micrometastatic disease in the original ACOSOG
Z0011 trial1 2 and other studies.25 26 Cowher et al29
published a retrospective analysis of patients
who underwent mastectomy and conservative
axillary regional excision (ie, removal of SLNs and
other palpable nodes). Among 144 patients with
pathological N1 disease, a small proportion (24%)
had micrometastatic disease; only three axillary
recurrences (2.1%) were reported.29 Notably, the
low locoregional failure rate was not attributed to
post-mastectomy irradiation25 26 27 28 29 or increased use of
chemotherapy.26 27 28
Intrinsic differences in tumour characteristics between different patient populations
In our previous study, we demonstrated differences
in clinical characteristics between Asian and
Western populations.15 In the present study, our
breast-conserving treatment group had a higher
rate of clinical T2 tumours and more frequent
lymphovascular invasion compared with the
Western population. Similar findings were observed
in Korean30 and Japanese31 studies, which revealed
larger and higher-grade tumours, increased
lymphovascular permeation, and more frequent
SLN macrometastases. Despite these disparities, the
Korean30 and Japanese31 studies both demonstrated
safe application of ACOSOG Z0011 criteria in
Asia, with low incidences of disease recurrence.
These intrinsic differences in tumour characteristics
between Eastern and Western populations have
presumably reduced the gap in clinicopathological
features between patients undergoing mastectomy
and those undergoing breast-conserving surgery.
In the head-to-head comparison between our
mastectomy cohort and our breast-conserving
treatment group, the only notable difference involved
the mean pathological size of the invasive focus
(2.2 cm vs 1.8 cm; P=0.005); the clinical tumour
stage distribution did not differ (P=0.69) [Table 2].
The small difference in mean MSKCC breast cancer
nomogram probability (37.1% vs 31.4%; P=0.03)
could also be related to the difference in pathological
size, which is one of the nine variables considered
in the nomogram. Therefore, we believe that a non-ALND approach in this low-risk subset of SLN-positive
mastectomy patients is acceptable.
Residual non–sentinel lymph node metastasis in non–axillary lymph node dissection approach
The primary concern regarding extrapolation of this
non-ALND approach is the risk of undertreatment
for patients with an extensive nodal burden. The original ACOSOG Z0011 trial revealed a non-SLN
macrometastasis rate of 27.3% in the ALND group.1 2
The AMAROS trial also showed that 33% of patients
in the ALND group had additional positive lymph
nodes.12 Importantly, the axillary recurrence rate
remained low in both of these studies. In our
SLN-positive mastectomy and breast-conserving
treatment groups, the proportions of patients with
additional non-SLN metastases were 26.8% and
17.8%, respectively. Among patients undergoing
adjuvant irradiation and adjuvant systemic
treatment, it is likely that some non-SLN metastases
do not progress to clinically detectable disease.
Limitations of this study
This study had several limitations. First, its
retrospective design could result in recall bias
and the potential for missing clinical information.
Although data from the ACOSOG Z0011 trial were
limited with respect to HER2 status, extracapsular
extension, and multifocality, we attempted to
mitigate this issue by including some of the affected
variables in the comparison of our mastectomy and
breast-conserving treatment groups. Second, we
could not address the need for post-mastectomy
irradiation among patients in this study. The value
of such irradiation for breast cancer patients with
<4 positive lymph nodes remains controversial. The
meta-analysis by the Early Breast Cancer Trialists’
Collaborative Group,32 which included 1314 breast
cancer patients with one to three positive nodes
after mastectomy and ALND, suggested that
radiotherapy provided oncological benefit in terms
of locoregional recurrence, overall recurrence,
and breast cancer mortality. However, this meta-analysis
has been criticised for including some very
early studies from the 1970s, in which the reported
recurrence rates were much higher than rates in
later studies. In 2016, a focused update by the
American Society of Clinical Oncology, American
Society for Radiation Oncology, and Society of
Surgical Oncology acknowledged the use of post-mastectomy
radiotherapy for this group of patients
but recommended clinical judgement for patients
with a low risk of locoregional recurrence.33 In our centre, post-mastectomy irradiation was generally
administered to patients with pathological N1
disease during the study period; 86.6% of patients in
the present study underwent adjuvant radiotherapy.
Considering the similarities in clinicopathological
features and adjuvant systemic treatment use
between our SLN-positive mastectomy and breast-conserving
treatment groups, we suspect that it is
safe for selected low-risk SLN-positive mastectomy
patients to forgo ALND through the expansion of
AMAROS eligibility12 to ACOSOG Z0011–ineligible
patients. Several ongoing randomised studies, such
as the English POSNOC (POsitive Sentinel NOde: adjuvant therapy alone versus adjuvant therapy
plus Clearance or axillary radiotherapy)34 and the
Dutch BOOG 2013-07,35 are recruiting breast
cancer patients who undergo mastectomy and
have a maximum of two to three positive SLNs;
these studies aim to compare completion axillary
treatment (ALND or axillary radiotherapy) and the
lack of completion axillary treatment. Additionally,
the SINODAR-ONE trial36 recently published their
subgroup analysis and found non-inferior overall
survival and recurrence-free survival among
mastectomy patients receiving SLNB and ALND.
The ongoing studies are expected to provide more
robust evidence concerning the optimal treatment
for SLN-positive mastectomy patients.
Conclusion
This study demonstrated the clinicopathological
similarities between SLN-positive mastectomy and
breast-conserving treatment groups among breast
cancer patients in Hong Kong. Cautious application
of the non-ALND approach in mastectomy patients
with low-volume SLN disease is reasonable,
considering the low locoregional recurrence
rate. However, additional research is needed
to standardise the adjuvant post-mastectomy
radiotherapy protocol, especially among patients
who forego ALND.
Author contributions
Concept or design: V Man.
Acquisition of data: V Man.
Analysis or interpretation of data: V Man.
Drafting of the manuscript: Both authors.
Critical revision of the manuscript for important intellectual content: A Kwong.
Acquisition of data: V Man.
Analysis or interpretation of data: V Man.
Drafting of the manuscript: Both authors.
Critical revision of the manuscript for important intellectual content: A Kwong.
Both authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
Both authors have disclosed no conflicts of interest.
Declaration
This study has been presented and awarded the Young Investigator Award Best Scientific Paper in the Hong Kong Society of Breast Surgeons 5th Annual Scientific Meeting (19 September 2021, Hong Kong).
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the Institutional Review Board of The University of Hong Kong/Hospital Authority Hong Kong West Cluster, Hong Kong (Ref No.: HKU/HA HKW UW 09-045). Written informed consent was obtained from patients for all treatments, procedures, and publication.
References
1. Giuliano AE, Hunt KK, Ballman KV, et al. Axillary
dissection vs no axillary dissection in women with invasive
breast cancer and sentinel node metastasis: a randomized
clinical trial. JAMA 2011;305:569-75. Crossref
2. Guiliano AE, Ballman KV, McCall L, et al. Effect of axillary
dissection vs no axillary dissection on 10-year overall
survival among women with invasive breast cancer and
sentinel node metastasis: the ACOSOG Z0011 (Alliance)
randomized clinical trial. JAMA 2017;318:918-26. Crossref
3. Lyman GH, Termin S, Edge SB, et al. Sentinel lymph
node biopsy for patients with early-stage breast cancer:
American Society of Clinical Oncology clinical practice
guideline update. J Clin Oncol 2014;32:1365-83. Crossref
4. Gradishar WJ, Anderson BO, Balassanian R, et al. NCCN
guidelines insights: breast cancer, version 1.2017. J Natl
Compr Canc Netw 2017;15:433-51. Crossref
5. Camp MS, Greenup RA, Taghian A, et al. Application of
ACOSOG Z0011 criteria reduces perioperative costs. Ann
Surg Oncol 2013;20:836-41. Crossref
6. Delpech Y, Bricou A, Lousquy R, et al. The exportability
of the ACOSOG Z0011 criteria for omitting axillary
lymph node dissection after positive sentinel lymph node
biopsy findings: a multicenter study. Ann Surg Oncol
2013;20:2556-61. Crossref
7. Verheuvel NC, Voogd AC, Tjan-Heijnen VC, Roumen RM.
Potential impact of application of Z0011 derived criteria to
omit axillary lymph node dissection in node positive breast
cancer patients. Eur J Surg Oncol 2016;42:1162-8. Crossref
8. Hennigs A, Riedel F, Feißt M, et al. Evolution of the use of
completion axillary lymph node dissection in patients with
T1/2N0M0 breast cancer and tumour-involved sentinel
lymph nodes undergoing mastectomy: a cohort study. Ann
Surg Oncol 2019;26:2435-43. Crossref
9. Poodt IG, Spronk PE, Vugts G, et al. Trends on axillary
surgery in nondistant metastatic breast cancer patients
treated between 2011 and 2015: a Dutch population-based
study in the ACOSOG-Z0011 and AMAROS era. Ann
Surg 2018;268:1084-90. Crossref
10. Galimberti V, Cole BF, Zurrida S, et al. Axillary dissection
versus no axillary dissection in patients with sentinel-node
micrometastases (IBCSG 23-01): a phase 3 randomised
controlled trial. Lancet Oncol 2013;14:297-305. Crossref
11. Galimberti V, Cole BF, Viale G, et al. Axillary dissection
versus no axillary dissection in patients with breast cancer
and sentinel-node micrometastases (IBCSG 23-01):
10-year follow-up of a randomised, controlled phase 3
trial. Lancet Oncol 2018;19:1385-93. Crossref
12. Donker M, van Tienhoven G, Straver ME, et al.
Radiotherapy or surgery of the axilla after a positive
sentinel node in breast cancer (EORTC 10981-22023
AMAROS): a randomised, multicentre, open-label, phase
3 non-inferiority trial. Lancet Oncol 2014;15:1303-10. Crossref
13. Sinnadurai S, Kwong A, Hartman M, et al. Breast-conserving surgery versus mastectomy in young women with breast cancer in Asian settings. BJS Open 2018;3:48-55. Crossref
14. Chan SW, Cheung C, Chan A, Cheung PS. Surgical options
for Chinese patients with early invasive breast cancer: data
from the Hong Kong Breast Cancer Registry. Asian J Surg 2017;40:444-52.Crossref
15. Man V, Lo MS, Kwong A. The applicability of the ACOSOG
Z0011 criteria to breast cancer patients in Hong Kong.
Chin Clin Oncol 2021;10:27. Crossref
16. Moran MS, Schnitt SJ, Giuliano AE, et al. Society of
Surgical Oncology–American Society for Radiation
Oncology consensus guideline on margins for breast-conserving
surgery with whole-breast irradiation in stages I
and II invasive breast cancer. Ann Surg Oncol 2014;21:704-16. Crossref
17. Memorial Sloan Kettering Cancer Center. Breast cancer
nomogram: breast additional non-SLN metastases.
Available from: https://nomograms.mskcc.org/breast/BreastAdditionalNonSLNMetastasesPage.aspx. Accessed 19 Mar 2024.
18. Gur AS, Unal B, Ozbek U, et al. Validation of breast cancer
nomograms for predicting the non–sentinel lymph node
metastases after a positive sentinel lymph node biopsy in a
multi-center study. Eur J Surg Oncol 2010;36:30-5. Crossref
19. Kuo YL, Chen WC, Yao WJ, et al. Validation of Memorial
Sloan-Kettering Cancer Center nomogram for prediction
of non–sentinel lymph node metastasis in sentinel lymph
node positive breast cancer patients: an international
comparison. Int J Surg 2013;11:538-43. Crossref
20. Bi X, Wang Y, Li M, et al. Validation of the Memorial
Sloan Kettering Cancer Center nomogram for predicting
non–sentinel lymph node metastasis in sentinel lymph
node–positive breast-cancer patients. Onco Targets Ther
2015;8:487-93. Crossref
21. Schmidt-Hansen M, Bromham N, Hasler E, Reed MW.
Axillary surgery in women with sentinel node–positive
operable breast cancer: a systematic review with meta-analyses.
Springerplus 2016;5:85. Crossref
22. Solá M, Alberro JA, Fraile M, et al. Complete axillary lymph
node dissection versus clinical follow-up in breast cancer
patients with sentinel node micrometastasis: final results
from the multicenter clinical trial AATRM 048/13/2000.
Ann Surg Oncol 2013;20:120-7. Crossref
23. Huang TW, Kuo KN, Chen KH, et al. Recommendation for
axillary lymph node dissection in women with early breast
cancer and sentinel node metastasis: a systematic review
and meta-analysis of randomized controlled trials using
the GRADE system. Int J Surg 2016;34:73-80. Crossref
24. Jagsi R, Chadha M, Moni J, et al. Radiation field design
in the ACOSOG Z0011 (Alliance) trial. J Clin Oncol
2014;32:3600-6. Crossref
25. Milgrom S, Cody H, Tan L, et al. Characteristics and
outcomes of sentinel node–positive breast cancer patients
after total mastectomy without axillary-specific treatment. Ann Surg Oncol 2012;19:3762-70. Crossref
26. FitzSullivan E, Bassett RL, Kuerer HM, et al. Outcomes
of sentinel lymph node–positive breast cancer patients
treated with mastectomy without axillary therapy. Ann
Surg Oncol 2017;24:652-9. Crossref
27. Snow R, Reyna C, Johns C, et al. Outcomes with and without
axillary node dissection for node-positive lumpectomy and
mastectomy patients. Am J Surg 2015;210:685-93. Crossref
28. Joo JH, Kim SS, Son BH, et al. Axillary lymph node
dissection does not improve post-mastectomy overall or
disease-free survival among breast cancer patients with 1-3
positive nodes. Cancer Res Treat 2019;51:1011-21. Crossref
29. Cowher MS, Grobmyer SR, Lyons J, O’Rourke C, Baynes D,
Crowe JP. Conservative axillary surgery in breast cancer
patients undergoing mastectomy: long-term results. J Am
Coll Surg 2014;218:819-24. Crossref
30. Jung J, Han W, Lee ES, et al. Retrospectively validating the
results of the ACOSOG Z0011 trial in a large Asian Z0011-eligible cohort. Breast Cancer Res Treat 2019;175:203-15. Crossref
31. Kittaka N, Tokui R, Ota C, et al. A prospective feasibility
study applying the ACOSOG Z0011 criteria to Japanese
patients with early breast cancer undergoing breast-conserving
surgery. Int J Clin Oncol 2018;23:860-6. Crossref
32. EBCTCG (Early Breast Cancer Trialists’ Collaborative
Group); McGale P, Taylor C, et al. Effect of radiotherapy
after mastectomy and axillary surgery on 10-year
recurrence and 20-year breast cancer mortality: meta-analysis
of individual patient data for 8135 women in 22
randomised trials. Lancet 2014;383:2127-35. Crossref
33. Recht A, Comen EA, Fine RE, et al. Postmastectomy
radiotherapy: an American Society of Clinical Oncology,
American Society for Radiation Oncology, and Society of
Surgical Oncology focused guideline update. Pract Radiat
Oncol 2016;6:e219-34. Crossref
34. Goyal A, Dodwell D. POSNOC: a randomised trial looking
at axillary treatment in women with one or two sentinel
nodes with macrometastases. Clin Oncol (R Coll Radiol)
2015;27:692-5. Crossref
35. van Roozendaal LM, de Wilt JH, van Dalen T, et al. The
value of completion axillary treatment in sentinel node
positive breast cancer patients undergoing a mastectomy:
a Dutch randomized controlled multicentre trial (BOOG
2013-07). BMC Cancer 2015;15:610. Crossref
36. Tinterri C, Canavese G, Gatzemeier W, et al. Sentinel
lymph node biopsy versus axillary lymph node dissection
in breast cancer patients undergoing mastectomy with one
to two metastatic sentinel lymph nodes: sub-analysis of the
SINODAR-ONE multicentre randomized clinical trial and
reopening of enrolment. Br J Surg 2023;110:1143-52. Crossref

