© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Detection of significant liver fibrosis in Chinese
psoriasis patients receiving methotrexate: a comparison between transient elastography and liver histology
Christina SM Wong, FRCP (Edin), FHKAM (Medicine)# 1; Loey LY Mak, MD, FRCP (Glasg)# 2; Victor KH Lee, FRCR, FHKAM (Radiology)3; Regina CL Lo, MD, FHKAM (Pathology)4; Martin MH Chung, MRCP, FHKAM (Medicine)1; Ferdinand Chu, FRCR, FACLM5,6; CK Yeung, MD, FRCP1; MF Yuen, PhD, FRCP2; Henry HL Chan, MD, PhD1
1 Division of Dermatology, Department of Medicine, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
2 Division of Gastroenterology and Hepatology, Department of Medicine, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
3 Imaging and Interventional Radiology Centre, CUHK Medical Centre, Hong Kong SAR, China
4 Department of Pathology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
5 Department of Radiology, Queen Mary Hospital, Hong Kong SAR, China
6 St Vincent’s Hospital, Sydney, Australia
# Equal contribution
Corresponding author: Prof Henry HL Chan (hhlchan@hku.hk)
Abstract
Introduction: Methotrexate (MTX) is effective
for treating psoriasis and psoriatic arthritis, but
its potential hepatoxicity remains a concern. Liver
biopsy, the gold standard for detecting MTX-induced
liver injury, is invasive and carries considerable risk.
Transient elastography (TE) offers a non-invasive
alternative for detecting advanced liver fibrosis. This
study investigated the performance of TE in detecting
MTX-induced liver fibrosis among Chinese psoriasis
patients, compared with liver biopsy.
Methods: This study included adult patients with
clinical psoriasis. Liver stiffness measurement using
TE was performed in patients receiving MTX.
Exclusion criteria were known liver cirrhosis,
positive viral hepatitis carrier status, or conditions
influencing TE performance. Liver biopsy was
performed when liver stiffness was ≥7.1 kilopascals
(kPa) or when the total cumulative dose (TCD) of
MTX was ≥3.5 g.
Results: A total of 228 patients were screened;
among 34 patients who met the inclusion criteria,
nine (26.5%) had significant liver fibrosis (Roenigk
grade ≥3a). The area under the receiver operating
characteristic curve was 0.76 (95% confidence
interval=0.59-0.93; P=0.021), indicating that TE had
satisfactory performance in detecting liver fibrosis.
A cut-off value of 7.1 kPa of liver stiffness yielded
100% sensitivity and 68% specificity. Liver fibrosis
was not correlated with the TCD of MTX or the
duration of MTX use; it was significantly correlated
with obesity and diabetes status (body mass index ≥30 kg/m2, waist circumference ≥138 cm, and
glycated haemoglobin level ≥7.8%).
Conclusions: Transient elastography is reliable and
superior to the TCD for detecting liver fibrosis in
Chinese psoriasis patients receiving MTX. Liver
biopsy should be reserved for high-risk patients or
patients with liver stiffness ≥11.7 kPa on TE.
New knowledge added by this study
- Transient elastography (TE) exhibits satisfactory performance in the detection of methotrexate (MTX)-induced liver fibrosis among Chinese psoriasis patients receiving MTX.
- Although current guidelines state that liver biopsy should be considered if the total cumulative dose of MTX is ≥3.5 g, it is shown that the dose was not correlated with the degree of liver fibrosis in Chinese psoriasis patients. Importantly, liver stiffness (LS) measurement by TE is a more appropriate method for monitoring MTXinduced liver fibrosis.
- Chinese patients with obesity who exhibit a high body mass index and large abdominal circumference have a higher risk of liver fibrosis and should be closely monitored when receiving MTX.
- Transient elastography can be used to monitor MTX-induced liver fibrosis, whereas liver biopsy should be reserved for high-risk psoriasis patients.
- Yearly TE monitoring is recommended for patients with LS ≥7.1 kilopascals (kPa), while liver biopsy should be considered for patients with LS ≥11.7 kPa.
Introduction
Methotrexate (MTX) is an effective
immunosuppressive drug for moderate to severe
psoriasis and psoriatic arthritis.1 2 It is considered
relatively safe and cost-effective for long-term use.
However, prolonged used of MTX can potentially
cause liver fibrosis and fatty changes without
alterations in liver enzymes. The mechanism of
MTX-induced liver fibrosis is suspected to involve
the production of extracellular adenosine, a pro-fibrotic
agent.3
Although liver fibrosis can be evaluated by
imaging or biochemical parameters, liver biopsy
remains the gold standard for diagnosing liver
fibrosis.4 However, it has limitations such as sampling
error and poor feasibility for serial assessments.
The rates of liver biopsy–associated morbidity and
mortality are approximately 1% and 0.01% to 0.1%,
respectively.5 6 The Roenigk scale is generally used to
grade MTX-induced liver fibrosis,7 but other systems
(eg, Ishak and METAVIR) have also been used for
fibrosis assessment.8 9
The measurement of serum level of specific
biomarkers such as the amino terminal of type III
procollagen peptide can be used as alternative
methods to assess liver fibrosis.10 11 12 13 However, these
measurements are not widely available in many
regions. Therefore, a sensitive and specific non-invasive
technique is required to detect MTX-induced
liver injury.
Transient elastography (TE) is a non-invasive
method for assessing liver ‘hardness’ or stiffness that
involves measuring the velocity of a vibration wave
(ie, a shear wave) when travelling to a particular
depth inside the liver, based on the principle that
the wave velocity is greater in fibrotic tissue than in
normal liver tissue. This technique has been used
as a screening tool for liver cirrhosis in various
conditions.14 15 16 17 In contrast to liver biopsy, TE allows
the simultaneous assessment of a larger sampling
area, is easily reproducible, and has low inter-observer
variability and a low failure rate (<5%).15
Generally, a liver stiffness (LS) of ≤5 kilopascals
(kPa) suggests a low probability of fibrosis, whereas
a value of ≥7 kPa suggests a high likelihood of
advanced fibrosis in patients with various chronic
liver disorders.14 15 16 17 18 Thus far, there are limited data
regarding TE accuracy and cut-off threshold in
terms of detecting MTX-induced liver injury among
Chinese psoriasis patients.
This study aimed to evaluate the performance
(reliability) of TE in detecting significant liver
fibrosis among psoriasis patients in the Chinese
population, compared with gold-standard liver
biopsy assessment using the Roenigk classification.
Methods
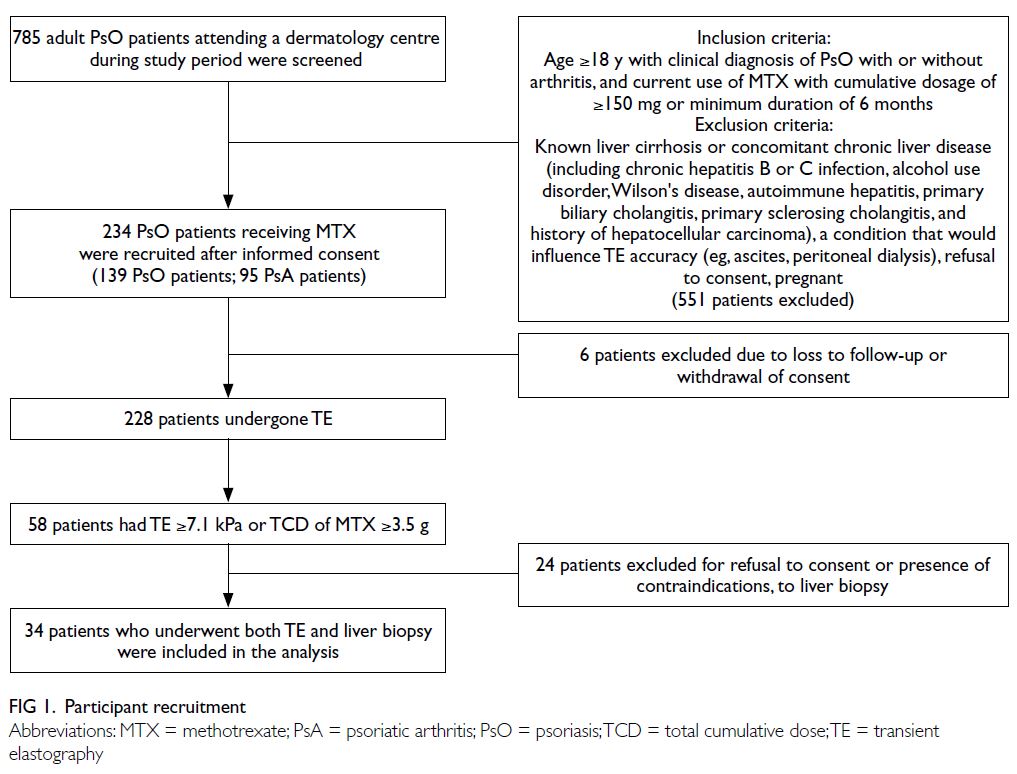
This cross-sectional single-centre study was conducted from 1 December 2019 to 31 March 2021.
Patients with psoriasis and/or psoriatic arthritis
undergoing regular follow-up in the dermatological
clinic at a major tertiary hospital in Hong Kong
were consecutively screened for inclusion (Fig 1).
The inclusion criteria were as follows: age ≥18 years,
clinical diagnosis of psoriasis with or without arthritis,
and current use of MTX with a cumulative dosage of
≥150 mg or minimum duration of 6 months. Patients
were excluded if they had known liver cirrhosis or
concomitant chronic liver disease (including chronic
hepatitis B or C infection, alcohol use disorder,
Wilson’s disease, autoimmune hepatitis, primary
biliary cholangitis, primary sclerosing cholangitis,
and a history of hepatocellular carcinoma), had a
condition that could influence TE accuracy (eg,
ascites, severe renal disease, peritoneal dialysis,
or severe cardiovascular insufficiency),14 15 16 17 18 refused
to give consent, were pregnant, or had not been
receiving MTX. After the acquisition of informed
consent, all participants provided a thorough history
and underwent a comprehensive examination.
Clinical and laboratory data
Upon recruitment, clinical and metabolic measurements, including body weight, body mass
index (BMI), systolic and diastolic blood pressure,
and waist circumference, were recorded. The
duration and cumulative dosage of MTX treatment,
disease severity (measured by the Psoriasis Area
and Severity Index), and body surface area (BSA)
were documented. Laboratory parameters assessed
included hepatitis B/C infection status, complete
blood count, serum fasting glucose level, glycated
haemoglobin (HbA1c) level, triglyceride level, low- and
high-density lipoprotein cholesterol levels, liver
enzyme levels (eg, aspartate aminotransferase and
alanine aminotransferase), and renal function. Patient
demographic data, co-morbidities (hypertension,
diabetes, cardiovascular disease, chronic liver/renal
disease, and alcoholic liver disease), and concomitant
medications were collected from electronic medical
records.
Liver stiffness measurement by transient elastography
Transient elastography assessments were conducted
within 1 month after recruitment. Liver stiffness
was evaluated using the FibroScan ultrasonic
imaging device (Echosens, Paris, France) and
expressed as the median value (in kPa) after at
least 10 successful acquisitions. Measurements were considered reliable if the success rate was
≥60%, combined with an interquartile range (IQR)
of ≤30%.15 16 For patients with a BMI of <30 kg/m2,
an M probe was used; for those with a BMI of
≥30 kg/m2, an XL probe was used. The controlled
attenuation parameter (CAP) was also recorded to
estimate liver steatosis, expressed in decibels per
metre (dB/m).16 The investigators (senior research
assistants trained to perform TE) were blinded to the
patients’ clinical characteristics, MTX dosage, and
previous liver ultrasound or biopsy results. Patients
were instructed to fast for 4 hours prior to LS
measurements. In previous studies involving Asian
populations, significant liver fibrosis or cirrhosis was
indicated by LS values of ≥7.1 kPa19 and >14.0 kPa,20
respectively. Therefore, in our study, significant liver
fibrosis on TE was defined as LS ≥7.1 kPa.
Liver biopsy
A liver biopsy (to assess the degree of liver fibrosis
by histology) was performed within 3 months after
TE in patients who had significant liver fibrosis
(ie, LS ≥7.1 kPa on TE) or a total cumulative dose
(TCD) of MTX ≥3.5 g, with or without additional
risk factors. This protocol was adopted based on
international guidelines1 2 18 19 20 21 22 and previous studies
in Asian populations.21 22 Ultrasound-guided core-needle liver biopsy procedures were performed by
an experienced radiologist using a percutaneous
approach.5 6
Histological assessments were conducted
by a pathologist with expertise in hepatobiliary
disorders, who was blinded to the TE results. The
Roenigk scale was used to grade liver fibrosis/cirrhosis, defined as grade 1 (no fibrosis, no or mild
fatty infiltration, no or mild nuclear variability, and
no or mild portal inflammation), grade 2 (no fibrosis,
but moderate to severe fatty infiltration, nuclear
pleomorphism, and portal inflammation), grade 3a
(mild fibrosis, moderate to severe fatty infiltration,
portal tract enlargement, and lobular necrosis),
grade 3b (moderate to severe fibrosis), and grade 4
(cirrhosis).7 For patients with MTX-induced liver
injury (Roenigk grade 3a), MTX could be continued,
with follow-up liver biopsy repeated in 6 months.
For those with significant liver fibrosis or cirrhosis
(Roenigk grades 3b or 4), MTX was discontinued
and alternative treatment was initiated.2
Data analysis and statistics
For statistical analysis, continuous variables were
expressed as median (range or IQR, as specified) or
mean (± standard deviation) values, as appropriate.
Receiver operating characteristic (ROC) curves were
used to determine the predictive ability of TE-based
LS relative to histopathology (Roenigk grade ≥3a),
with 95% confidence intervals (CIs). Correlations
between two variables were calculated using Pearson
or Spearman rank correlation coefficients. The Chi
squared test or Fisher’s exact test was used for
comparisons of categorical variables, as appropriate.
Quantitative variables were subjected to normality
assessment via the Shapiro–Wilk test; non–normally
distributed variables were compared between
groups using the Wilcoxon rank-sum test. Statistical
analyses were conducted using SPSS software
(Windows version 26.0; IBM Corp, Armonk [NY],
United States). P values <0.05 were considered
statistically significant.
Results
Baseline characteristics
In total, 785 psoriasis patients were recruited into the
study; 234 fulfilled the screening criteria and were
included in the analysis. Six patients subsequently
withdrew their consent (Fig 1).
Among 228 patients who underwent TE
evaluation, 140 were diagnosed with psoriasis and
88 were diagnosed with psoriatic arthritis (Table 1). Fifty-eight patients, who either had LS ≥7.1 kPa
or received a TCD of MTX ≥3.5 g, were advised to
undergo liver biopsy; 24 patients who refused or
had contraindications to liver biopsy were excluded
from the study (Fig 1). Thus, 34 patients (24 fulfilling the TE criteria and 15 fulfilling the TCD criteria of
MTX) who had undergone both TE and liver biopsies
were included in further analyses. The clinical and
demographic details of the study participants are
summarised in Table 1.
Among the 34 patients, the median values of
LS and CAP were 8.80 kPa (IQR, 7.1-12.4; range,
3.5-30.4) and 321.0 dB/m (IQR, 262-357; range,
200-400), respectively. Furthermore, 24 patients
(70.6%) had a high LS value (ie, ≥7.1 kPa, indicative
of significant fibrosis); 24 patients (70.6%) had a CAP
value of ≥268 dB/m, indicative of moderate to severe
steatosis.
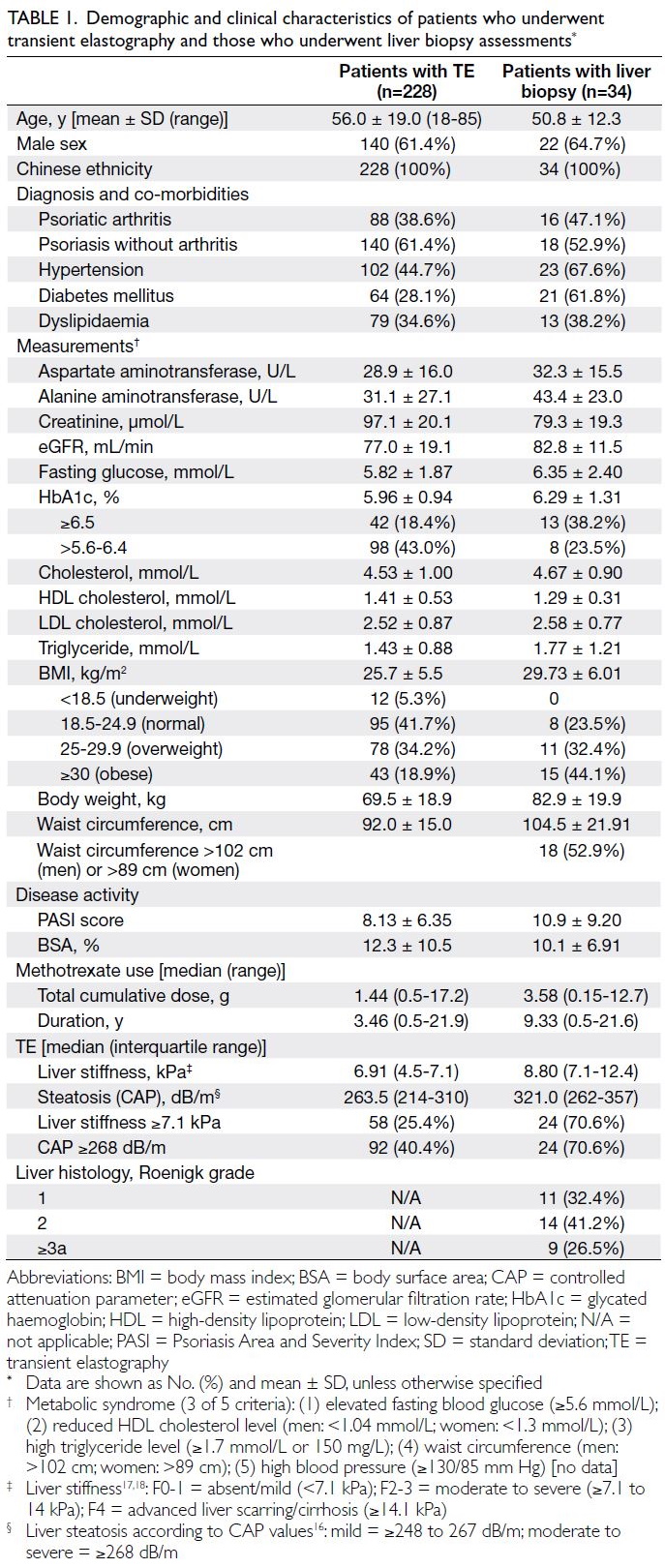
Table 1. Demographic and clinical characteristics of patients who underwent transient elastography and those who underwent liver biopsy assessments
Histology evaluation revealed liver fibrosis in
nine of 34 (26.5%) patients: six had Roenigk grade 3a,
three had Roenigk grade 3b, and none had Roenigk
grade 4 (cirrhosis).
Correlations of liver stiffness with clinical
and laboratory parameters
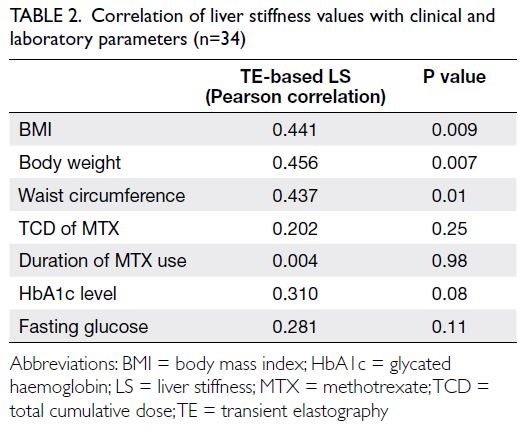
Liver stiffness measurements via TE showed moderate correlations with BMI (r=0.441; P=0.009),
waist circumference (r=0.437; P=0.01), and body
weight (r=0.456; P=0.007); there were no correlations
with the TCD of MTX or duration of MTX use (Table 2).
Patients with LS ≥7.1 kPa had a higher
BMI (P=0.01), body weight (P=0.01), and waist
circumference (P=0.03). They also exhibited greater
disease severity with a higher Psoriasis Area and
Severity Index score (P=0.02) and more extensive
BSA involvement (P=0.03). Furthermore, patients
with LS ≥7.1 kPa had higher fasting glucose (P=0.03)
and HbA1c levels (P=0.02), but they did not show
significant differences in serum lipid levels (Table 3).
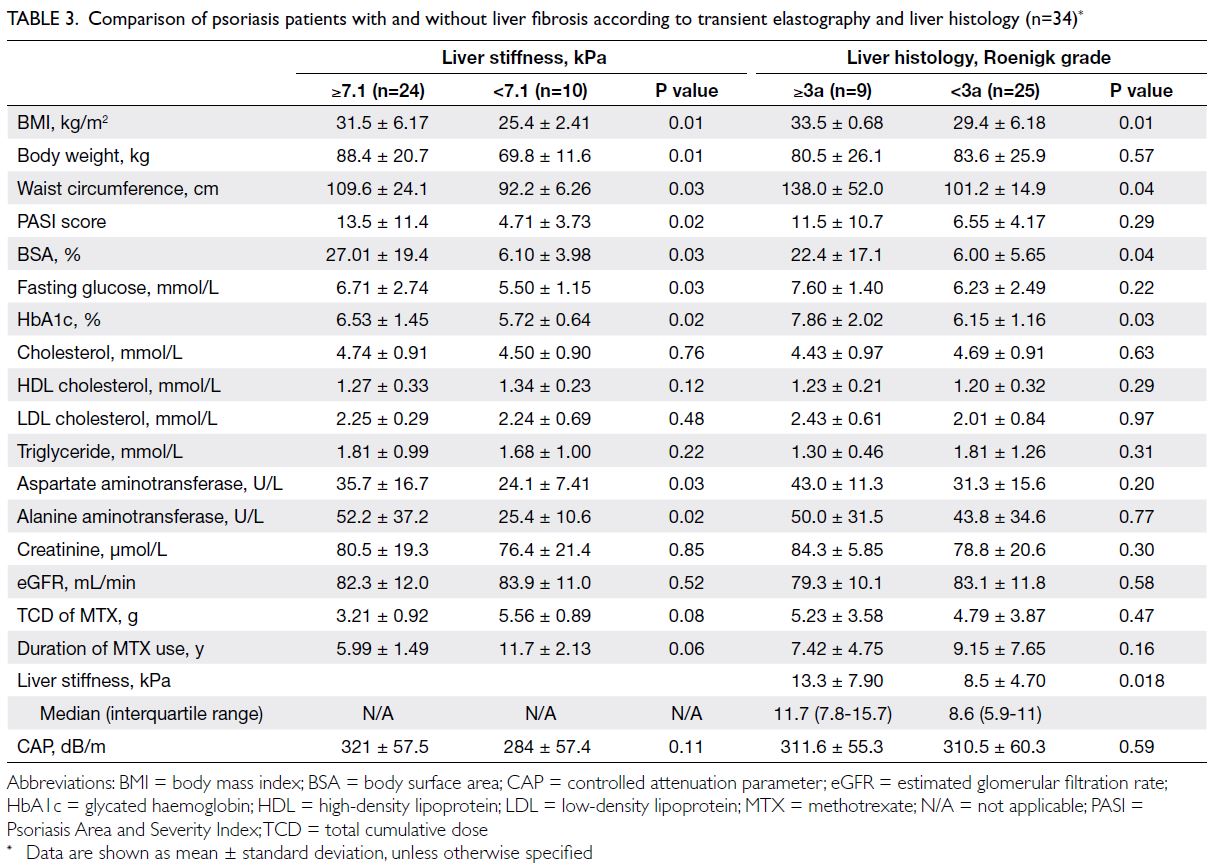
Table 3. Comparison of psoriasis patients with and without liver fibrosis according to transient elastography and liver histology (n=34)
In terms of histology findings, patients with
a Roenigk grade ≥3a had significantly greater
BMI (P=0.01), waist circumference (P=0.04), BSA
(P=0.04), and HbA1c values (P=0.03), compared
with those who exhibited lower stages of histological fibrosis. Notably, there were no significant differences
in the TCD of MTX, lipid profiles, or liver and renal
biochemistries between the two groups. Patients
with Roenigk grade ≥3a (LS: 11.7 kPa; range, 7.8-15.7) had higher LS values than those with Roenigk
grade <3a (LS: 8.6 kPa; range, 5.9-11) [P=0.018]
(Table 3).
Performance characteristics of transient
elastography for diagnosing significant/advanced liver fibrosis
Comparison of TE-based LS values (in kPa) with
histopathology revealed an area under the ROC
curve of 0.76 (95% CI=0.59-0.93; P=0.021) [Fig 2],
which demonstrated the satisfactory performance
of TE in detecting significant liver fibrosis. An LS
cut-off value of 7.1 kPa yielded 100% sensitivity and
68% specificity for diagnosing Roenigk grade ≥3a.
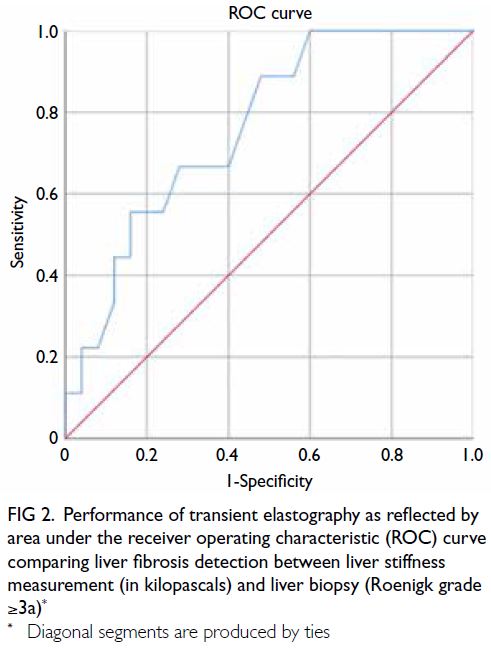
Fig 2. Performance of transient elastography as reflected by area under the receiver operating characteristic (ROC) curve comparing liver fibrosis detection between liver stiffness measurement (in kilopascals) and liver biopsy (Roenigk grade ≥3a)
In a subgroup analysis of patients with LS
≥7.1 kPa, excluding patients who only met the TCD
criteria of MTX (n=24), the area under the ROC
curve with reference to Roenigk grade ≥3a was
0.702 (95% CI=0.47-0.94); an LS cut-off value of
10.7 kPa yielded 86% sensitivity and 59% specificity.
In contrast, when ROC analysis was focused on the
subgroup of patients with a TCD of MTX ≥3.5 g
(n=14, excluding patients who only met the TE
criteria), the area under the ROC curve was 0.622
(95% CI=0.31-0.94); an LS cut-off value of 8.2 g
yielded 60% sensitivity and 67% specificity.
Discussion
Use of transient elastography to monitor
liver fibrosis in psoriasis patients receiving methotrexate
International guidelines recommend using TE for
routine monitoring of MTX therapy.2 4 According to
the Australasian position statement, TE monitoring
is recommended every 3 years if initial LS is
<7.5 kPa and yearly if LS is ≥7.5 kPa; liver biopsy is
recommended if LS is >9.5 kPa.23
Currently, there is no guideline for monitoring
MTX-induced liver fibrosis among Chinese psoriasis
patients in Hong Kong. On the basis of previous
studies,2 4 23 the present study utilised a modified
approach that reflects our department’s routine
practice of conducting liver biopsy for patients who
have TE-based LS ≥7.1 kPa or a TCD of MTX ≥3.5 g,
with or without abnormal liver biochemistry.
Our study confirmed the robust performance
of TE in detecting significant liver fibrosis among
Chinese psoriasis patients receiving MTX; the LS
cut-off value of 7.1 kPa yielded 100% sensitivity
and 68% specificity. These findings are consistent
with a recent review and studies in other countries
(Table 4).7 19 20 According to these published data, TE
demonstrated fair to good performance in detecting liver fibrosis, with high negative predictive values
(NPVs) [83% to 96%] but generally low positive
predictive values (PPVs),19 20 21 22 23 24 which might be
explained by the overall low prevalence of significant
liver fibrosis in this population and the higher rate
of TE failure among patients with obesity. Obesity
might also influence diagnostic performance; a
recent review noted that obesity could substantially
reduce TE accuracy.19 Lee et al25 showed that,
compared with control participants, independent
risk factors for liver fibrosis included diabetes
mellitus (odds ratio [OR]=30.4), obesity with high
BMI (OR=8.3), and overweight (OR=6.3). Our results
supported previous observations that TE-based LS
measurements were not correlated with the TCD
of MTX, although they were associated with BMI,
diabetes mellitus, and obesity.19 24 Rongngern et al19
analysed 41 Asian psoriasis patients receiving MTX;
they demonstrated that TE had good performance
in detecting MTX-induced liver injury, with an area
under the ROC curve of 0.78.19
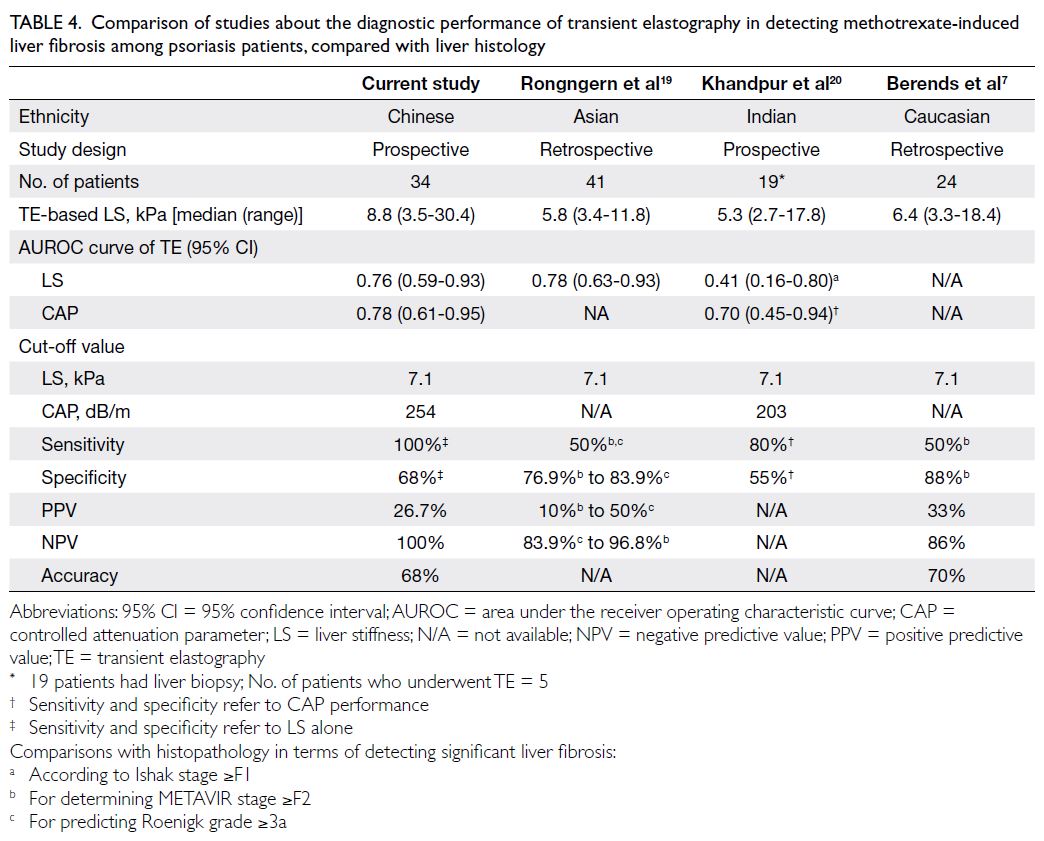
Table 4. Comparison of studies about the diagnostic performance of transient elastography in detecting methotrexate-induced liver fibrosis among psoriasis patients, compared with liver histology
In this same study,19 using an LS cut-off
value of 7.1 kPa, for detecting MTX-induced liver
injury, defined as Roenigk grade ≥3a, TE provided a sensitivity and specificity of 50% and 83.9%,
respectively, and a PPV of 50% and a NPV of 84%;
in addition, the use of TE values ≥7.1 yielded 50%
sensitivity and 76.9% specificity for detecting
significant liver fibrosis, defined as METAVIR stage
≥F2; and giving a PPV of 10% and a NPV of 96.8%.
Similarly, our study showed a PPV of 26.7% and a
NPV of 100%. In the study of 53 psoriasis patients
receiving MTX, Khandpur et al20 identified median
LS values of 5.3 kPA (range, 2.7-17.8); TE could only
detect 4 (21%) of 19 patients with liver fibrosis (Ishak
stage ≥F1).20
Because the median LS for our patients with
significant liver fibrosis (Roenigk grade ≥3a) was
11.7 kPa (IQR, 7.8-15.7), we recommend yearly
TE monitoring for patients with LS ≥7.1 kPa. Liver
biopsy should be considered for patients with LS
≥11.7 kPa, instead of a TCD ≥3.5 g or LS ≥7.1 kPa;
earlier biopsy is suggested for high-risk patients
(eg, patients with high BMI, abdominal obesity, or
diabetes mellitus).
Associations of body mass index, abdominal
obesity, and glycated haemoglobin level with liver fibrosis
The median LS in this study (n=228) was 6.91 kPa (IQR, 4.5-7.1). Among the 34 patients who
underwent liver biopsy, the median LS was 8.80 kPa
(IQR, 7.1-12.4). Overall, 9 of 34 patients (26.5%) had
Roenigk grade 3a (mild)/3b (moderate to severe) liver
fibrosis, whereas 14 (41.2%) patients had moderate
to severe fatty infiltration (Roenigk grade 2) [Table 1]. The prevalences of liver fibrosis and steatotic
changes in our study were higher than the rates
reported in a 2015 review, where histology showed
that only 5% of patients (range, 0%-33%) receiving
MTX had developed significant liver fibrosis.14
Impacts of body mass index and coexisting non-alcoholic steatohepatitis or fatty liver disease on liver stiffness measurement
The aetiology of liver fibrosis can be multifactorial.
Previous studies have shown that obesity, combined
with other metabolic risk factors, is associated with
liver fibrosis in non-alcoholic fatty liver disease
(NAFLD) patients and psoriasis patients.26 27 28 The
potential contributions of coexisting non-alcoholic
steatohepatitis or NAFLD and metabolic syndrome
to liver fibrosis have been suggested. In our cohort,
>40% of psoriasis patients had a CAP of ≥268 dB/m,
indicative of moderate to severe steatosis. The
liver fibrosis could be attributed to coexisting non-alcoholic steatohepatitis or NAFLD, in addition to
MTX-induced changes. The work of Wong et al26
demonstrated that NAFLD patients with BMI
≥30 kg/m2 had higher LS compared with normal
healthy individuals. Our findings corroborate these
observations; we found that BMI, body weight, and
waist circumference were moderately correlated
with TE-based LS measurements (all r>0.40; all
P<0.05) [Table 2]. Intriguingly, although 70.6% of
our biopsy cohort had moderate to severe hepatic
steatosis (Table 1), CAP values were not significantly
associated with LS >7.1 kPa or Roenigk grade ≥3a
(Table 3). Therefore, the adverse effect of BMI on LS cannot be explained by concomitant hepatic
steatosis alone.
In our study, patients with clinically
significant liver fibrosis more frequently displayed
characteristics of metabolic syndrome compared
with patients lacking histologically confirmed liver
fibrosis, as evidenced by significantly greater BMI
(33.5 ± 0.68 kg/m2 vs 29.4 ± 6.18 kg/m2; P=0.01), waist circumference (138.0 ± 52.0 cm vs 101.2 ±
14.9 cm; P=0.04), and HbA1c values (7.86 ± 2.02% vs
6.15 ± 1.16%; P=0.03) [Table 3]. Sub-analysis showed
that all patients with histologically confirmed liver
fibrosis had a BMI ≥25 kg/m2 (Table 5), suggesting
that psoriasis patients should maintain a normal
BMI to decrease the risk of liver fibrosis.
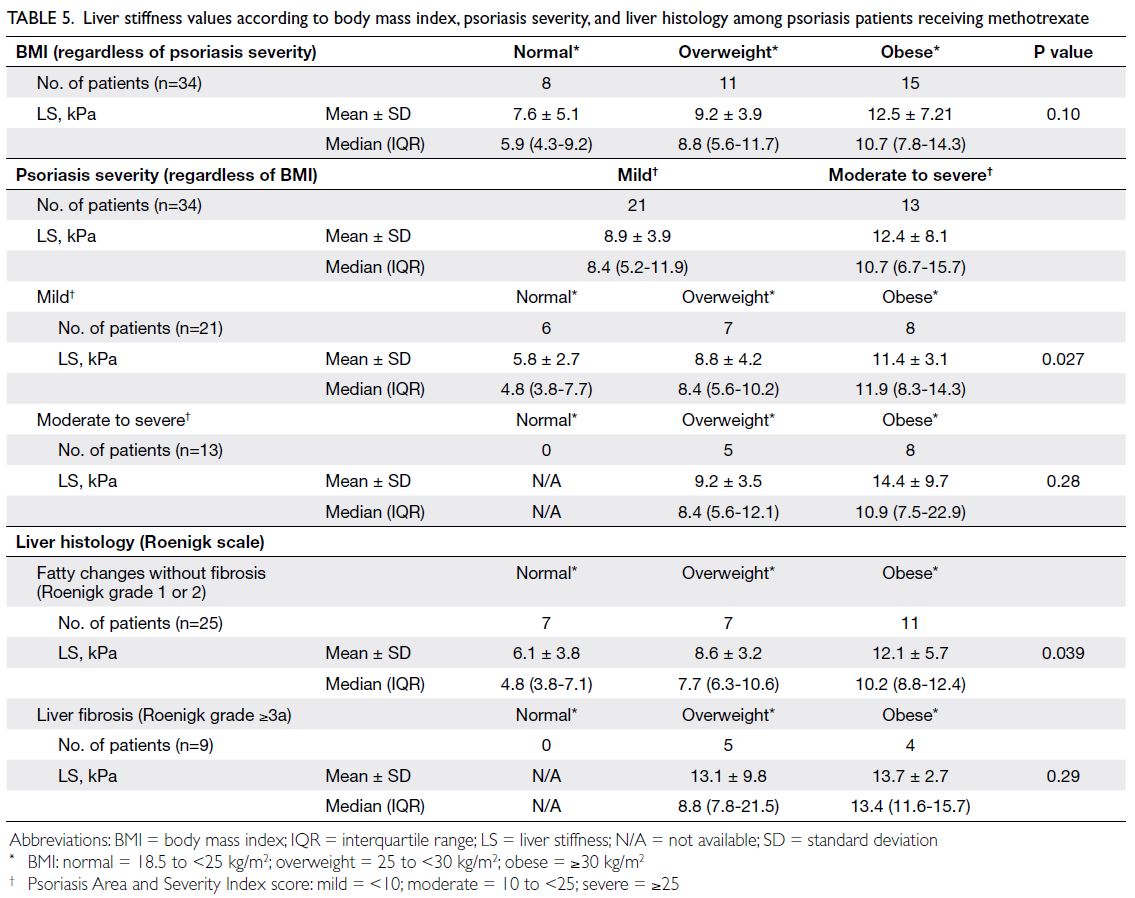
Table 5. Liver stiffness values according to body mass index, psoriasis severity, and liver histology among psoriasis patients receiving methotrexate
Role of transient elastography: liver stiffness measurements to guide liver biopsy considerations
Despite the recommendation to perform a screening
liver biopsy for exclusion of possible liver cirrhosis
among patients receiving long-term MTX therapy
(with a TCD >3.5 g),1 2 our study did not identify
a positive correlation between significant liver
fibrosis and the TCD of MTX. Similarly, a study
involving 420 patients with inflammatory arthritis
receiving MTX revealed no significant correlation
between cumulative MTX dosage and TE-based
LS measurements.24 In the present study, although the TCD of MTX was higher in patients with liver
fibrosis (5.23 g vs 4.79 g in those without), the
difference was not statistically significant. In this
context, LS measurements may be superior to the
TCD of MTX when considering the need for liver
biopsy to rule out advanced liver fibrosis.
Contrary to concerns about hepatotoxicity
during long-term MTX therapy, we did not find
a correlation between LS values and the duration
of MTX use. The duration of MTX use in patients
without liver fibrosis was nearly 10 years, indicating
that the drug is generally well-tolerated in psoriasis
patients. Patients who tended to continue MTX
treatment belonged to an MTX-responsive group
without significant adverse events, such as liver
derangement identified via blood tests. In contrast,
patients experiencing liver derangement or other
adverse events might have discontinued MTX
treatment earlier and were thus excluded from
the study. Although we excluded patients with
chronic hepatitis B, TE-based LS measurement
is a widely validated non-invasive tool for real-world
assessments of liver fibrosis in such patients;
it allows the prediction of advanced fibrosis and
disease progression.29 Therefore, TE should also be
considered a valuable tool in guiding treatment for
psoriasis patients with chronic hepatitis B who are
receiving MTX.
In addition to LS measurement, TE can assess
the degree of steatosis through CAP. This assessment
was beneficial among patients with psoriasis in
the present study; 26.5% (9 of 34) of the patients
had metabolic syndrome and were predisposed to
concomitant hepatic steatosis, regardless of MTX
use. The median CAP value in our study was 321 ±
95 dB/m (range, 200-400). The area under the ROC
curve of TE was 0.783 (95% CI=0.61-0.95; P<0.01);
the cut-off of 254 dB/m for detecting steatosis
yielded 91% sensitivity and 60% specificity. However,
the presence of simple hepatic steatosis alone does
not warrant liver biopsy, and management decisions
should follow appropriate clinical guidelines.30 31 32
Limitations
This study had some limitations. Notably, its sample
size was small. Although liver fibrosis is generally
uncommon in patients with psoriasis receiving MTX
therapy, a larger sample size may be required for
more definitive conclusions. Additionally, sampling
error and inter- and intra-observer variabilities in
histological assessment of liver tissue may have
influenced the findings.
Conclusion
Transient elastography is a reliable screening tool
for detecting significant liver fibrosis in Chinese
psoriasis patients receiving MTX. When considering liver biopsy to rule out the possibility of clinically
significant liver fibrosis, TE-based LS measurements
provide superior reference information, compared
with the TCD of MTX. Patients with high BMI,
body weight, and abdominal obesity have a higher
risk of liver fibrosis. Therefore, these factors should
be considered when monitoring MTX-related liver
fibrosis in psoriasis patients.
Author contributions
Concept or design: CSM Wong, LLY Mak, CK Yeung, HHL Chan, MF Yuen.
Acquisition of data: CSM Wong, MMH Chung, LLY Mak.
Analysis or interpretation of data: CSM Wong, MMH Chung, LLY Mak.
Drafting of the manuscript: CSM Wong, LLY Mak, F Chu, VKH Lee, RCL Lo.
Critical revision of the manuscript for important intellectual content: CK Yeung, HHL Chan, MF Yuen.
Acquisition of data: CSM Wong, MMH Chung, LLY Mak.
Analysis or interpretation of data: CSM Wong, MMH Chung, LLY Mak.
Drafting of the manuscript: CSM Wong, LLY Mak, F Chu, VKH Lee, RCL Lo.
Critical revision of the manuscript for important intellectual content: CK Yeung, HHL Chan, MF Yuen.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
The authors thank Ms Rachael Yu, Ms Davis Wong, and Ms
Ivy Cheng from Division of Dermatology, Department of
Medicine, School of Clinical Medicine, Li Ka Shing Faculty
of Medicine, The University of Hong Kong for their assistance
with patient recruitment, data acquisition, and analysis.
Declaration
The preliminary data of this research were presented as ePoster
presentation in the 31st European Academy of Dermatology
and Venereology Congress 2022 (7-10 September 2022,
Milan, Italy and online).
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the Institutional Review Board of
The University of Hong Kong and Hong Kong West Cluster,
Hospital Authority, Hong Kong (Ref No.: UW19-390) and was
conducted in full compliance with the International Council
for Harmonisation E6 guideline for Good Clinical Practice
and the principles of the Declaration of Helsinki. Patient
consent has been obtained for all clinical information and
images reported in this article. All participant information has
been deidentified and remains anonymous.
References
1. Kalb RE, Strober B, Weinstein G, Lebwohl M. Methotrexate
and psoriasis: 2009 National Psoriasis Foundation
Consensus Conference. J Am Acad Dermatol 2009;60:824-37. Crossref
2. Warren RB, Weatherhead SC, Smith CH, et al. British
Association of Dermatologists’ guidelines for the safe and
effective prescribing of methotrexate for skin disease 2016.
Br J Dermatol 2016;175:23-44. Crossref
3. Themido R, Loureiro M, Pecegueiro M, Brandão M,
Campos MC. Methotrexate hepatotoxicity in psoriatic
patients submitted to long-term therapy. Acta Derm
Venereol 1992;72:361-4. Crossref
4. Kremer JM, Alarcón GS, Lightfoot RW Jr, et al.
Methotrexate for rheumatoid arthritis. Suggested
guidelines for monitoring liver toxicity. American College
of Rheumatology. Arthritis Rheum 1994;37:316-28. Crossref
5. Howlett DC, Drinkwater KJ, Lawrence D, Barter S,
Nicholson T. Findings of the UK national audit evaluating
image-guided or image-assisted liver biopsy. Part II. Minor
and major complications and procedure-related mortality.
Radiology 2013;266:226-35. Crossref
6. Regev A, Berho M, Jeffers LJ, et al. Sampling error and
intraobserver variation in liver biopsy in patients with
chronic HCV infection. Am J Gastroenterol 2002;97:2614-8. Crossref
7. Berends MA, Snoek J, de Jong EM, et al. Biochemical and
biophysical assessment of MTX-induced liver fibrosis in
psoriasis patients: FibroTest predicts the presence and
FibroScan predicts the absence of significant liver fibrosis.
Liver Int 2007;27:639-45. Crossref
8. Ishak K, Baptista A, Bianchi L, et al. Histological grading
and staging of chronic hepatitis. J Hepatol 1995;22:696-9. Crossref
9. Goodman ZD. Grading and staging systems for
inflammation and fibrosis in chronic liver diseases. J
Hepatol 2007;47:598-607. Crossref
10. Carneiro SC, Cássia FF, Lamy F, Chagas VL, Ramos-e-Silva M. Methotrexate and liver function: a study of 13 psoriasis cases treated with different cumulative dosages.
J Eur Acad Dermatol Venereol 2008;22:25-9. Crossref
11. Chou R, Wasson N. Blood tests to diagnose fibrosis or
cirrhosis in patients with chronic hepatitis C virus infection:
a systematic review. Ann Intern Med 2013;158:807-20. Crossref
12. Fung J, Lai CL, Fong DY, Yuen JC, Wong DK, Yuen MF.
Correlation of liver biochemistry with liver stiffness in
chronic hepatitis B and development of a predictive model
for liver fibrosis. Liver Int 2008;28:1408-16. Crossref
13. Martyn-Simmons CL, Rosenberg WM, Cross R, Wong T,
Smith CH, Barker JN. Validity of noninvasive markers
of methotrexate-induced hepatotoxicity: a retrospective
cohort study. Br J Dermatol 2014;171:267-73. Crossref
14. Brener S. Transient elastography for assessment of liver
fibrosis and steatosis: an evidence-based analysis. Ont
Health Technol Assess Ser 2015;15:1-45.
15. Foucher J, Castéra L, Bernard PH, et al. Prevalence
and factors associated with failure of liver stiffness
measurement using FibroScan in a prospective study
of 2114 examinations. Eur J Gastroenterol Hepatol
2006;18:411-2. Crossref
16. Karlas T, Petroff D, Sasso M, et al. Individual patient data
meta-analysis of controlled attenuation parameter (CAP)
technology for assessing steatosis. J Hepatol 2017;66:1022-30. Crossref
17. Castéra L, Vergniol J, Foucher J, et al. Prospective
comparison of transient elastography, Fibrotest, APRI,
and liver biopsy for the assessment of fibrosis in chronic
hepatitis C. Gastroenterology 2005;128:343-50. Crossref
18. Friedrich-Rust M, Ong MF, Martens S, et al. Performance
of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology 2008;134:960-74. Crossref
19. Rongngern P, Chularojanamontri L, Wongpraparut C,
et al. Diagnostic performance of transient elastography
for detection of methotrexate-induced liver injury using
Roenigk classification in Asian patients with psoriasis: a
retrospective study. Arch Dermatol Res 2017;309:403-8. Crossref
20. Khandpur S, Yadav D, Jangid B, et al. Ultrasound liver
elastography for the detection of liver fibrosis in patients
with psoriasis and reactive arthritis on long-term
methotrexate therapy: a cross-sectional study. Indian J
Dermatol Venereol Leprol 2020;86:508-14. Crossref
21. Marsh RL, Kelly S, Mumtaz K, Kaffenberger J. Utility and
limitations of transient elastography to monitor hepatic
steatosis, hepatic fibrosis, and methotrexate-associated
hepatic disease in psoriasis: a systematic review. J Clin
Aesthet Dermatol 2021;14:24-8.
22. Cheng HS, Rademaker M. Monitoring methotrexate-induced
liver fibrosis in patients with psoriasis: utility of
transient elastography. Psoriasis (Auckl) 2018;8:21-9. Crossref
23. Rademaker M, Gupta M, Andrews M, et al. The
Australasian Psoriasis Collaboration view on methotrexate
for psoriasis in the Australasian setting. Australas J
Dermatol 2017;58:166-70. Crossref
24. Darabian S, Wade JP, Kur J, Wade SD, Sayre EC, Badii M.
Using FibroScan to assess for the development of liver
fibrosis in patients with arthritis on methotrexate: a single-center
experience. J Rheumatol 2022;49:558-65. Crossref
25. Lee JH, Loo CH, Tan WC, Lee CK, Jamil A, Khor YH.
Comparison of noninvasive screening tools for hepatic
fibrosis, association with methotrexate cumulative dose,
and risk factors in psoriasis patients. Dermatol Ther
2022;35:e15203. Crossref
26. Wong GL, Chan HL, Choi PC, et al. Association between
anthropometric parameters and measurements of liver
stiffness by transient elastography. Clin Gastroenterol
Hepatol 2013;11:295-302.e1-3. Crossref
27. Rodríguez-Zúñiga MJ, García-Perdomo HA. Systematic
review and meta-analysis of the association between
psoriasis and metabolic syndrome. J Am Acad Dermatol
2017;77:657-66.e8. Crossref
28. Malatjalian DA, Ross JB, Williams CN, Colwell SJ,
Eastwood BJ. Methotrexate hepatotoxicity in psoriatics:
report of 104 patients from Nova Scotia, with analysis of
risks from obesity, diabetes and alcohol consumption during
long term follow-up. Can J Gastroenterol 1996;10:369-75. Crossref
29. Wong GL. Non-invasive assessments for liver fibrosis:
the crystal ball we long for. J Gastroenterol Hepatol
2018;33:1009-15. Crossref
30. Cusi K, Isaacs S, Barb D, et al. American Association of
Clinical Endocrinology Clinical Practice Guideline for
the diagnosis and management of nonalcoholic fatty liver
disease in primary care and endocrinology clinical settings:
co-sponsored by the American Association for the Study of
Liver Diseases (AASLD). Endocr Pract 2022;28:528-62. Crossref
31. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and
management of nonalcoholic fatty liver disease: practice
guidance from the American Association for the Study of
Liver Diseases. Hepatology 2018;67:328-57. Crossref
32. European Association for the Study of the Liver; Clinical
Practice Guideline Panel; Berzigotti A, et al. EASL Clinical
Practice Guidelines on non-invasive tests for evaluation
of liver disease severity and prognosis—2021 update. J
Hepatol 2021;75:659-89. Crossref