Hong Kong Med J 2024 Apr;30(2):102–9 | Epub 26 Mar 2024
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Stevens–Johnson syndrome and toxic epidermal necrolysis in Hong Kong
Christina MT Cheung, MB, ChB, MRCP1; Mimi M Chang, MB, ChB, FRCP1; Joshua JX Li, MB, ChB, FHKCPath2; Agnes WS Chan, MB, ChB, MRCP1
1 Department of Medicine and Therapeutics, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong SAR, China
2 Department of Anatomical and Cellular Pathology, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong SAR, China
Corresponding author: Dr Agnes WS Chan (agneswschan@cuhk.edu.hk)
Abstract
Introduction: Stevens–Johnson syndrome (SJS) and
toxic epidermal necrolysis (TEN) [hereafter, SJS/TEN] are uncommon but severe mucocutaneous
reactions. Although they have been described in
many populations worldwide, data from Hong Kong
are limited. Here, we explored the epidemiology,
disease characteristics, aetiology, morbidity, and
mortality of SJS/TEN in Hong Kong.
Methods: This retrospective cohort study included
all hospitalised patients who had been diagnosed
with SJS/TEN in Prince of Wales Hospital from 1
January 2004 to 31 December 2020.
Results: There were 125 cases of SJS/TEN during
the 17-year study period. The annual incidence was
5.07 cases per million. The mean age at onset was
51.4 years. The mean maximal body surface area of
epidermal detachment was 23%. Overall, patients in
32% of cases required burns unit or intensive care unit
admission. Half of the cases involved concomitant
sepsis, and 23.2% of cases resulted in multiorgan
failure or disseminated intravascular coagulation.
The mean length of stay was 23.9 days. The cause of
SJS/TEN was attributed to a drug in 91.9% of cases,
including 84.2% that involved anticonvulsants,
allopurinol, antibiotics, or analgesics. In most cases, patients received treatment comprising either
best supportive care alone (35.2%) or combined
with intravenous immunoglobulin (43.2%). The in-hospital
mortality rate was 21.6%. Major causes of
death were multiorgan failure and/or fulminant
sepsis (81.5%).
Conclusion: This study showed that SJS/TEN are
uncommon in Hong Kong but can cause substantial
morbidity and mortality. Early recognition, prompt
withdrawal of offending agents, and multidisciplinary
supportive management are essential for improving
clinical outcomes.
New knowledge added by this study
- Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare severe cutaneous adverse reactions in Hong Kong, with a combined annual incidence of 5.07 cases per million.
- Stevens–Johnson syndrome and TEN cause considerable burdens on the Hong Kong healthcare system due to their prolonged length of stay, high demand for intensive care, and substantial mortality.
- Clinicians should be aware of the early signs and symptoms of SJS and TEN to enable rapid recognition of the disease and prompt withdrawal of culprit drugs.
- Dedicated multidisciplinary teams should be established in tertiary centres to optimise patient outcomes.
Introduction
Stevens–Johnson syndrome (SJS) and toxic
epidermal necrolysis (TEN) are uncommon but
potentially life-threatening severe mucocutaneous
reactions characterised by extensive epidermal
necrosis and detachment. Both entities are
considered variants of a single disease continuum
and are classified according to the percentage of body
surface area (BSA) with epidermal detachment.1 2
Although SJS and TEN (hereafter, SJS/TEN) have
been described in all ethnicities worldwide,3 studies of these reactions in Hong Kong have been limited.4 5 The incidence, clinical characteristics, aetiology,
treatment regimen, morbidity, and mortality in the
territory are largely unknown. This pilot study aimed
to review cases of SJS/TEN over a 17-year period at
a tertiary referral centre in Hong Kong, and to aid
future research in Hong Kong focused on severe
cutaneous adverse reactions.
Methods
This retrospective cohort study included all hospitalised patients who had been diagnosed
with SJS/TEN and were treated in Prince of Wales
Hospital (PWH), a major regional hospital under
the New Territories East Cluster (NTEC), from 1
January 2004 to 31 December 2020.
Patient identification
Patients with clinical and histological diagnoses of
SJS/TEN were identified from the Hospital Authority
database using International Classification of Diseases,
Ninth Revision, Clinical Modification diagnosis codes
and the database of the Department of Anatomical
and Cellular Pathology of PWH, respectively.
Inclusion criteria
Diagnoses of SJS/TEN were based on consensus
guidelines.1 Patients were diagnosed with SJS,
SJS/TEN overlap, and TEN when they exhibited
epidermal detachment levels of <10%, 10% to 30%,
and >30%, respectively, with consistent histological
features (if skin biopsy was performed). Consistent
histological features were regarded as partial- to full-thickness
epidermal necrosis.
Exclusion criteria
Patients were excluded if they had an alternative
diagnosis, such as severe cutaneous adverse
reactions other than SJS/TEN (eg, drug reaction with
eosinophilia and systemic symptoms syndrome/acute generalised exanthematous pustulosis/generalised bullous fixed drug eruption), erythema
multiforme major, autoimmune blistering disease,
acute graft-versus-host disease, and infections such
as staphylococcal scalded skin syndrome.
Data collection and statistical analysis
Clinical characteristics were collected from
electronic records and, when available, hospital
case notes. The following clinical characteristics
were recorded and analysed: age at onset, sex,
ethnicity, maximal BSA of detached or detachable
skin, SCORTEN (Severity-of-Illness Score for Toxic
Epidermal Necrolysis) prognostic score,6 mucosa
involved, histology results if available, causative
drugs, time from exposure to onset, time from onset
to admission and treatment, treatment regimen,
disease complications, mortality and its cause, and
length of stay. Efforts to identify causative drugs
were guided by the ALDEN (algorithm of drug
causality for epidermal necrolysis) score,7 which
was retrospectively calculated by two independent
investigators. All clinical data were expressed as
percentages or means ± standard deviations unless
otherwise specified.
This article was written in compliance with
the STROBE (Strengthening the Reporting of
Observational Studies in Epidemiology) reporting
guidelines.
Results
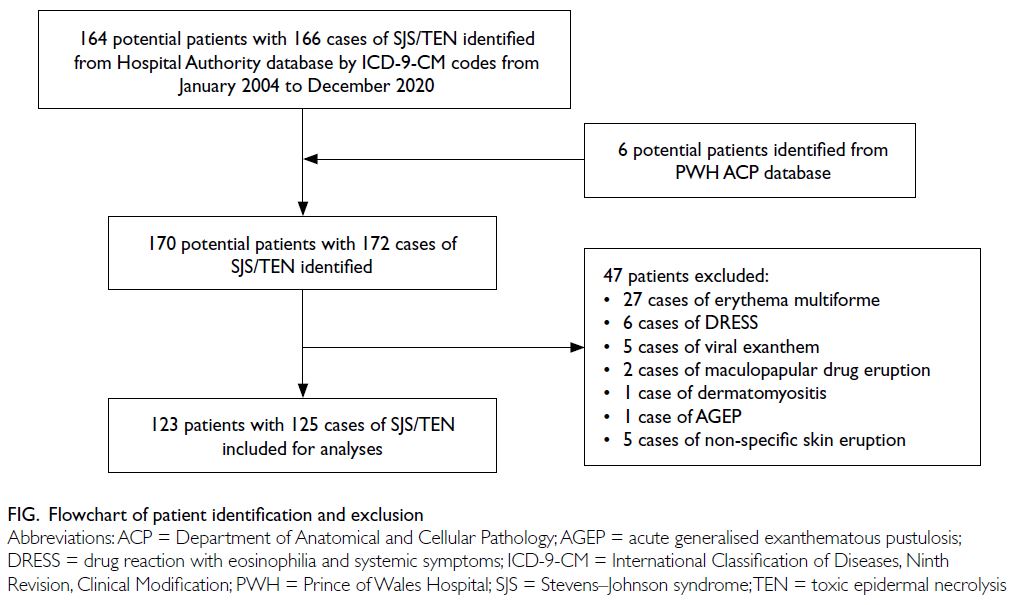
Using the International Classification of Diseases,
Ninth Revision, Clinical Modification diagnosis
codes for SJS/TEN, 164 potential patients with 166
cases of SJS/TEN during the period from January
2004 to December 2020 were initially identified. Six
additional patients with SJS/TEN were identified
from the database of the Department of Anatomical
and Cellular Pathology of PWH. Forty-seven
patients were excluded due to alternative diagnoses.
In total, 123 patients with 125 cases of SJS/TEN were
included in the study (Fig).
Demographic characteristics and disease
classification
Among the 123 patients with SJS/TEN, 53 were men
and 70 were women; the female-to-male ratio was
1.32:1 (Table 1). The mean age at onset was 51.4
years, and most patients were Chinese. Of the 125
cases, 59 were SJS, 27 were SJS-TEN overlap, and
39 were TEN. A small number of patients (n=18,
14.4%) were admitted for other medical issues but
developed SJS/TEN after hospitalisation.
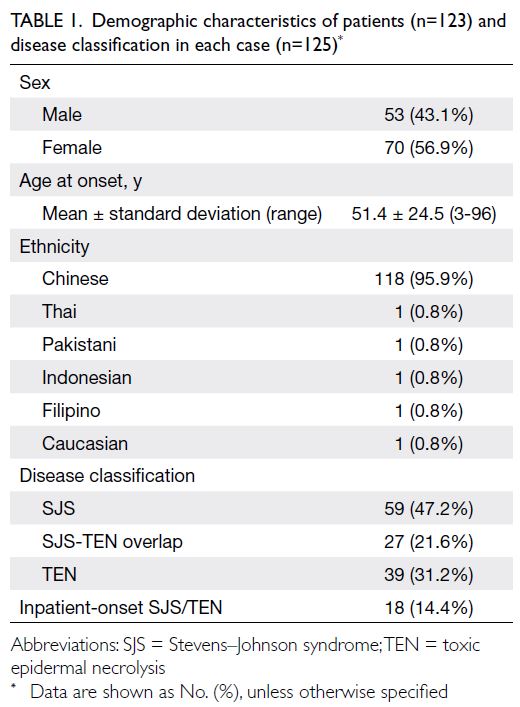
Table 1. Demographic characteristics of patients (n=123) and disease classification in each case (n=125)
Clinical characteristics and clinical course
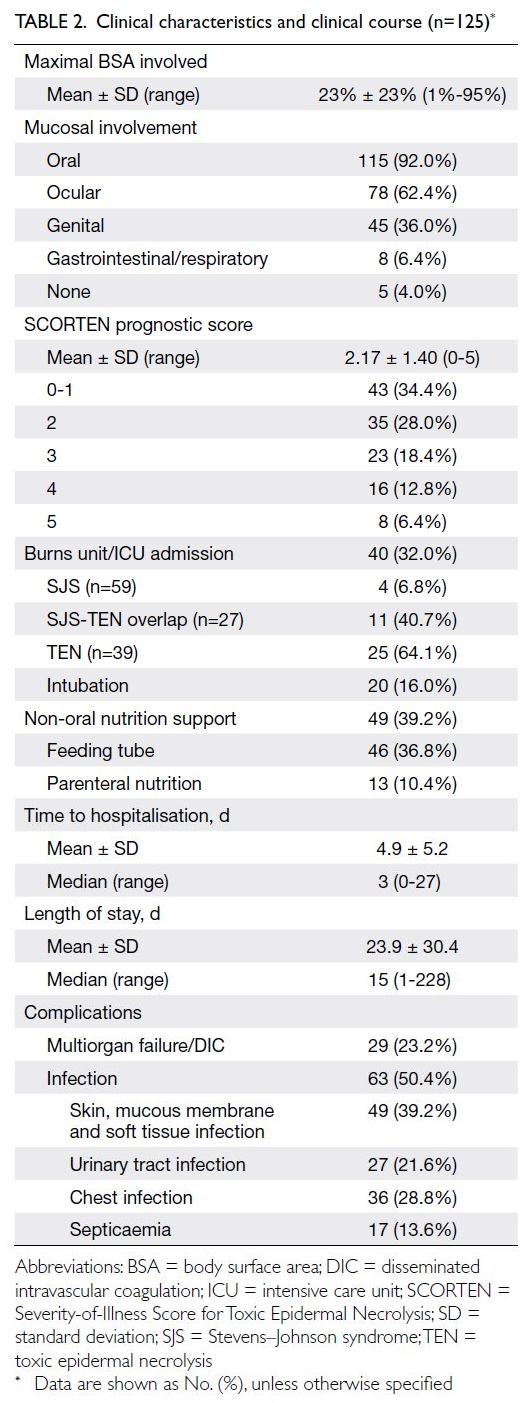
The mean time from disease onset to hospitalisation was 4.9 days (Table 2). Fever was present on
admission in 88 cases (70.4%). The mean maximal
BSA of epidermal detachment was 23%. Mucosal
involvement was common; only five cases (4.0%)
lacked mucosal involvement. Skin biopsy was performed in 87 cases (69.6%) and the mean SCORTEN prognostic score was 2.17.
Burns unit or intensive care unit admission was
required in 40 cases (32.0%) and half of these cases
required invasive mechanical ventilation. In total, 63
cases (50.4%) involved concomitant infection from
various sources. Multiorgan failure or disseminated
intravascular coagulation occurred in 29 cases
(23.2%). The mean length of stay in the hospital was
23.9 days (Table 2).
Aetiology
Stevens–Johnson syndrome and TEN onset was attributed to a drug in 114 of 124 cases (91.9%); one
patient developed a second case of SJS/TEN upon
accidental re-exposure to the same culprit drug
(paracetamol). Four cases (3.2%) were caused by
infection, and no cause was identified in six cases
(4.8%). The identified culprit drugs are shown in
Table 3. The mean time from initiation of the culprit
drug to onset of SJS/TEN was 20.5 ± 16.7 days (range,
1-87; median, 15.5).
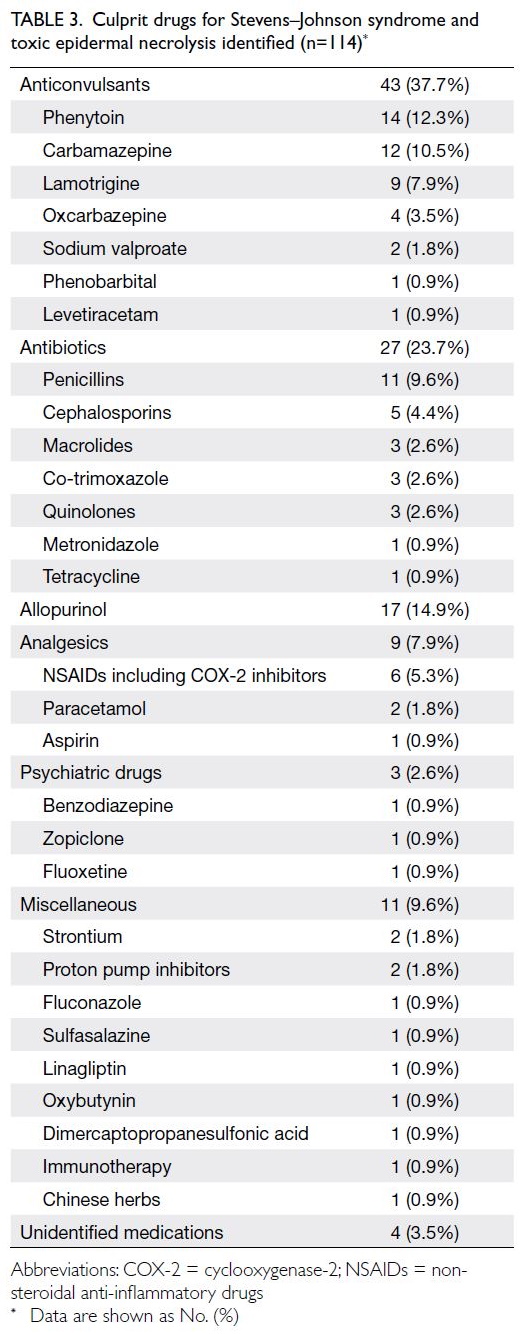
Table 3. Culprit drugs for Stevens–Johnson syndrome and toxic epidermal necrolysis identified (n=114)
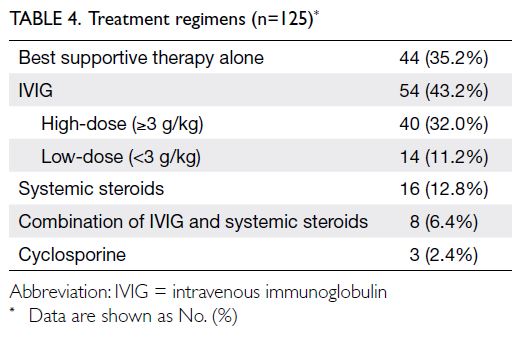
Treatment
All patients received the best supportive medical
care available. In some cases, patients received
additional treatment. The numbers and proportions
of cases treated with different regimens are shown in
Table 4. The mean time between disease onset and
active treatment initiation was 7.4 ± 6.1 days (range,
1-34; median, 6).
Intravenous immunoglobulin (IVIG) was administered in 54 cases. The mean total dose of IVIG was 3.2 g/kg (range, 1.5-6; administered over
2-6 days). High-dose IVIG, defined as ≥3 g/kg, was
administered in 40 cases. Systemic steroid regimens considerably varied, with daily doses of prednisolone ranging from 20 to 120 mg (or an equivalent dose). The cyclosporine regimen was 3 mg/kg/day, tapered
over 20 to 30 days.
Mortality
There were 27 deaths in the study cohort, and the
overall mortality rate was 21.6%. The mean time from
SJS/TEN onset to death was 23.6 ± 19.0 days (range,
5-76). Most patients died from multiorgan failure
and/or fulminant sepsis (n=22, 81.5%); other causes
of death were acute coronary syndrome (n=2),
liver failure (n=1), and sudden cardiac arrest
(n=2).
The observed mortality rates were 16.9%,
22.2%, and 28.2% for SJS, SJS-TEN overlap, and
TEN, respectively. The SCORTEN-based predicted
mortality rates were 14.1%, 28.5%, and 36.9% for SJS,
SJS-TEN overlap, and TEN, respectively. Inpatient-onset
SJS/TEN had a high mortality rate of 77.8%: 14
deaths among 18 patients who developed SJS/TEN
after admission.
Discussion
Epidemiology
Stevens–Johnson syndrome and TEN are recognised
worldwide, with several epidemiological studies
conducted in Europe and the US. In the 1990s,
Roujeau et al8 reported that the annual incidence of
TEN in France was 1.2 cases per million; during the
same period, the estimated overall annual incidences
of SJS/TEN were 1.89 cases per million in Germany9
and 4.2 cases per million in the US.10 In the past
decade, two large epidemiological studies in the US11
and United Kingdom12 revealed that the overall annual
incidences of SJS/TEN were 12.7 and 5.76 cases per
million, respectively. In contrast, the epidemiology
of SJS/TEN in Asia is not well-documented.13 In
Singapore, based on a small retrospective hospital-based
study of 20 patients with TEN, the estimated
annual incidence of TEN was 1.4 cases per million14;
in Korea, a large population-based study indicated
that the overall annual incidence of SJS/TEN was 4.9
to 6.5 cases per million.15 In the present study, there
were 125 cases of SJS/TEN during the 17-year study
period. Notably, 13 of these cases were transferred
from hospitals outside of the NTEC: one was SJS, three were SJS/TEN overlap, and nine were TEN.
The NTEC serves a population of 1.3 million.16
The estimated annual incidence of TEN alone and
combined annual incidence of SJS, SJS-TEN overlap,
and TEN were 1.36 and 5.07 cases per million,
respectively; these incidences are comparable with
findings from studies in other countries.
Stevens–Johnson syndrome is approximately
threefold more common than TEN.15 17 However,
in the current study, fewer than half of the cases
(47.2%) were SJS, whereas 31.2% were TEN. This
may be related to referral bias, whereby more
severe cases were transferred to the study hospitals,
whereas ‘milder’ cases were managed in regional
hospitals where the patients were initially admitted.
Prince of Wales Hospital is a tertiary referral centre
and one of the few hospitals in Hong Kong with
both on-site dermatologists and burns unit support.
In our cohort, 31 cases (24.8%) were transferred
from peripheral hospitals: 18 (14.4%) arrived from
hospitals within the NTEC, whereas 13 (10.4%)
arrived from hospitals outside of the NTEC.
Aetiology
Stevens–Johnson syndrome and TEN are most often drug-induced, and a culprit drug is identified
in approximately 85% of cases.7 18 The reactions
usually occur between 7 days and 8 weeks after drug
ingestion.19 However, upon rechallenge with the
culprit drug, SJS/TEN can develop within hours.17 19
Efforts to identify causative drugs were guided by
the ALDEN score.7 In cases of SJS/TEN, the most
commonly implicated high-risk medications are
anticonvulsants, allopurinol, antimicrobials, and
oxicam non-steroidal anti-inflammatory drugs.19 20
In the present study, SJS/TEN onset was attributed
to a drug in 114 of 124 cases (91.9%). The mean time
between drug initiation and SJS/TEN onset was 20.5
days. Among these 114 cases, 81.6% were caused
by the high-risk medications listed above. These
findings are comparable with previous reports.
Mortality
Stevens–Johnson syndrome and TEN are associated with high mortality rates, with 1% to 5% in cases of SJS and 25% to 30% in cases of TEN. Survival analyses
in multinational European studies (EuroSCAR
[European Study of Severe Cutaneous Adverse
Reactions] and RegiSCAR [Registry of Severe
Cutaneous Adverse Reactions]) have indicated that
the overall mortality rate in cases of SJS/TEN is
approximately 22% to 23%.18 20 21 22 23 In Asia, reported
overall mortality rates vary from 12.3% to 25%.24 25 26 27
Sepsis leading to multiorgan failure is the most
common cause of death.21 Despite the substantial
mortality, there currently is no therapeutic regimen
with a clear benefit for patients with SJS/TEN.18 21
Considering the rarity of these diseases, it is difficult to conduct randomised trials. Early recognition,
rapid withdrawal of offending agents, and best
supportive care remain the primary components of
clinical management.
In the current study, the overall mortality rate
was 21.6%; in 81.5% of these cases, the patient died of
fulminant sepsis or multiorgan failure. These findings
are consistent with existing literature. However,
the mortality rate in cases of SJS was much higher
in the present study than in previous studies. In the
59 cases of SJS, there were 10 deaths; the mortality
rate was 16.9%. Among the 10 patients who died, six
experienced complete skin re-epithelisation before
death from other medical conditions, which include
massive duodenal ulcer bleeding, acute coronary
syndrome, metastatic lung cancer, acute liver and
renal failure due to herbs, aspiration pneumonia,
and sudden cardiac arrest. The remaining four
patients had inpatient-onset SJS; they were initially
admitted for traumatic intracranial haemorrhage,
post-hepatectomy liver failure, convulsions caused
by metastatic lung cancer, and post-stroke seizure,
respectively. These patients exhibited skin-specific
improvements but soon died of aspiration pneumonia
and acute renal failure, liver failure, metastatic lung
cancer with respiratory failure and liver failure, and sudden cardiac arrest, respectively. The high
mortality rate among patients with SJS in the present
study could be related to referral bias (as noted in
the Epidemiology subsection above); specifically,
more severe cases of SJS with co-morbidities and/or complications may have been transferred to our
tertiary centre for medical care, whereas less severe
cases of SJS might have been managed in regional
hospitals where the patients were initially admitted.
Indeed, the predicted mortality rate (based on the
SCORTEN prognostic score) among cases of SJS
in our cohort was 14%; this rate was similar to the
observed mortality rate.
In the present study, inpatient-onset SJS/TEN had a high mortality rate (77.8%). Although
high mortality of inpatient-onset SJS/TEN was not
previously described in the literature, we speculate
that this high mortality was related to the underlying
medical conditions for which patients were initially
admitted. The clinical characteristics of the 14
patients who died are presented in Table 5.
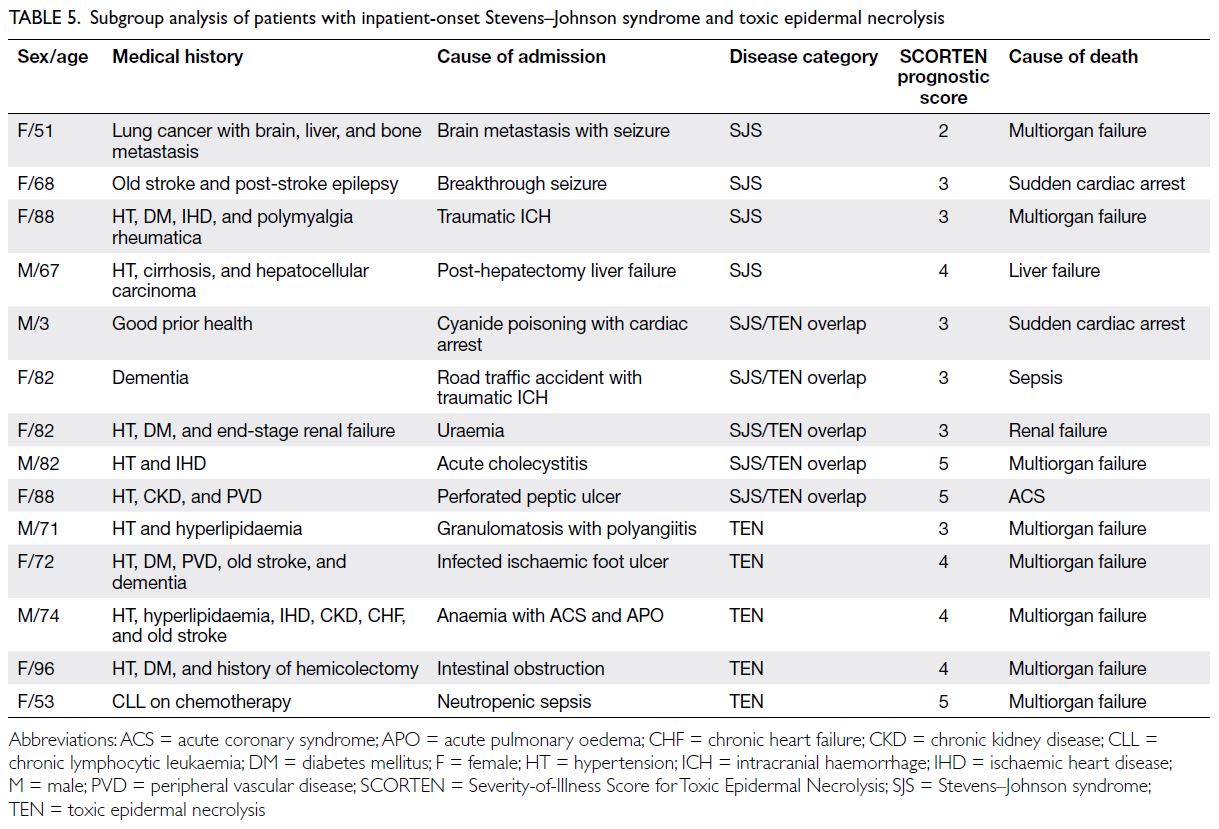
Table 5. Subgroup analysis of patients with inpatient-onset Stevens–Johnson syndrome and toxic epidermal necrolysis
In addition to high mortality, SJS/TEN were
associated with high rates of burns unit/intensive care
unit admission (32%) and prolonged length of stay
(mean=23.9 days) [Table 2], placing a considerable
burden on the public healthcare system.
Limitations and strengths
As a retrospective cohort study, the present study
had some intrinsic limitations. Some hospital case
notes (ie, from earlier in the study period) were no
longer retrievable. Clinical characteristics such as
the exact date of disease onset, precise total BSA
involved, and detailed drug history (including over-the-counter medications/medications prescribed
by private doctors) might not have been available
for some of these older cases. Skin biopsies were
performed in 70% of cases and might have been
omitted in cases of terminal illness. Many patients
with milder cases were lost to follow-up after
discharge; thus, long-term sequelae were not well-documented.
Additionally, referral bias may have been
present because PWH is a tertiary referral centre.
Such bias could have led to underestimation of
the true incidence of SJS and overestimation of
the incidence of TEN; milder cases of SJS might
have been managed in regional hospitals, whereas
more severe cases of TEN were transferred to our
centre for better care. Similarly, there may have
been overestimation of various outcome measures
including length of stay, complications, and
mortality.
Nonetheless, this study had several strengths.
To our knowledge, this is one of the largest single-centre
studies regarding SJS/TEN in Asia; it included
a homogenous group of predominantly Chinese
patients. The patients were managed by the same
dermatology team with a consistent diagnostic and
therapeutic approach throughout the study period.
Data collection was adequate, and exhaustive
evaluation of drug history was feasible for cases with
access to both electronic records and hospital case
notes. To ensure accurate identification of causative
drugs, the ALDEN score was retrospectively
evaluated by two independent dermatology doctors
during the study.
Conclusion
This is the first large study in Hong Kong to
provide data regarding the epidemiology, disease
characteristics and clinical course, aetiology,
treatment regimen, and mortality of SJS/TEN.
Although uncommon, SJS/TEN is associated with
substantial morbidity and mortality. Therefore,
in addition to increasing awareness of SJS/TEN
among patients and clinicians, efforts should be
made to optimise inpatient care among public
hospitals in Hong Kong by establishing dedicated
multidisciplinary teams that are experienced in the
management of SJS/TEN.
Author contributions
Concept or design: CMT Cheung, MM Chang.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: CMT Cheung, AWS Chan.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: CMT Cheung, AWS Chan.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This research was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee (Ref No.: 2017.424). The requirement for informed consent was waived by the Committee due to the
retrospective nature of the research.
References
1. Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L,
Roujeau JC. Clinical classification of cases of toxic
epidermal necrolysis, Stevens–Johnson syndrome, and
erythema multiforme. Arch Dermatol 1993;129:92-6. Crossref
2. Roujeau JC. Stevens–Johnson syndrome and toxic
epidermal necrolysis are severity variants of the same
disease which differs from erythema multiforme. J
Dermatol 1997;24:726-9. Crossref
3. Roujeau JC, Chosidow O, Saiag P, Guillaume JC. Toxic
epidermal necrolysis (Lyell syndrome). J Am Acad
Dermatol 1990;23:1039-58. Crossref
4. Ying S, Ho W, Chan HH. Toxic epidermal necrolysis: 10
years experience of a burns centre in Hong Kong. Burns
2001;27:372-5. Crossref
5. Yeung CK. Intravenous immunoglobulin treatment for
Stevens–Johnson syndrome and toxic epidermal necrolysis
[dissertation]. Queen Mary Hospital, The University of
Hong Kong; 2004.
6. Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC,
Revuz J, Wolkenstein P. SCORTEN: a severity-of-illness
score for toxic epidermal necrolysis. J Invest Dermatol
2000;115:149-53. Crossref
7. Sassolas B, Haddad C, Mockenhaupt M, et al. ALDEN,
an algorithm for assessment of drug causality in Stevens–Johnson syndrome and toxic epidermal necrolysis:
comparison with case-control analysis. Clin Pharmacol
Ther 2010;88:60-8. Crossref
8. Roujeau JC, Guillaume JC, Fabre JP, Penso D, Fléchet ML,
Girre JP. Toxic epidermal necrolysis (Lyell syndrome).
Incidence and drug etiology in France, 1981-1985. Arch
Dermatol 1990;126:37-42. Crossref
9. Rzany B, Mockenhaupt M, Baur S, et al. Epidemiology
of erythema exsudativum multiforme majus, Stevens–Johnson syndrome, and toxic epidermal necrolysis
in Germany (1990-1992): structure and results of a
population-based registry. J Clin Epidemiol 1996;49:769-73. Crossref
10. Chan HL, Stern RS, Arndt KA, et al. The incidence of
erythema multiforme, Stevens–Johnson syndrome, and
toxic epidermal necrolysis. A population-based study with
particular reference to reactions caused by drugs among
outpatients. Arch Dermatol 1990;126:43-7. Crossref
11. Hsu DY, Brieva J, Silverberg NB, Silverberg JI. Morbidity
and mortality of Stevens–Johnson syndrome and toxic
epidermal necrolysis in United States adults. J Invest
Dermatol 2016;136:138797. Crossref
12. Frey N, Jossi J, Bodmer M, et al. The epidemiology of
Stevens–Johnson syndrome and toxic epidermal necrolysis
in the UK. J Invest Dermatol 2017;137:1240-7. Crossref
13. Lee HY, Martanto W, Thirumoorthy T. Epidemiology of
Stevens–Johnson syndrome and toxic epidermal necrolysis
in Southeast Asia. Dermatologica Sinica 2013;31:217-20. Crossref
14. Chan HL. Toxic epidermal necrolysis in Singapore, 1989
through 1993: incidence and antecedent drug exposure.
Arch Dermatol 1995;131:1212-3. Crossref
15. Yang MS, Lee JY, Kim J, et al. Incidence of Stevens–Johnson
syndrome and toxic epidermal necrolysis: a nationwide
population-based study using national health insurance
database in Korea. PLoS One 2016;11:e0165933. Crossref
16. Hospital Authority, Hong Kong SAR Government. New Territories East Cluster biennial report 2018-2020. Available from: https://www3.ha.org.hk/ntec/clusterreport/clusterreport2018-20/index.html. Accessed 8 Feb 2024.
17. Schwartz RA, McDonough PH, Lee BW. Toxic epidermal
necrolysis: Part I. Introduction, history, classification,
clinical features, systemic manifestations, etiology, and
immunopathogenesis. J Am Acad Dermatol 2013;69:173.
e1-13; quiz 185-6. Crossref
18. Creamer D, Walsh SA, Dziewulski P, et al. UK guidelines
for the management of Stevens–Johnson syndrome/toxic epidermal necrolysis in adults 2016. J Plast Reconstr
Aesthet Surg 2016;69:e119-53. Crossref
19. Roujeau JC, Kelly JP, Naldi L, et al. Medication use and the risk of Stevens–Johnson syndrome or toxic epidermal necrolysis. N Engl J Med 1995;333:1600-7. Crossref
20. Mockenhaupt M, Viboud C, Dunant A, et al. Stevens–Johnson syndrome and toxic epidermal necrolysis:
assessment of medication risks with emphasis on recently
marketed drugs. The EuroSCAR study. J Invest Dermatol
2008;128:35-44. Crossref
21. Schwartz RA, McDonough PH, Lee BW. Toxic epidermal
necrolysis: Part II. Prognosis, sequelae, diagnosis,
differential diagnosis, prevention, and treatment. J Am
Acad Dermatol 2013;69:187.e1-16; quiz 203-4. Crossref
22. Roujeau JC, Stern RS. Severe adverse cutaneous reactions
to drugs. N Engl J Med 1994;331:1272-85. Crossref
23. Sekula P, Dunant A, Mockenhaupt M, et al. Comprehensive
survival analysis of a cohort of patients with Stevens–Johnson syndrome and toxic epidermal necrolysis. J Invest
Dermatol 2013;133:1197-204. Crossref
24. Barvaliya M, Sanmukhani J, Patel T, Paliwal N, Shah H,
Tripathi C. Drug-induced Stevens–Johnson syndrome
(SJS), toxic epidermal necrolysis (TEN), and SJS-TEN
overlap: a multicentric retrospective study. J Postgrad Med
2011;57:115-9. Crossref
25. Roongpisuthipong W, Prompongsa S, Klangjareonchai T.
Retrospective analysis of corticosteroid treatment in
Stevens–Johnson syndrome and/or toxic epidermal
necrolysis over a period of 10 years in Vajira Hospital,
Navamindradhiraj University, Bangkok. Dermatol Res
Pract 2014;2014:237821. Crossref
26. Suwarsa O, Yuwita W, Dharmadji HP, Sutedja E. Stevens–Johnson syndrome and toxic epidermal necrolysis in Dr
Hasan Sadikin General Hospital Bandung, Indonesia from
2009-2013. Asia Pac Allergy 2016;6:43-7. Crossref
27. Lee HY, Fook-Chong S, Koh HY, Thirumoorthy T, Pang SM.
Cyclosporine treatment for Stevens–Johnson syndrome/toxic epidermal necrolysis: retrospective analysis of a
cohort treated in a specialized referral center. J Am Acad
Dermatol 2017;76:106-13. Crossref