Hong Kong Med J 2024 Feb;30(1):10–5 | Epub 8 Feb 2024
https://doi.org/10.12809/hkmj2210034
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Non–vitamin K oral anticoagulants versus
warfarin for the treatment of left ventricular thrombus
Kevin KH Kam, MB, ChB, MRCP1; Jeffrey SK Chan, MB, ChB1; Alex PW Lee, MD, FRCP1,2
1 Division of Cardiology, Department of Medicine and Therapeutics, Prince of Wales Hospital, Hong Kong SAR, China
2 Laboratory of Cardiac Imaging and 3D Printing, Li Ka Shing Institute of Health Sciences, The Chinese University of Hong Kong, Hong Kong SAR, China
Corresponding author: Prof Alex PW Lee (alexpwlee@cuhk.edu.hk)
Abstract
Introduction: Left ventricular thrombus (LVT) is
associated with significant morbidity and mortality.
Conventional treatment comprises warfarin-mediated
anticoagulation; it is unclear whether
non–vitamin K oral anticoagulants (NOACs) exhibit
comparable efficacy and safety. Limited data are
available for Asian patients. This study compared
NOACs with warfarin in terms of clinical efficacy
and safety for managing LVT.
Methods: Clinical and echocardiographic
records were retrieved for all adult patients with
echocardiography-confirmed LVT at a major
regional centre in Hong Kong from January 2011 to
January 2020. Discontinuation of anticoagulation
by 1 year was recorded. Outcomes were compared
between patients receiving NOACs and those
receiving warfarin. Primary outcomes were
cumulative mortality and net adverse clinical events
(NACEs). Secondary outcomes were complete LVT
resolution and percentage reduction in LVT size at
3 months.
Results: Forty-three patients were included; 28
received warfarin and 15 received NOACs, with
follow-up periods (mean ± standard deviation) of
20 ± 12 months and 22 ± 9 months, respectively
(P=0.522). Use of NOACs was associated with significantly lower NACE risk (hazard ratio
[HR]=0.111, 95% confidence interval [CI]=0.012-0.994; P=0.049) and a tendency towards lower
cumulative mortality (HR=0.184, 95% CI=0.032-1.059; P=0.058). There were no significant differences
in secondary outcomes. Considering LVT resolution,
discontinuation of anticoagulation by 1 year was not
significantly associated with different outcomes.
Conclusion: Non–vitamin K oral anticoagulants
may be an efficacious and safe alternative to warfarin
for LVT management. Future studies should
explore the safety and efficacy of anticoagulation
discontinuation by 1 year as an overall strategy.
New knowledge added by this study
- In a Hong Kong cohort, non–vitamin K oral anticoagulant users had fewer net adverse clinical events and tended to exhibit lower mortality, compared with warfarin users.
- Considering left ventricular thrombus (LVT) resolution, discontinuation of anticoagulation by 1 year may be a safe overall strategy.
- Non–vitamin K oral anticoagulants may be an efficacious and safe alternative to warfarin for LVT management.
- Further studies are needed to explore the safety and efficacy of anticoagulant discontinuation by 1 year as an overall strategy for patients with LVT resolution.
Introduction
Left ventricular thrombus (LVT) primarily
occurs in patients who exhibit heart failure with
reduced ejection fraction, particularly when these
conditions are secondary to dilated cardiomyopathy
or myocardial infarction. Recent advances in the
treatment of myocardial ischaemia and heart failure
have reduced the estimated incidence to 7 cases
per 10 000 patients.1 However, this lower incidence does not reduce the importance of identifying and
treating LVT; one study has shown very high risks of
major cardiovascular or cerebrovascular events and
mortality in patients with LVT.2
Although LVT has conventionally been
managed with warfarin, multiple guidelines suggest
different treatment algorithms based on expert
opinion and small-scale studies, reflecting the lack
of evidence that underlies such recommendations.3 4 This lack of evidence is partly related to the low
incidence of LVT, which hinders adequately
powered research with high evidence quality.
Considering the growing popularity of non–vitamin K oral anticoagulants (NOACs), there has
been increasing interest in the use of NOACs as
an alternative to warfarin for LVT management.5
A systematic review in 2020, which involved only
relevant case series and case reports, concluded
that NOACs constitute a ‘reasonable alternative’ to
warfarin for LVT management.6 However, another
2020 study of >500 patients showed that NOACs
increased the incidence of stroke or systematic
embolism compared with warfarin.7 Nonetheless,
only thromboembolic events were compared in that
study; safety outcomes, specifically bleeding events,
were not investigated. Thus, it remains unclear
whether NOACs exhibit efficacy and safety similar
to warfarin for LVT management. This retrospective
cohort study aimed to evaluate the efficacy and
safety of NOACs versus warfarin for the treatment
of LVT.
Methods
Patient population
This retrospective cohort study included all patients with LVT diagnosed by echocardiography from
January 2011 to January 2020 at our institution, a
major tertiary university hospital in Hong Kong.
Only patients aged ≥18 years were included.
Patients were excluded if baseline echocardiography,
pharmacotherapy regimen or clinical records were
non-retrievable, or if the type of anticoagulation
therapy (warfarin or NOACs) was switched within
the first 2 years after LVT diagnosis.
At our institution, all patients began
anticoagulation therapy upon echocardiography-based
diagnosis of LVT. Patients either received
warfarin with titration and maintenance of a
therapeutic international normalised ratio of 2-3,
or they received NOAC therapy. Because there are
no specific treatment recommendations in current
guidelines, anticoagulant selection was performed
at the treating physicians’ discretion, generally
considering patient-specific factors such as renal
function, presence of other indications, and drug
compliance. Follow-up echocardiography was
performed 3 months after diagnosis of LVT, and
further follow-up echocardiography was performed
as clinically indicated. Anticoagulation was only
discontinued if LVT had been resolved; this step
required a shared, informed decision between
the patient and the physician. Anticoagulation
discontinuation was not considered for patients with
persistent LVT.
Outcomes and measurements
All patients were followed up for ≤3 years.
Echocardiographic images of all included patients at
baseline and the 3-month follow-up were reviewed.
The left ventricular ejection fraction, baseline size
of LVT, and any resolution of LVT by the 3-month
follow-up or the size of residual LVT at the
3-month follow-up were recorded. Clinical records
of all patients were reviewed using the Clinical
Management System of the Hong Kong Hospital
Authority; important pre-morbid conditions, types
of anticoagulants used, and pre-specified clinical
outcomes were recorded. Any discontinuation of
anticoagulation by 1 year was recorded.
The primary outcomes were cumulative
mortality and net adverse clinical events (NACEs),
defined as any of the following: ischaemic
stroke, intracranial haemorrhage, systemic
thromboembolism other than cerebral embolism,
fatal bleeding (Bleeding Academic Research
Consortium class 58), and major non-fatal bleeding
(Bleeding Academic Research Consortium class
38). Secondary outcomes were complete resolution
of LVT and percentage reduction of LVT size
at the 3-month follow-up. Outcomes were also
compared between patients who had discontinued
anticoagulation by 1 year and those who continued
anticoagulation for >1 year.
Statistical analysis
Unless otherwise specified, all continuous variables
are expressed as mean ± standard deviation. Pre-morbid
conditions and clinical outcomes in the two
anticoagulation therapy groups were compared using
Fisher’s exact test (for dichotomous variables) or
Mann-Whitney U test (for continuous variables); the
Mann-Whitney U test was chosen over parametric
tests because the sample sizes were unlikely to
support an assumption of data normality. Kaplan-Meier survival curves were used to visualise survival
status and freedom from NACEs throughout the
study period; Cox regression was used to compare
mortality and NACE use between the two groups.
Cases with missing values were excluded from
analysis of the respective variables; no imputation
was performed. All P values were two-sided, and
P<0.05 was considered statistically significant. All
statistical analyses were performed using SPSS
software (Windows version 25.0; IBM Corp, Armonk
[NY], United States).
Results
In total, 43 patients (37 men) with LVT were
included in this study: 28 received warfarin and 15
received NOACs. No patients were excluded for
switching anticoagulant therapy during the first 2
years after LVT diagnosis. Of the patients treated
with NOACs, 10 received apixaban, four received
dabigatran, and one received rivaroxaban. Their
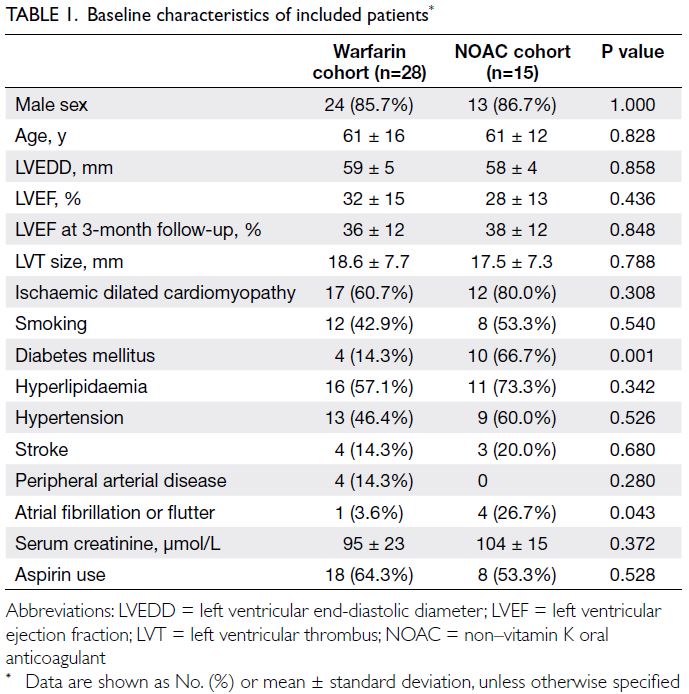
baseline characteristics are summarised in Table 1;
the two cohorts were generally comparable, except
the NOAC cohort included more patients with
diabetes mellitus (P=0.001) and atrial fibrillation
or flutter (P=0.043). Eleven patients in the warfarin
cohort and three patients in the NOAC cohort had
non-ischaemic cardiomyopathy (P=0.308), including
one patient with non-compaction cardiomyopathy
and another patient (lost to follow-up after 6 months)
with myocarditis. Both of these patients were in the
warfarin cohort.
Three patients (all in the warfarin group)
were lost to follow-up: one after 6 months (as
noted above), one after 22 months, and one after
26 months. One of these patients had discontinued
anticoagulation therapy by 1 year. The warfarin and
NOAC cohorts were followed up for mean intervals
of 20 ± 12 months (median, 20; interquartile range,
7-33) and 22 ± 9 months (median, 19; interquartile
range, 15-31), respectively (P=0.522). All patients
were examined by follow-up echocardiography at 3
months after initiation of anticoagulation therapy,
except one patient in the warfarin cohort who died
1 month after diagnosis of LVT. In total, 14 deaths
were observed in the NOAC (n=2; 13.3%) and
warfarin (n=12; 42.9%) cohorts during the study
period. Causes of death in the NOAC cohort were
cardiovascular (sudden death; n=2); in the warfarin cohort, the causes of death were cardiovascular (n=8),
intracerebral haemorrhage (n=3), gastrointestinal
haemorrhage (n=1), and malignancy (n=2). Of the
34 patients who completed 1 year of follow-up, nine
had discontinued anticoagulation therapy.
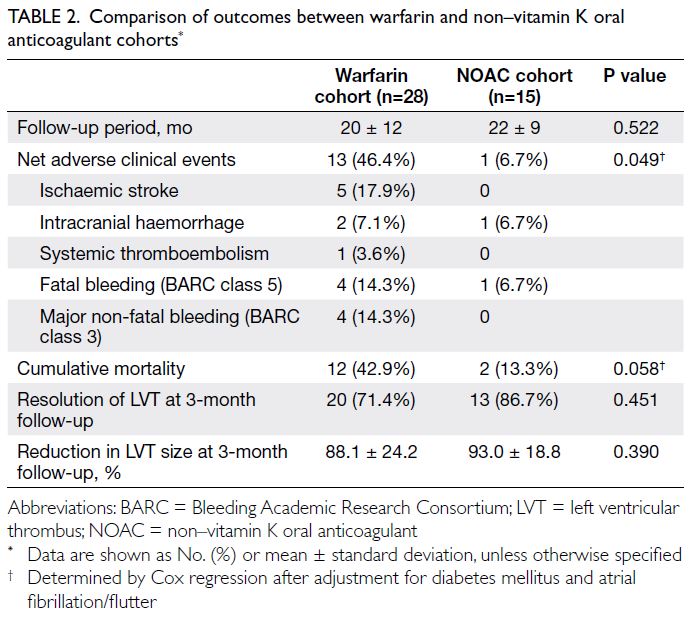
All primary and secondary outcomes are
summarised in Table 2. We observed a significantly lower risk of NACEs in the NOAC cohort (n=1 [6.7%]
in the NOAC cohort vs n=13 [46.4%] in the warfarin
cohort; hazard ratio [HR]=0.124, 95% confidence
interval [CI]=0.016-0.952; P=0.045), which
remained statistically significant after adjustment
for the clinical statuses of diabetes mellitus and atrial fibrillation or flutter (HR=0.111, 95% CI=0.012-0.994; P=0.049) [Fig 1]. There was a tendency
towards lower mortality in the NOAC cohort (n=2
[13.3%] in the NOAC cohort vs n=12 [42.9%] in the
warfarin cohort; HR=0.285, 95% CI=0.064-1.275;
P=0.101 [before adjustment of clinical statuses]),
which remained similar after adjustment for the
clinical statuses of diabetes mellitus and atrial
fibrillation or flutter (HR=0.184, 95% CI=0.032-1.059; P=0.058) [Fig 2]. Numerically lower rates of
ischaemic stroke (n=0 [0%] in the NOAC cohort
vs n=5 [17.9%] in the warfarin cohort), major non-fatal
bleeding (n=0 [0%] in the NOAC cohort vs n=4
[14.3%] in the warfarin cohort), and fatal bleeding
(n=1 [6.7%] in the NOAC cohort vs n=4 [14.3%] in
the warfarin cohort) were observed among patients
receiving NOACs.
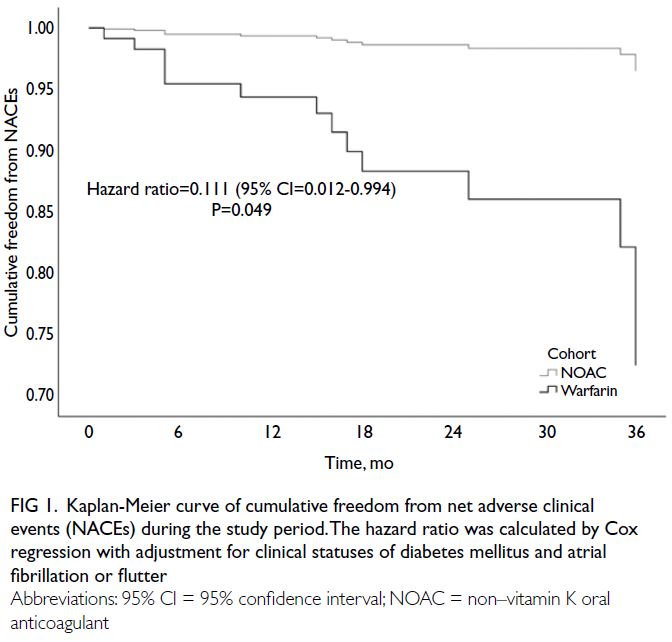
Fig 1. Kaplan-Meier curve of cumulative freedom from net adverse clinical events (NACEs) during the study period. The hazard ratio was calculated by Cox regression with adjustment for clinical statuses of diabetes mellitus and atrial fibrillation or flutter
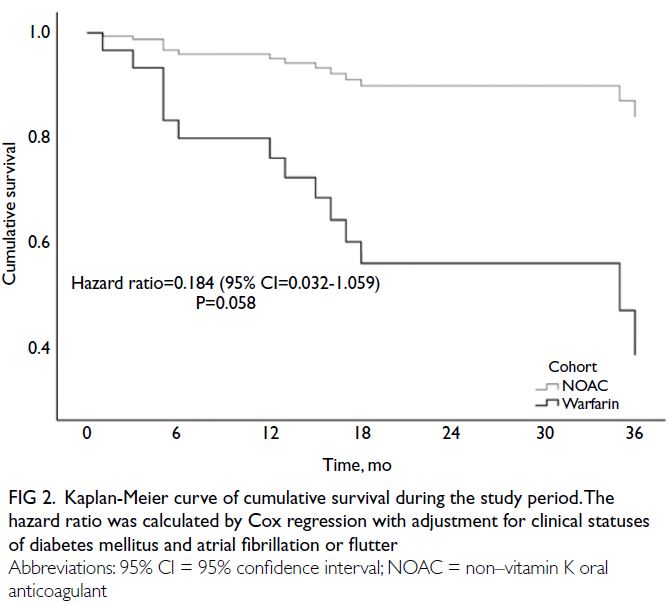
Fig 2. Kaplan-Meier curve of cumulative survival during the study period. The hazard ratio was calculated by Cox regression with adjustment for clinical statuses of diabetes mellitus and atrial fibrillation or flutter
Concerning secondary outcomes, there were
no significant differences between the two cohorts
in LVT resolution (P=0.451) or percentage reduction
in LVT size (P=0.390) at the 3-month follow-up.
The outcomes of patients whose had or had
not discontinued anticoagulation therapy by 1 year
are summarised in Table 3. There were no significant
differences between the two cohorts.
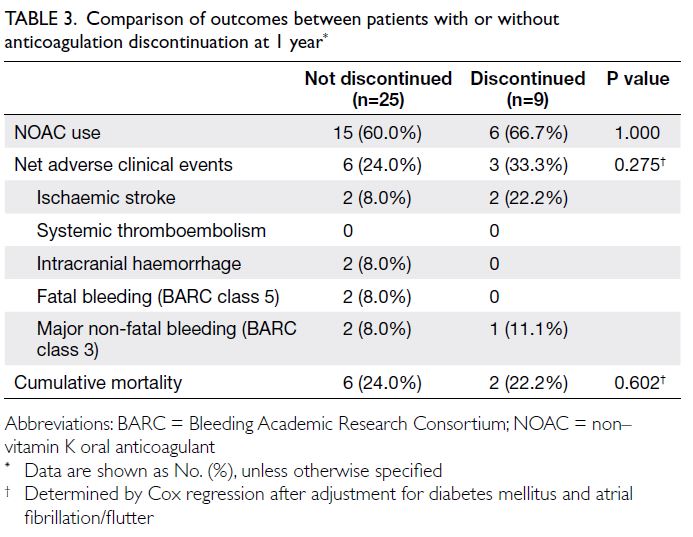
Table 3. Comparison of outcomes between patients with or without anticoagulation discontinuation at 1 year
Discussion
In this retrospective cohort study, we explored the
use of NOACs as an alternative to warfarin for LVT
management in a Hong Kong hospital. Although the
sample size was limited, we found that NOAC use was
associated with significantly fewer NACEs, with a
tendency towards differences in cumulative survival.
Additionally, anticoagulation discontinuation by
1-year post-diagnosis was not associated with
significantly different clinical outcomes.
Our results confirm and extend previous
findings concerning similar rates of LVT regression
between NOAC and warfarin therapies; moreover, it
has been reported that NOAC use is at least non-inferior
to warfarin in terms of cumulative survival.2
Importantly, we demonstrated significantly lower
rates of NACEs in NOAC users, a key finding that
was likely driven by tendencies towards reductions
in ischaemic stroke and major non-fatal bleeding.
The numerically lower rate of major non-fatal
bleeding in NOAC users was consistent with
previous findings of lower bleeding risk among
patients receiving NOACs compared with patients
receiving warfarin.9 10 11 This reduction in bleeding
risk is more prominent among Asian individuals
than among non-Asian individuals.12 Therefore, it is
possible that clinical practice recommendations for
Asian individuals should be different from that for
non-Asian individuals.
A recent study by Abdelnabi et al13
demonstrated significantly more effective resolution
of LVT with rivaroxaban. We did not observe such difference, consistent with recent findings by
Iqbal et al.14 These discrepancies may be related
to differences in imaging intervals: we repeated
echocardiography at 3 months and Iqbal et al14
repeated imaging at a mean interval of 233 days,
whereas Abdelnabi et al13 repeated imaging at 1
month. Importantly, Abdelnabi et al13 observed
converging rates of thrombus resolution by 3 and 6
months after initiation of anticoagulation, when they
performed additional imaging. It is thus possible that
frequent imaging intervals (more frequent than that
recommended by societal guidelines3 4) are required
to demonstrate differences in the rate of thrombus
resolution. Although the clinical benefits of NOACs
in our cohort were mainly driven by a reduction in
bleeding events, more rapid thrombus resolution
may be relevant in other populations. Further
investigation in this area may be warranted.
Another recent study by Robinson et al7
revealed significantly higher rates of systemic
thromboembolism among patients receiving
NOACs, compared with those receiving warfarin. In
the present study, systemic embolism was rare, and
there were no pronounced numerical differences in
the rates of systemic embolism between cohorts.
Although this finding may be partly related to our small
sample size, ethnic differences in thromboembolic
tendencies could also play important roles. It has
been observed that Asian individuals are generally
less susceptible to thromboembolism than Caucasian
and Hispanic individuals,15 consistent with the rarity
of systemic thromboembolism in our cohort. These
findings may imply that any increase in systemic
thromboembolism associated with NOAC use, as
detected by Robinson et al,7 is less relevant for Asian
patients. Considering this lack of relevance and the
reduction in NACEs observed in the present study,
NOAC use may be preferrable to warfarin in Asian
patients. Further studies with larger cohorts should
be conducted to confirm these findings.
Additionally, we observed that considering the
resolution of LVT, anticoagulation discontinuation by
1 year probably did not lead to significantly different
rates of adverse outcomes, despite the numerically
higher rate of cerebrovascular accidents. Although
Lattuca et al2 showed that anticoagulation for ≥3
months reduced the incidence of major adverse
cardiovascular events, it has been unclear whether
anticoagulation can be discontinued after resolution
of LVT. Our results, derived from a small cohort,
warrant further investigation in larger cohorts.
Limitations
There were several limitations in this study. First,
the sample size was limited, primarily due to the
rarity of LVT—although the study was conducted
in a large tertiary hospital, only 43 patients could
be included over a 9-year period. Second, various NOACs were used. Nonetheless, subgroup analysis
was precluded by the small sample size; the present
study design remains valid as a general comparison
of vitamin K versus non–vitamin K anticoagulants,
especially because all included NOACs are
commonly prescribed. Third, more patients in
the NOAC cohort had diabetes mellitus and atrial
fibrillation or flutter. Despite these co-morbidities,
we found that NOACs remained statistically
superior to warfarin for NACEs; we also found a
tendency for better cumulative mortality among
patients receiving NOACs after adjustment for these
two co-morbidities. Thus, our results remain valid
in terms of demonstrating the probable superiority
of NOACs over warfarin for LVT management in
Asian patients.
Conclusion
The use of NOACs to treat patients with LVT was
associated with significantly fewer NACEs, with
a tendency towards lower cumulative mortality.
Additionally, anticoagulation discontinuation by 1
year might be safe for patients with LVT resolution.
Overall, NOACs may be superior to warfarin for LVT
management. Further studies are required to confirm
our findings and determine the optimal duration of
anticoagulation therapy for LVT management.
Author contributions
Concept or design: KKH Kam, JSK Chan.
Acquisition of data: KKH Kam.
Analysis or interpretation of data: JSK Chan.
Drafting of the manuscript: JSK Chan.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: KKH Kam.
Analysis or interpretation of data: JSK Chan.
Drafting of the manuscript: JSK Chan.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
KKH Kam and JSK Chan have disclosed no conflicts of interest. APW Lee received grants, consulting fees/honoraria, and research support from Boehringer Ingelheim, Bayer, and
Pfizer.
Declaration
This research was presented as a poster at the European Society of Cardiology Congress 2021 (27-30 August 2021, online).
Funding/support
This research was supported by the Hong Kong Special
Administrative Region Government Health and Medical
Research Fund (Grant No.: 05160976). The funder had no
role in study design, data collection/analysis/interpretation or
manuscript preparation.
Ethics approval
This research was approved by The Joint Chinese University of
Hong Kong–New Territories East Cluster Clinical Research
Ethics Committee (Ref No.: 2020.425). The need for individual
patient consent was waived by the Committee due to the
retrospective nature of the study.
References
1. Lee JM, Park JJ, Jung HW, et al. Left ventricular thrombus
and subsequent thromboembolism, comparison of
anticoagulation, surgical removal, and antiplatelet agents.
J Atheroscler Thromb. 2013;20:73-93. Crossref
2. Lattuca B, Bouziri N, Kerneis M, et al. Antithrombotic
therapy for patients with left ventricular mural thrombus. J
Am Coll Cardiol 2020;75:1676-85. Crossref
3. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for
the management of acute myocardial infarction in patients
presenting with ST-segment elevation: The Task Force for
the management of acute myocardial infarction in patients
presenting with ST-segment elevation of the European
Society of Cardiology (ESC). Eur Heart J 2018;39:119-77. Crossref
4. Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the
prevention of stroke in patients with stroke and transient
ischemic attack: a guideline for healthcare professionals
from the American Heart Association/American Stroke
Association. Stroke 2014;45:2160-236. Crossref
5. McCarthy CP, Murphy S, Venkateswaran RV, et al. Left
ventricular thrombus: contemporary etiologies, treatment
strategies, and outcomes. J Am Coll Cardiol 2019;73:2007-9. Crossref
6. Kajy M, Shokr M, Ramappa P. Use of direct oral
anticoagulants in the treatment of left ventricular
thrombus: systematic review of current literature. Am J
Ther 2020;27:e584-90. Crossref
7. Robinson AA, Trankle CR, Eubanks G, et al. Off-label use
of direct oral anticoagulants compared with warfarin for
left ventricular thrombi. JAMA Cardiol 2020;5:685-92. Crossref
8. Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding
definitions for cardiovascular clinical trials: a consensus
report from the bleeding academic research consortium.
Circulation 2011;123:2736-47. Crossref
9. Adeboyeje G, Sylwestrzak G, Barron JJ, et al. Major bleeding
risk during anticoagulation with warfarin, dabigatran,
apixaban, or rivaroxaban in patients with nonvalvular atrial
fibrillation. J Manag Care Spec Pharm 2017;23:968-78. Crossref
10. Chan YH, See LC, Tu HT, et al. Efficacy and safety of
apixaban, dabigatran, rivaroxaban, and warfarin in Asians
with nonvalvular atrial fibrillation. J Am Heart Assoc
2018;7:e008150.Crossref
11. Patel P, Pandya J, Goldberg M. NOACs vs. warfarin for
stroke prevention in nonvalvular atrial fibrillation. Cureus
2017;9:e1395. Crossref
12. Yamashita Y, Morimoto T, Toyota T, et al. Asian patients
versus non-Asian patients in the efficacy and safety of
direct oral anticoagulants relative to vitamin K antagonist
for venous thromboembolism: a systemic review and
meta-analysis. Thromb Res 2018;166:37-42. Crossref
13. Abdelnabi M, Saleh Y, Fareed A, et al. Comparative study
of oral anticoagulation in left ventricular thrombi (No-LVT
trial). J Am Coll Cardiol 2021;77:1590-2. Crossref
14. Iqbal H, Straw S, Craven TP, Stirling K, Wheatcroft SB,
Witte KK. Direct oral anticoagulants compared to vitamin
K antagonist for the management of left ventricular
thrombus. ESC Hear Fail 2020;7:2032-41. Crossref
15. Zakai NA, McClure LA. Racial differences in venous
thromboembolism. J Thromb Haemost 2011;9:1877-82. Crossref