Hong Kong Med J 2024 Apr;30(2):120–9 | Epub 9 Apr 2024
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Exploration of clinical and ethical issues in an expanded newborn metabolic screening programme: a qualitative interview study of healthcare professionals in Hong Kong
Olivia MY Ngan, PhD1,2; Ching Janice Tam, MSc (Medical Genetics), BNurs3; CK Li, MD, FRCPCH4,5
1 Medical Ethics and Humanities Unit, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
2 Centre for Medical Ethics and Law, The University of Hong Kong, Hong Kong SAR, China
3 Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
4 Department of Paediatrics, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
5 Hong Kong Hub of Paediatric Excellence, The Chinese University of Hong Kong, Hong Kong SAR, China
Corresponding author: Dr Olivia MY Ngan (olivian1@hku.hk)
Abstract
Introduction: The Newborn Screening Programme
for Inborn Errors of Metabolism (NBSIEM) enables
early intervention and prevents premature mortality.
Residual dried bloodspots (rDBS) from the heel prick
test are a valuable resource for research. However,
there is minimal data regarding how stakeholders
in Hong Kong view the retention and secondary use
of rDBS. This study aimed to explore views of the
NBSIEM and the factors associated with retention
and secondary use of rDBS among healthcare
professionals in Hong Kong.
Methods: Between August 2021 and January 2022,
semi-structured interviews were conducted with 30
healthcare professionals in obstetrics, paediatrics,
and chemical pathology. Key themes were identified
through thematic analysis, including views towards
the current NBSIEM and the retention and secondary
use of rDBS.
Results: After implementation of the NBSIEM,
participants observed fewer patients with acute
decompensation due to undiagnosed inborn errors
of metabolism. The most frequently cited clinical
utilities were early detection and improved health
outcomes. Barriers to rDBS storage and its secondary use included uncertain value and benefits, trust
concerns, and consent issues.
Conclusion: This study highlighted healthcare
professionals’ concerns about the NBSIEM and
uncertainties regarding the handling or utilisation of
rDBS. Policymakers should consider these concerns
when establishing new guidelines.
New knowledge added by this study
- After implementation of the Newborn Screening Programme for Inborn Errors of Metabolism, participants observed fewer patients with acute decompensation due to undiagnosed inborn errors of metabolism.
- The obligation to know more about a child’s health and the drive for an altruistic contribution to science were factors supporting the retention of residual dried bloodspots (rDBS) for secondary research use.
- Uncertain value and benefits of rDBS, along with concerns regarding trust, privacy, and consent, were cited as barriers to the retention of rDBS for secondary research use.
- The retention of rDBS requires inherent trust based on public support, with strict clinical and ethical parameters.
- Concerns about privacy and consent issues related to genomic information should be addressed before next-generation sequencing is integrated into clinical care for newborns.
Introduction
Inborn errors of metabolism (IEM) are rare genetic
diseases arising from congenital deficiencies of
certain enzymes or cofactors. The accumulation
of excessive toxic substances and the absence of
essential metabolites may damage vital organs,
impair normal metabolism, or increase risks of morbidity and mortality. A small proportion of IEM
cases can be diagnosed and treated early through
dietary interventions. Patients substantially benefit
from early diagnosis and appropriate disease
monitoring.
The incidence of IEM in Hong Kong is 1 in 1682 newborns.1 In response to public health concerns, a territory-wide free voluntary Newborn
Screening Programme for IEM (NBSIEM) was
implemented for all newborns born in public
birthing units, beginning in 2017.2 This programme
covers 27 conditions, including severe combined
immunodeficiency (SCID).3 Spots of blood are
collected from newborns within 24 to 72 hours after
birth, preferably following 24 hours of milk feeding,
using a heel prick test; these samples are discarded
after hospital laboratories perform quality control
and assurance monitoring (online supplementary Table 1).
The materials on dried bloodspots provide
clinical benefits and lifelong healthcare research
opportunities that are advantageous to individuals
and the population. However, the retention and
use of residual DBS (rDBS) has led to controversies
regarding privacy, transparency, consent, misuse
of, and unauthorised access to information,
unclear research purposes, and the absence of data
management and governance protocols.4 Despite
these concerns, it is common for rDBS to be routinely
stored and used for research purposes in some
regions. For example, in Denmark, a nationwide
newborn screening programme was implemented in
1975; it currently screens for 17 diseases.5 Samples
are stored indefinitely with consent in the Danish
Newborn Screening Biobank at the State Serum
Institute.6 Similarly, a programme in the Netherlands
screens for 31 conditions.7 Although participation is
voluntary, the participation rate has reached 99.3%.8 In the Netherlands, rDBS samples are stored for
1 year to facilitate quality control. Most samples
are stored for an additional 4 years for secondary
uses, such as disease-specific biomedical research
and patient-specific diagnostic purposes, after the
acquisition of parental consent.9 The International
Society for Neonatal Screening compared national
newborn screening policies, revealing great variation
in programme acceptance, consent procedure,
storage, and length of storage.8 The Society’s findings
highlight the importance of incorporating local
views during policy development.
Two empirical studies in Hong Kong revealed
that parents were unaware of the expanded newborn
screening programme and the potential value of
rDBS.10 11 The secondary use of rDBS in medical and
health research is well-supported, mainly on the basis
of altruism. However, participation does not provide
direct individual benefits. Factors contributing to
parental support towards retention and secondary
use of rDBS include parental consent and trust
in the relevant authority. If explicit permission is
obtained, parents are more willing to contribute
their child’s rDBS card. An opt-out approach and
broad consent for unspecified use were considered
unfavourable options.11 Although multiple rDBS-focused
studies in Hong Kong have included public
stakeholders, few have assessed the attitudes of
healthcare professionals (HCPs)12; none have been
conducted since implementation of the territory-wide
screening programme. To address this gap, the
present study explored views of the NBSIEM and the
factors associated with retention and secondary use
of rDBS cards among HCPs in Hong Kong.
Methods
Sampling and recruitment
Semi-structured interviews were conducted among
30 HCPs in obstetrics, paediatrics, and chemical
pathology practising in eight public and two private
institutions in Hong Kong between August 2021 and
January 2022. Purposeful sampling was used to select
key stakeholders involved in the NBSIEM according
to disciplines and responsibilities. Initial invitations
were sent through the two local medical universities
(ie, The University of Hong Kong and The Chinese
University of Hong Kong) and associated hospitals.
Referrals via snowballing were also performed to
recruit additional participants.
The study inclusion criteria were academics
and HCPs (eg, medical, nursing, and laboratory
staff) involved in IEM-related research or clinical
work. Individuals who did not meet the criteria were
excluded. Overall, this study recruited participants
who were involved in recruitment and counselling
within the NBSIEM, laboratory data analysis and
interpretation, or academic research related to IEM.
Each participant received a detailed
description of the research. All participants gave
written informed consent before taking part in the
study. The second author conducted the interviews
at locations convenient for participants, such as
meeting rooms, offices, and coffee shops. The
interview length ranged from 37 to 71 minutes.
Upon completion of the interview, each participant
received a supermarket voucher for HK$200.
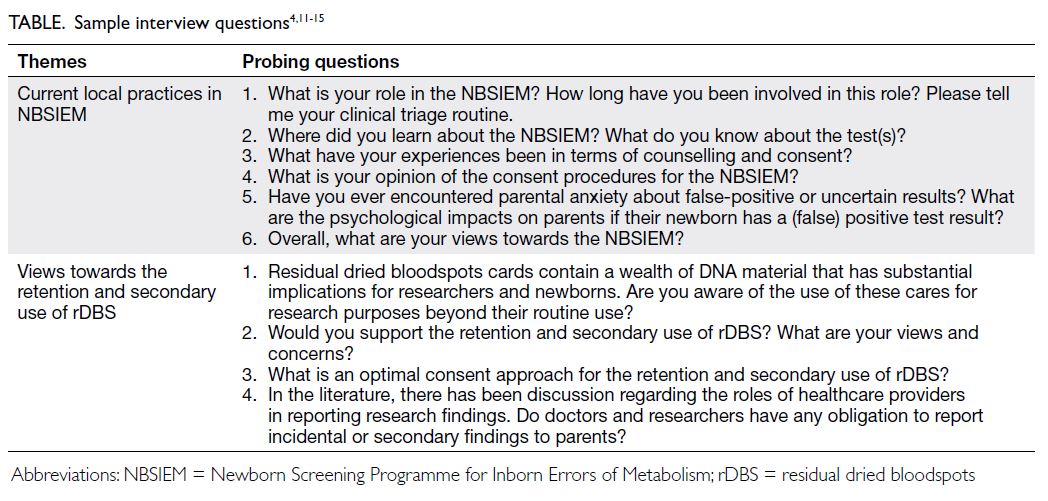
Interview guide
A semi-structured topic guide was developed based
on existing literature concerning newborn screening
and ethical considerations (Table).4 11 12 13 14 15 Prior to
data collection, the guide was reviewed by a senior
paediatrician to ensure its content validity, relevance,
clarity, and cultural sensitivity.
Data analysis
Interviews were digitally recorded, transcribed
verbatim in the original language (Cantonese),
and then translated into English. Transcripts
were anonymised and assigned an identification
code. The interviewer and another member of the
research team reviewed the transcription accuracy.
Two independent researchers read and coded
the transcripts and audio recordings via thematic
analysis, in which textual data were coded and labelled
in an inductive manner. New thematic codes that did
not fit into predetermined categories were created
and refined, as necessary. Codes were compared
and discussed among research members until a
consensus was reached. Reflexivity was maintained
during the discussion and data analysis process. The
entire research team identified emerging themes
from the early and intermediate stages of interviews,
then recruited HCPs to represent each new theme until theoretical saturation was achieved (ie, no new
themes emerged in the discussions and existing
themes were consistently observed).
Results
Interviewee characteristics
Thirty HCPs were recruited and interviewed. The
study sample was diverse. Among the interviewees,
15 (50.0%) were doctors and 13 (43.3%) were nurses
or midwives; 13 (43.3%) worked in obstetrics and
gynaecology and 13 (43.3%) worked in paediatrics;
28 worked in the public sector (93.3%); and 14
(46.7%) had >10 years of clinical experience (online supplementary Table 2). Two major thematic themes
were identified, namely, views towards the current
NBSIEM and views towards the retention and
secondary use of rDBS. Illustrative quotations are
used to support the themes.
Theme 1: views towards the current newborn
screening programme
Perceived clinical utility
The implementation of the NBSIEM was a public
health achievement, and interviewees showed a
positive attitude towards the programme. Early
detection and improved health outcomes were the
most commonly reported clinical utility outcomes
(n=21, 70.0%). Some interviewees, primarily
paediatric HCPs, noted a difference between
the periods before and after territory-wide test
implementation. Before NBSIEM, it was not
uncommon for doctors working in intensive care
units to encounter cases of acute decompensation
due to undiagnosed IEM. Considering the broad
phenotypes involved in IEM, such conditions may
not be recognised in early stages.
‘I have been working in the ward for ages and
observed that many patients with IEM deteriorated
to an irreversible stage. With this NBSIEM, we
can screen out IEM cases and offer treatment. The
patients achieve normal development like others.
In other words, the screening helped many people.’
(Interview 25, paediatric nurse)
Affected families previously endured a long
wait for diagnosis before the NBSIEM; there was a
substantial psychological burden involved. Parental
distress was observed.
‘From hospital admission to disease diagnosis,
it takes 2 weeks. The whole process involved hospital
transfer from the (suspected IEM clinic) to the
specialised team at (Hong Kong) Children’s Hospital,
conducting blood investigation, and making a
diagnosis.’ (Interview 20, paediatric doctor)
Some limitations were noted, including false-positive
and false-negative results, as well as call-back
rates. Interviewees cited the reduction of
recall caseloads as an advantage associated with
implementation of second-tier tests.
‘It is best if we can eliminate the false-positives.
To achieve this purpose, we implement aggressive
second-tier testing. Our pathologists also make
stringent interpretations. Without pathologists
working in laboratories, clinicians can only draw
reference from the pre-set levels.’ (Interview 30,
pathology doctor)
Screening panel
The programme covers 27 conditions, including
SCID.3 Interviewees generally agreed that the
benefits of screening for lethal IEM conditions
outweigh the costs of screening, despite the very low
incidence of IEMs. When asked about their views
on the current panel, interviewees emphasised that
disease selection must be based on public health
principles, supported by Wilson and Jungner’s
screening criteria.16 Diseases in the panel should be
treatable and have a high prevalence in Hong Kong.
‘I support the NBSIEM. I believe (the experts)
came up with the 27 conditions based on robust
considerations, including incidence and prevalence,
availability of treatment, mortality prevention, cost-effectiveness,
etc. It is beneficial to patients.’ (Interview
13, obstetrics and gynaecology doctor)
Healthcare professionals have encountered
parents who have completed the publicly funded
IEM test and selected an additional private IEM test
solely based on the number of conditions. For such
parents, the underlying motivation is that ‘screening
more conditions is perceived to be more definitive’.
If I were a mother with a child, I would like to
know whether my child was affected by these diseases.
The more conditions the panel includes, the better.
One can prevent the onset of disease. Parents are
helpless when diseases occur suddenly.’ (Interview 24, obstetrics and gynaecology doctor)
‘Some mothers compared the list of conditions
between the public and private sectors. I am talking
about the difference between 26 and 30 conditions,
respectively. They would rather pay out of pocket and
send the baby to retake the test in the private sector.’
(Interview 16, obstetrics and gynaecology academic)
One paediatrician questioned whether a
genetic test with a larger number of conditions
contributes to enhanced parental control and
confidence regarding the newborn’s health. She was
aware of some urine tests available in the direct-to-consumer
market that screen for so-called ‘non-diseases’—short-/branched-chain acyl-coenzyme A
dehydrogenase and 3-methylcrotonyl-coenzyme A
carboxylase deficiency—although such conditions
do not require follow-up. She highlighted the
importance of periodically reviewing conditions
on the panel according to locality-specific factors,
including disease prevalence, clinical sensitivity and
specificity, treatment, and cost-effectiveness.
‘Running an analysis on a rDBS card is not
difficult. What is more challenging is the post-analysis
follow-up. Compared with other NBSIEMs,
the United States screens for the greatest number
of conditions, maybe 40, while the United Kingdom
screens for five conditions. Instead of adding
conditions, should we also consider taking out some
(non-disease) conditions from the list?’ (Interview 27,
paediatric doctor)
Source of information
Three HCPs (10.0%) reported that most Hospital
Authority staff received NBSIEM information
through departmental seminars and training
sessions, which prepared them to complete the
consent procedure with parents. When asked about
their understanding of IEMs, knowledge levels varied
among frontline staff involved in the NBSIEM. Some
were uncertain what the test evaluated.
‘What is it (NBSIEM) testing for…? Is it
checking for chromosomal defects? Or is it checking
for lack of (metabolites)?’ (Interview 15, obstetrics
and gynaecology nurse)
Some also mistakenly thought that the NBSIEM analysed genes.
‘It is a filter paper with some dried bloodspots,
testing IEM genes.’ (Interview 29, obstetrics and
gynaecology doctor)
Some frontline staff wanted additional
information beyond the procedure. They felt
unprepared for questions about the diseases,
symptoms, test procedures, and care for patients
with IEMs. They felt anxious or uncomfortable
explaining these aspects to parents with some level
of understanding.
‘After implementing this programme, what the
patients will undergo, where they will be referred to, what to do with a confirmed diagnosis… to be honest,
I learned everything from the protocol. In actual
settings, parents asked many practical questions,
such as “what to be cautious about during daily
care”—I do not know how to answer them. These are
not common diseases observed in the ward, but we
are asked to counsel parents.’ (Interview 24, obstetrics
and gynaecology doctor)
A senior doctor responsible for providing
educational seminars noted that training should
not be an isolated event; periodic refresher training
should be provided.
‘We provide intensive training for nurses, all of
them. (In the training), we give clear explanations,
conduct videotaping (for review), and address
inquiries and questions. In addition, we also plan
to host refresher courses every few years. Hong Kong
requires more observation before moving forward.’
(Interview 27, paediatric doctor)
Experience with parental counselling and consent
procedure
Interviewees felt that the educational pamphlet and
consent material are easy to read. During parental
counselling, HCPs were prepared to answer parents’
enquiries. Most parents supported the NBSIEM.
Parents wanted to identify IEM conditions in their
newborns because they felt that early detection
could facilitate autonomous decision-making related
to their child and other family members.
‘I observed that most parents enrol in the
NBSIEM as they would like to know sooner if their
babies are affected. If a diagnosis is confirmed, we run
a genetic test to predict the risk of recurrence. Only a
few refuse to take part in the programme.’ (Interview
23, paediatric doctor)
Midwives played an important role in obtaining
parental consent in antenatal clinics. Refusals of
the current NBSIEM are infrequent. Five frontline
staff (16.7%) involved in the recruitment process
observed that only a small number of parents, who
were sceptical about medical interventions or had
religious affiliation–based reservations, declined to
join the programme.
‘Some people who advocate minimal
medicalisation do not consent to procedures in our
hospital. For example, they refuse vaccinations and
vitamin K injections.’ (Interview 10, obstetrics and
gynaecology nurse)
Participants involved in recruitment
recognised that informed consent procedures
were intended to enable parents to make informed
choices. The current opt-in consent approach allows
HCPs to obtain explicit permission from parents.
The participants observed that parents, especially
Hong Kong Chinese individuals, were vocal about
the patient’s right to know.
‘Nowadays, patients put a strong emphasis on patient’s rights, thinking that “you need my consent before carrying out a procedure".’ (Interview 5,
obstetrics and gynaecology nurse)
In particular, six HCPs (20.0%) speculated that
opt-in consent was more accepted by parents and
thus easier to obtain. It provided parents with a sense
of personal control by allowing them to give explicit
permission. Opt-in consent has been used in many
medical settings. It is more familiar to and accepted
by community members with respect to studies of
genetic material.
‘Must I choose a consent model for handling
genetic materials? It would be an opt-in approach.’
(Interview 6, obstetrics and gynaecology doctor)
Seven HCPs (23%) felt that consent was needed because of the invasiveness of the procedure, but some HCPs felt that the procedure involved minimal harm.
‘(The phlebotomists) perform an invasive
procedure on the infant, which may cause discomfort
or pain. Opt-in is preferable to an opt-out approach.
(Interview 27, paediatric doctor)
‘It is just a heel prick test and will not affect the
baby. I cannot see the downsides (of the screening).’
(Interview 16, obstetrics and gynaecology academic)
Opt-in consent was perceived to be more
efficient. There may be opposition to an opt-out
approach. Some HCPs (n=6, 20.0%) felt that an
opt-out approach would increase sample sizes and
contribute to advances in medical research.
‘Inborn errors of metabolism would be a
prevalent issue, and therefore, opt-out is better than the
opt-in approach. Like an human immunodeficiency
virus test with an opt-out approach, one can refuse to
take the test for a valid reason. There are treatments
for IEMs; opt-out is a desirable consent model.’
(Interview 24, obstetrics and gynaecology doctor)
One doctor pondered the adoption of different
methods in obtaining informed consent because
opt-in and opt-out approaches are ‘like two sides
of the same coin’. He stated that the key aspect of
selecting an appropriate consent approach is the
parental counselling process. He also emphasised
that the consent procedure is not absent from the
opt-out approach and that effective communication
remains important.
Disclosure of confirmed results of inborn errors of
metabolism to affected families
When a confirmed IEM diagnosis was disclosed to an
affected family, parents often felt shocked, stressed,
and guilty about having an ‘abnormal’ baby. They
then began to explore the financial implications; for
example, some worried about treatment costs and
uncertainties. Counselling is limited to discussing
the diagnosis; it also includes psychological support
through follow-up care involving a multidisciplinary
team.
‘Many families were worried when they heard
about the IEM diagnosis, as they knew it was a life-long
condition. It is tough to handle (bad news). Their
child will be different from other peers, and finances
will be affected. They must self-finance the drugs.’
(Interview 28, paediatric nurse)
On some occasions, the NBSIEM is beneficial
to the newborn and has implications for the entire
family. Some interviewees reported disclosing an
IEM result relevant to the mother, rather than the
infant. In one case, the HCP informed the involved
family members and provided follow-up care for the
newborn’s siblings.
‘Sometimes, a secondary finding is related to
the mother instead of the child. When maternal blood
contamination is present (the baby is not affected),
the mother is referred to relevant specialists for
medical follow-up.’ (Interview 23, paediatric doctor)
‘We had a positive result for citrullinemia
deficiency. The newborn had three brothers and a
sister with the same disease. Now we are following
them.’ (Interview 27, paediatric doctor)
Theme 2: acceptance of retention and secondary use of residual dried bloodspots
Motivations for storage
Interviewees were asked about their views of rDBS
storage. Healthcare professionals supported the
long-term storage of rDBS through the NBSIEM to
facilitate advances in public health epidemiology,
forensic purposes, familial disease analysis, and
development of other screening tests. Some HCPs
highlighted the importance of rDBS in supporting
scientific advances. Stored rDBS could be used to
enhance healthcare management and clinical testing,
such as establishment of local reference standards.
‘We did not know how to define the cut-off values
at first. Within the United States, the cut-off values
differ by state. The initial cut-off values we chose may
not reflect local needs. Residual dried bloodspots
(storage) is essential to develop a large data pool that
supports a control pool when technology advances.’
(Interview 19, pathology doctor)
Healthcare professionals observed that
most parents demonstrated substantial interest in
knowledge about their children. They thought that
parents would like to have the right to obtain medical
information regarding their children.
‘I believe that most parents would agree to save
(their) genetic material...perhaps... they would not
mind if the laboratory preserved the DNA material
and let them know the findings of future screening
tests.’ (Interview 17, paediatric doctor)
Barriers to storage
Uncertain value of retention
Four HCPs (13.3%) were worried that the public lacked an understanding of how rDBS could generate
knowledge. This lack of awareness may be linked to
an unwillingness among parents to permit the use of
their children’s rDBS samples.
‘Many laypeople may not understand why
they should engage in research studies. They may be
reluctant to take part in research studies due to their
own beliefs.’ (Interview 3, obstetrics and gynaecology
nurse)
Two-fifths of HCPs (n=12) questioned the need for the long-term storage programme.
‘Several ongoing studies on population
genetics use a wide-consent approach, supported by
government funding. Does every newborn have to
provide data (to support this research)? I doubt it.’
(Interview 18, paediatric doctor)
‘With strong opposition, I dissent to the storage
(of rDBS) as I see no value at all. Perhaps it offers
convenience for research, but it provides no personal
benefits.’ (Interview 30, pathology doctor)
Interviewees believed that genetic material is
very stable and does not easily degrade, despite long
storage periods. Although storage is possible, some
concerns were raised about its cost-effectiveness.
‘I heard researchers (scientists) mention that
proper sample storage incurs a considerable cost.’
(Interview 13, obstetrics and gynaecology doctor)
No direct benefit to patients or parents
Around one-fourth of HCPs (n=7, 23.3%) would
only support clinical research if the findings could
be used to help their children and patients. Parents
were not expected to be interested in research,
especially if it did not provide direct clinical benefit
to their children.
‘Parents care about whether the disease can
be treated or not. Knowledge of disease aetiology is
only relevant to public health or research institutes.’
(Interview 2, obstetrics and gynaecology nurse)
‘Would I receive the data if I donated a sample?
I would donate a sample if the researcher would
return the data. I must know every single conclusion
or diagnosis from data generated using the rDBS. I
would refuse if no data were returned.’ (Interview 13,
obstetrics and gynaecology doctor)
Trust and privacy concerns regarding responsible
authorities
Another recurring theme was trust in the context of primary privacy concerns, such as data leakage
and misuse of private information generated from
sensitive genetic materials.
‘It may not be desirable to store (genetic
materials) for a long time. The longer it is stored, the
more concerns arise. Immediate disposal would be
more reassuring in terms of the protection of privacy.’
(Interview 12, paediatric nurse)
‘Some people may steal genetic information
(rDBS cards) for illegal (or unauthorised) purposes.’
(Interview 12, paediatric nurse)
Obligation to return research findings
Generally, around 30% of the interviewed doctors and
researchers (n=9) believed they have a duty to warn
research participants upon finding abnormalities,
enabling parents to take appropriate action after
receiving relevant test results, including secondary
findings.
There is an obligation to inform the patients
(of medically actionable findings) because we work in
this profession. First, we do no harm. If a significant
finding warrants medical attention, we should be
responsive and responsible.’ (Interview 24, obstetrics
and gynaecology doctor)
Issues with obtaining consent for storage purposes
The importance of consent was acknowledged, but
there was disagreement concerning the need for
broad or specific consent. Interviewees frequently
noted that broad consent is convenient for
researchers.
‘Our understanding of IEMs or diagnostic tests
increases as time goes by. The advantage of broad
consent is that we do not need to obtain consent
when new technology evolves. Like the recently added
SCID, we do not need to redesign or implement a new
consent procedure again when adding new conditions
to the panel.’ (Interview 9, paediatric doctor)
Despite the view that broad consent may permit
more efficient use of biospecimens and relevant data,
there were concerns about public acceptance. Some
interviewees stated that it would be challenging to
obtain consent for all future research and explain the
need for a change in consent approach.
‘Broad consent implies uncertainty in the
research scope, which leads to parental concern.
Parents are uncertain how the rDBS will be used
or handled. I feel uneasy during counselling. I am
not sure how their blood will be used in a research
project, but in short, it will be helpful.’ (Interview 7,
paediatric doctor)
‘Broad consent entails an unknown. As such,
parents might be unwilling to sign the consent form
(and contribute the rDBS).’ (Interview 27, paediatric doctor)
For HCPs, the legitimacy and scope of consent
are key considerations. Specific consent is commonly
exercised in clinical or research procedures in Hong
Kong. It is recognised as the most appropriate
procedure because it ensures patients receive
information about the study. A few interviewees
mentioned that no existing framework recommends
the use of broad consent; thus, they favoured the use
of specific consent.
‘Specific consent may not provide sufficient coverage of all possible research. If there is a breach
in the protocol, it may bring about ethical and legal
issues.’ (Interview 26, obstetrics and gynaecology doctor)
‘I found that specific consents were more
protective for HCPs.’ (Interview 15, obstetrics and
gynaecology nurse)
‘I have never sought ethical approval for broad
consent from institutional research boards.’ (Interview
28, paediatric nurse)
The level of public knowledge regarding
the NBSIEM requires further analysis. Education
and counselling might be intended to address
problems that arise from long-term storage. One
doctor emphasised that proper counselling on tests
involving genetic material should be considered best
practice. Some interviewees expressed a desire to
prepare themselves to address parents’ concerns.
‘The drawbacks of the NBSIEM should be
discussed, apart from privacy and personal genetic
information. Parents should be aware that there
are many unknowns in genetics. (As medical
professionals) we have, of course, fewer concerns.
Suppose I have to conduct genetic counselling for an
IEM test. In that case, I will cover all the aspects,
including the basic understanding of genetics, even if
it is a selected target gene panel. I do not see much
difference in terms of counselling across all forms of
genetic tests.’ (Interview 9, paediatric doctor)
Discussion
This study explored the voices of HCPs from
various backgrounds and discussed clinical and
ethical issues during the early implementation
phase of the NBSIEM. Similar to professionals in
the United Kingdom,13 HCPs in Hong Kong did not
exhibit extensive knowledge and awareness of IEM
conditions, which may have detrimental effects
on patient-centred care. First, parental autonomy
might be undermined because parents are not
adequately informed about the test procedure
and conditions. Second, a lack of understanding
regarding IEMs can lead to suboptimal clinical care.
Children with IEMs attend multiple specialist clinics
to manage multiple co-morbidities. Caregivers
encounter difficulties, such as miscommunication
or inconsistent information about medications or
dietary restrictions, when attending non–IEM-specific
clinics.14 They face numerous psychosocial
challenges in caring for their children,15 17 and
increased awareness of these stressors among
healthcare providers could improve communication
for the entire family. More than four-fifths of
individuals in Hong Kong attend medical services
at public hospitals,18 and many parents are expected
to participate in the NBSIEM. The establishment
of training or educational interventions and
a centralised pipeline to coordinate care are essential considerations for patient-centred care
that focuses on caregivers of children with IEMs.
Because hospitals are expanding screening for other
uncommon disorders, such as SCID,19 the results of
the present study may inform the development of a
family-oriented framework for IEM management.
Development of the current NBSIEM was
based on a stringent infrastructure and second-tier
testing pipeline.20 Samples with borderline or
ambiguous results were sent for further genetic tests
to confirm the diagnosis and carrier status. Carnitine
deficiency, citrin deficiency, methylmalonic
acidaemia, and glutaric aciduria type I are examples
of diseases with relatively high incidences of
false-positives or false-negatives.1 21 After the
implementation of stringent second-tier tests, the
recall rate has declined to 0.3% to 0.4%, similar to
the standards of international IEM programmes.22
This work has been successful, and the retention of
rDBS to create a large-scale genetic biobank will be
the next focus of public health dialogue.
It is important to note that territory-wide
biobanks are not common; biobank platforms in
Hong Kong currently are operated by individual
hospitals or institutions. A notable example is
Children of 1997, a population-based birth cohort
study of local infants.23 Other existing platforms
include disease-oriented biobanks,24 which support
quality assurance and conduct epidemiology studies;
they also identify risk factors, novel molecular
markers, and genetic variants associated with diabetes
and related complications. The establishment of
biobanks at separate institutions has led to non-standardised
informed consent practices. Many
ethical and legal issues remain unresolved in efforts
to harmonise all regional biobanks. Public awareness
of the value of rDBS has been low11; improvements
in public acceptance and engagement are needed for
broad support of rDBS storage or biobanks.
The present study highlighted common ethical,
legal, and social concerns as barriers to the storage of
rDBS. Trust and low awareness of the potential value
of rDBS were cited as primary barriers. In contrast
to the assumptions of HCPs, parents generally agree
with academic researchers and doctors accessing their
children’s rDBS and health data after explicit consent
has been provided.11 The optimal consent model
for the use of rDBS outside of screening purposes
depends on cultural and social characteristics that
vary among regions. In the past three decades, some
countries have stored rDBS without consent, leading
to public controversy and lawsuits.25 Considering
these situations, the retention of rDBS requires
inherent trust based on public support, with strict
clinical and ethical parameters. Essential factors
in establishing trust are consent to participate in
the NBSIEM, as well as consent for rDBS retention
and secondary uses; questions remain regarding the optimal approach to obtaining consent.25 Other
factors involved in decision-making concerning
rDBS retention and secondary uses include timing of
consent, adequate communication and discussion of
potential uses, protection of privacy, and responsible
governance.9 11 26 These factors should be considered
in public policy initiatives.
Concerns about privacy issues and
discrimination related to genomic information
will be amplified as next-generation sequencing
is integrated into clinical care for newborns.27 28 In
South Korea, next-generation DNA sequencing
has been evaluated for use in primary newborn
screening.29 In the United Kingdom, Genomics
England plans to offer whole-genome sequencing to
newborns, identifying actionable genetic conditions
that may impact infants in early childhood.30
There is evidence that sequencing data provides
information about conditions not currently assessed
in newborns, as well as information with unclear
clinical significance.29 The previous regime agreed
that it was appropriate to disclose incidental research
findings if they would directly benefit the child after
considering risk and benefit. However, this approach
may differ in cultural and social settings when
considering the child’s future and non-therapeutic
genomic information.
Limitations and strengths of the study
Most participants in this study were HCPs working
in the public sector; they may have different views
regarding clinical utility, value, and perceived cost-benefit,
compared with stakeholders from other
healthcare settings. However, this study used various
sampling strategies to recruit a heterogeneous
group of HCPs with diverse specialities, roles, and
responsibilities in the screening programme, as well
as years of experience. A longitudinal study would
provide long-term insights concerning the NBSIEM.
Knowledge of heterogeneous IEMs and perception
of rDBS storage among HCPs could be analysed via
quantitative methods.
Conclusion
This study highlighted HCPs’ concerns about the
NBSIEM and uncertainties regarding the handling
or utilisation of rDBS. Policymakers should consider
these concerns when establishing new guidelines.
Future investigations should explore parents’
experiences with screening for rare metabolic
conditions and communication of positive results.
Author contributions
Concept or design: OMY Ngan.
Acquisition of data: CJ Tam.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: OMY Ngan, CJ Tam.
Critical revision of the manuscript for important intellectual content: CK Li.
Acquisition of data: CJ Tam.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: OMY Ngan, CJ Tam.
Critical revision of the manuscript for important intellectual content: CK Li.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors declared no conflicts of interest.
Acknowledgement
The authors thank all healthcare professionals who participated in the study.
Funding/support
This research was supported by the Direct Grant for Research
from the Faculty of Medicine at The Chinese University of
Hong Kong (2020/2021) [Ref No.: 2020.081]. The funder had
no role in study design, data collection/analysis/interpretation
or manuscript preparation.
Ethics approval
The research was approved by the Survey and Behavioural
Research Ethics Committee of The Chinese University of
Hong Kong (Ref No.: SBRE-20-846). All participants provided
written consent for interview and publication of the study.
Supplementary material
The supplementary material was provided by the authors and
some information may not have been peer reviewed. Any
opinions or recommendations discussed are solely those of the
author(s) and are not endorsed by the Hong Kong Academy
of Medicine and the Hong Kong Medical Association. The
Hong Kong Academy of Medicine and the Hong Kong Medical
Association disclaim all liability and responsibility arising from
any reliance placed on the content.
References
1. The Task Force on the Pilot Study of Newborn Screening for
Inborn Errors of Metabolism. Evaluation of the 18-month
“Pilot Study of Newborn Screening for Inborn Errors of
Metabolism” in Hong Kong. Hong Kong J Paediatr (new
series) 2020;25:16-22.
2. Belaramani KM, Chan TC, Hau EW, et al. Expanded
newborn screening for inborn errors of metabolism in
Hong Kong: results and outcome of a 7-year journey. Int J
Neonatal Screen 2024;10:23. Crossref
3. Hospital Authority. Newborn Screening Programme
for Inborn Errors of Metabolism (IEM). October 2023.
Available from: https://www21.ha.org.hk/smartpatient/SPW/MediaLibraries/SPW/SPWMedia/Education-pamphlet_NBS-IEM_Eng-(Oct-2023).pdf?ext=.pdf. Accessed 27 Mar 2024.
4. Ngan OM, Li CK. Ethical issues of dried blood spot
storage and its secondary use after newborn screening
programme in Hong Kong. Hong Kong J Paediatr (new
series) 2020;25:8-15.
5. Lund A, Wibrand F, Skogstrand K, et al. Danish expanded
newborn screening is a successful preventive public health
programme. Dan Med J 2020;67:A06190341.
6. Nordfalk F, Ekstrøm CT. Newborn dried blood spot
samples in Denmark: the hidden figures of secondary use
and research participation. Eur J Hum Genet 2019;27:203-10. Crossref
7. Jansen ME, Klein AW, Buitenhuis EC, Rodenburg W,
Cornel MC. Expanded neonatal bloodspot screening
programmes: an evaluation framework to discuss new
conditions with stakeholders. Front Pediatr 2021;9:635353. Crossref
8. Loeber JG, Platis D, Zetterström RH, et al. Neonatal
screening in Europe revisited: an ISNS perspective on the
current state and developments since 2010. Int J Neonatal
Screen 2021;7:15. Crossref
9. Jansen ME, van den Bosch LJ, Hendriks MJ, et al. Parental
perspectives on retention and secondary use of neonatal
dried bloodspots: a Dutch mixed methods study. BMC
Pediatr 2019;19:230. Crossref
10. Mak CM, Lam CW, Law CY, et al. Parental attitudes on
expanded newborn screening in Hong Kong. Public Health
2012;126:954-9. Crossref
11. Hui LL, Nelson EA, Deng HB, et al. The view of Hong Kong
parents on secondary use of dried blood spots in newborn
screening program. BMC Med Ethics 2022;23:105. Crossref
12. Mak CM, Law EC, Lee HH, et al. The first pilot study
of expanded newborn screening for inborn errors of
metabolism and survey of related knowledge and opinions
of health care professionals in Hong Kong. Hong Kong
Med J 2018;24:226-37. Crossref
13. Moody L, Atkinson L, Kehal I, Bonham JR. Healthcare
professionals’ and parents’ experiences of the confirmatory
testing period: a qualitative study of the UK expanded
newborn screening pilot. BMC Pediatr 2017;17:121. Crossref
14. Siddiq S, Wilson BJ, Graham ID, et al. Experiences of
caregivers of children with inherited metabolic diseases: a
qualitative study. Orphanet J Rare Dis 2016;11:168. Crossref
15. Zeltner NA, Landolt MA, Baumgartner MR, et al. Living
with intoxication-type inborn errors of metabolism: a
qualitative analysis of interviews with paediatric patients
and their parents. JIMD Rep 2017;31:1-9. Crossref
16. Petros M. Revisiting the Wilson–Jungner criteria: how
can supplemental criteria guide public health in the era of
genetic screening? Genet Med 2012;14:129-34. Crossref
17. Grant S, Cross E, Wraith JE, et al. Parental social support,
coping strategies, resilience factors, stress, anxiety and
depression levels in parents of children with MPS III
(Sanfilippo syndrome) or children with intellectual
disabilities (ID). J Inherit Metab Dis 2013;36:281-91. Crossref
18. Hospital Authority. Hospital Authority Strategic Plan 2022-2027: Towards Sustainable Healthcare. Available from: https://www.ha.org.hk/haho/ho/ap/HA_StrategicPlan2022-2027_Eng_211216.pdf. Accessed 16 Dec 2021.
19. Leung D, Lee PP, Lau YL. Review of a decade of international
experiences in severe combined immunodeficiency
newborn screening using T-cell receptor excision circle.
Hong Kong J Paediatr (new series) 2020;25:30-41.
20. Tsang KY, Chan TC, Yeung MC, Wong TK, Lau WT,
Mak CM. Validation of amplicon-based next generation
sequencing panel for second-tier test in newborn screening
for inborn errors of metabolism. J Lab Med 2021;45:267-74. Crossref
21. Hennermann JB, Roloff S, Gellermann J, Grüters A, Klein J.
False-positive newborn screening mimicking glutaric
aciduria type I in infants with renal insufficiency. J Inherit
Metab Dis 2009;32 Suppl 1:S355-9. Crossref
22. la Marca G. Mass spectrometry in clinical chemistry: the case of newborn screening. J Pharm Biomed Anal 2014;101:174-82. Crossref
23. Schooling CM, Hui LL, Ho LM, Lam TH, Leung GM. Cohort profile: ‘Children of 1997’: a Hong Kong Chinese birth cohort. Int J Epidemiol 2012;41:611-20. Crossref
24. Ng IH, Cheung KK, Yau TT, Chow E, Ozaki R, Chan JC.
Evolution of diabetes care in Hong Kong: from the Hong
Kong Diabetes Register to JADE-PEARL Program to
RAMP and PEP Program. Endocrinol Metab (Seoul)
2018;33:17-32. Crossref
25. Cunningham S, O’Doherty KC, Sénécal K, Secko D,
Avard D. Public concerns regarding the storage and
secondary uses of residual newborn bloodspots: an
analysis of print media, legal cases, and public engagement
activities. J Community Genet 2015;6:117-28. Crossref
26. Hendrix KS, Meslin EM, Carroll AE, Downs SM. Attitudes about the use of newborn dried blood spots for research: a survey of underrepresented parents. Acad Pediatr
2013;13:451-7. Crossref
27. Pereira S, Robinson JO, Gutierrez AM, et al. Perceived
benefits, risks, and utility of newborn genomic sequencing
in the BabySeq project. Pediatrics 2019;143(Suppl 1):S6-13. Crossref
28. Johnston J, Lantos JD, Goldenberg A, et al. Sequencing
newborns: a call for nuanced use of genomic technologies.
Hastings Cent Rep 2018;48 Suppl 2:S2-6. Crossref
29. Woerner AC, Gallagher RC, Vockley J, Adhikari AN. The
use of whole-genome and exome sequencing for newborn
screening: challenges and opportunities for population
health. Front Pediatr 2021;9:663752. Crossref
30. Biesecker LG, Green ED, Manolio T, Solomon BD, Curtis D.
Should all babies have their genome sequenced at birth?
BMJ 2021;375:n2679. Crossref