Hong
Kong Med J 2019 Aug;25(4):305–11 | Epub 5 Aug 2019
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE CME
Common urological problems in children: primary
nocturnal enuresis
Ivy HY Chan, MB, BS, FHKAM (Surgery); Kenneth KY
Wong, PhD, FHKAM (Surgery)
Department of Surgery, The University of Hong Kong,
Pokfulam, Hong Kong
Corresponding author: Dr Kenneth KY Wong (kkywong@hku.hk)
Abstract
Enuresis is a common complaint in children, with
a prevalence of around 15% at age 6 years. Evidence suggests that
enuresis could affect neuropsychiatric development. The condition may
represent an entire spectrum of underlying urological conditions. It is
important to understand the difference between monosymptomatic and
non-monosymptomatic enuresis. Primary monosymptomatic enuresis can be
managed efficaciously with care in different settings, like primary
care, specialist nursing, or paediatric specialists, while
non-monosymptomatic enuresis requires more complex evaluation and
treatment. The diagnosis, investigation, and management of
the two types of enuresis are discussed in this review.
Introduction
Enuresis is a common problem in children worldwide.
Despite this, many parents underestimate its impact. The reactions from
parents of children with enuresis are diverse, with various misconceptions
among patients and parents.1 Some
parents delay seeking medical assessment to avoid stigmatisation. Many
parents believe that enuresis will be cured with age and does not need any
treatment. Yet, left untreated, this problem may persist into adulthood.2
The most common enuresis condition is primary
monosymptomatic nocturnal enuresis (PMNE). It can also be a symptom of
other urological conditions like detrusor overactivity, neurogenic
bladder, or posterior urethral valve.
The underlying causes of PMNE and
non-monosymptomatic nocturnal enuresis are different. Their treatment and
prognosis thus differ. Both conditions nonetheless can affect patients’
psychological development, self-confidence, and participation in social
events.
What is enuresis?
The term ‘enuresis’ refers to nocturnal urinary
incontinence, while urinary incontinence is involuntary leakage of urine.
This differentiation is very important in establishing diagnosis and hence
formulating treatment plans. The American Psychiatric Association defines
enuresis as recurrent urine leakage in bed >2 nights per week for >3
weeks in patients aged ≥5 years.
In contrast, PMNE refers to those patients who have
never been dry for >6 months and have urinary leakage during sleep time
only. They do not have any other urinary symptoms like daytime urinary
leakage, urinary frequency, or urgency. Hence, patients with symptoms in
addition to isolated bedwetting warrant different evaluation and should be
regarded as having non-monosymptomatic nocturnal enuresis. The
International Children Continence Society (ICCS) has listed the
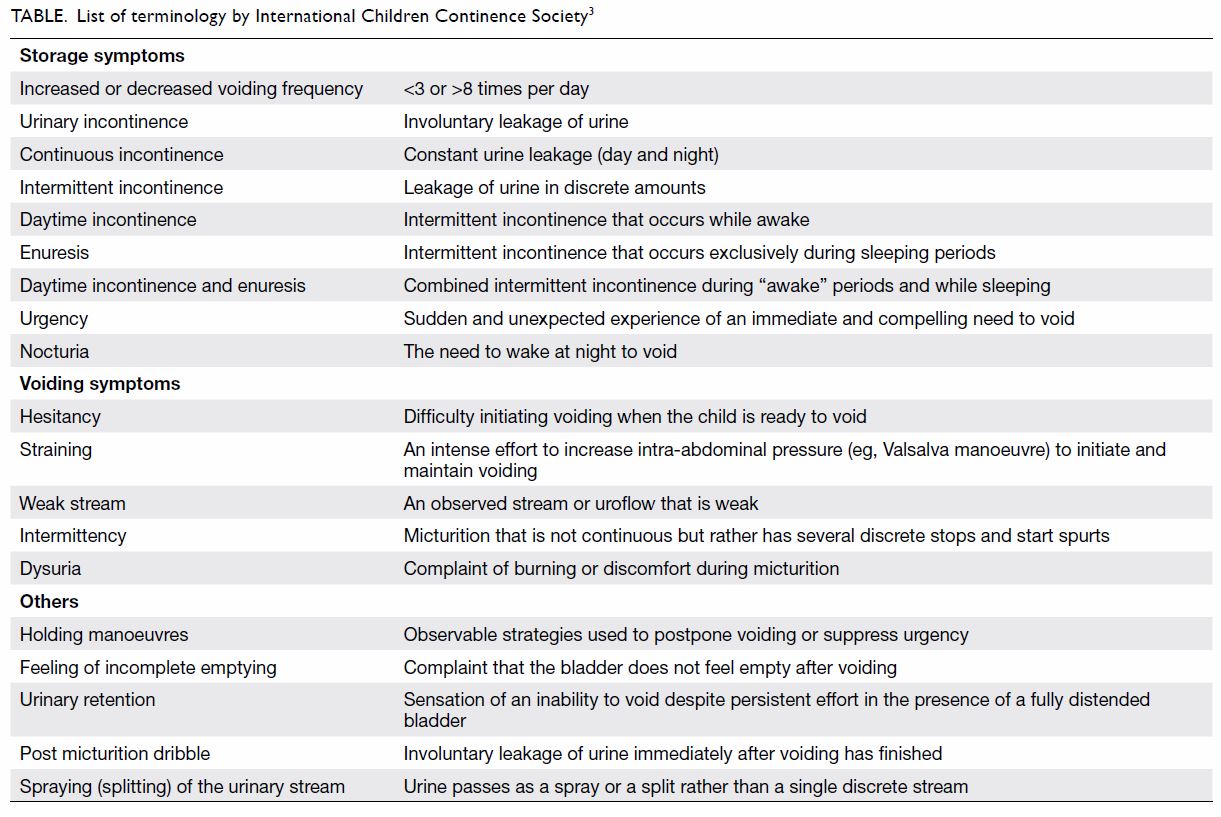
definitions of different terminology for all related symptoms and signs (Table 3).
Prevalence
The prevalence of enuresis in children is usually
quoted as 10% to 15% at age 6 years, 5% at age 10 years, and 0.5% to 1%
among teenagers/young adults.4 A
large-scale epidemiological study in Hong Kong carried out in 2006 showed
the prevalence of nocturnal enuresis as 16.1% at age 5 years, 3.14% at age
9 years, and 2.2% at age 19 years.5
It is more common in boys in all age-groups. The prevalence of nocturnal
enuresis decreases as age increases. However, patients who have daytime
urinary incontinence and those with enuresis >3 nights per week seem to
have symptoms that persist to adulthood, according to a 2004 study that
showed the static prevalence of enuresis over different age-groups from
age 16 to 40 years.6 Thus, the
phenomenon of ‘growing out of enuresis’ may not apply in all patients.
Aetiology and risk factors
Enuresis is a complex condition to which genetic,
physiological, and psychological factors contribute. Some patients have a
definite cause of enuresis or urinary incontinence, like neurogenic
bladder, detrusor overactivity, vaginal reflux, or stress incontinence.
Presence of daytime symptoms definitely warrants a detailed investigation.
it has been hypothesised that small bladder volume in children plays a
role in PMNE. Nonetheless, further studies showed that there was a genetic
component to this condition. Indeed, a Finnish twin study showed a higher
concordance rate of enuresis for monozygotic than dizygotic twins.7 Furthermore, there is a strong association with
parental history of childhood enuresis.8
In this study, the overall odds ratio of having nocturnal enuresis and
urinary incontinence was 10.1 times higher if the father or mother also
had a history of nocturnal enuresis or urinary incontinence.
It is also known that antidiuretic hormone, or
vasopressin, is very important in controlling and concentrating the urine
volume. The normal physiological increase in night-time vasopressin
level/diurnal level has been shown to be absent in some enuretic children.9 10
There is a phenomenon of ‘nocturnal polyuria’, characterised by increased
nocturnal urine output due to a dysregulated diurnal rhythm of
antidiuretic hormone, with a larger volume of urine production at night
time.11 Adults with nocturnal
polyuria syndrome will need to get up to urinate a few times every night.
This group of children will benefit from desmopressin treatment.
Enuresis is classified as a sleep disorder by the
American Psychiatric Association in the fifth edition of the Diagnostic
and Statistical Manual of Mental Disorders. Enuresis is correlated with
the quality of sleep and the arousability of the child. Poor sleep
quality, periodic movement during sleep, and inadequate sleep are factors
associated with enuresis and night-time diuresis.12
13 The awareness of the
relationship between enuresis and obstructive sleep apnoea is also
increasing.13 In 2014, Kovacevic
et al14 showed resolution of
nocturnal enuresis after adenotonsillectomy. Indeed, a systematic review
on this topic also showed that after adenotonsillectomy, an improvement of
nocturnal enuresis was seen in >60% of patients, with a complete
resolution rate in excess of 50%.15
There is also strong association between attention
deficit hyperactivity disorder (ADHD) and enuresis. Children with enuresis
had 2.88 times increased odds of having ADHD compared with those without
enuresis in a population study in the United States.16 Conversely, children with ADHD have also been shown
to have a 2.1 times higher risk of enuresis.17
In addition, treating ADHD with medications like atomoxetine can decrease
the number of wet nights in children with co-morbid enuresis.18
Until now, no conclusions about the causal
relationship between enuresis and childhood behavioural problems could be
drawn. Yet, patients with refractory enuresis may warrant neuropsychiatric
evaluation.19
Why do we need to treat enuresis?
The prevalence of enuresis decreases with age.
Parents may therefore ask whether enuresis really needs to be treated. The
answer is yes because, first, enuresis is a symptom but not a diagnosis.
After specialist medical assessment, other underlying urological problems
may be suspected and further investigations instigated. Second, enuresis
is often associated with poor school performance and decreased quality of
life, self-esteem, and psychosocial development.19
20 A study showed significant
improvement in self-esteem with higher self-concept scores after 6 months
of treatment with desmopressin or alarm therapy.21
Finally, enuresis can persist to adulthood if not treated properly.
About one third of the patients with childhood
enuresis reported symptoms of nocturia despite resolution of enuresis.
About one quarter of them still reported some kind of urinary
incontinence.
Initial assessment and evaluation
Detailed history taking is the key to success in
treating enuresis or urinary incontinence. Bladder function and cognitive
control of voiding take time to develop. By definition, we define enuresis
as persistent urinary incontinence in children aged >5 years. Before
the detailed history, there are three important questions that one should
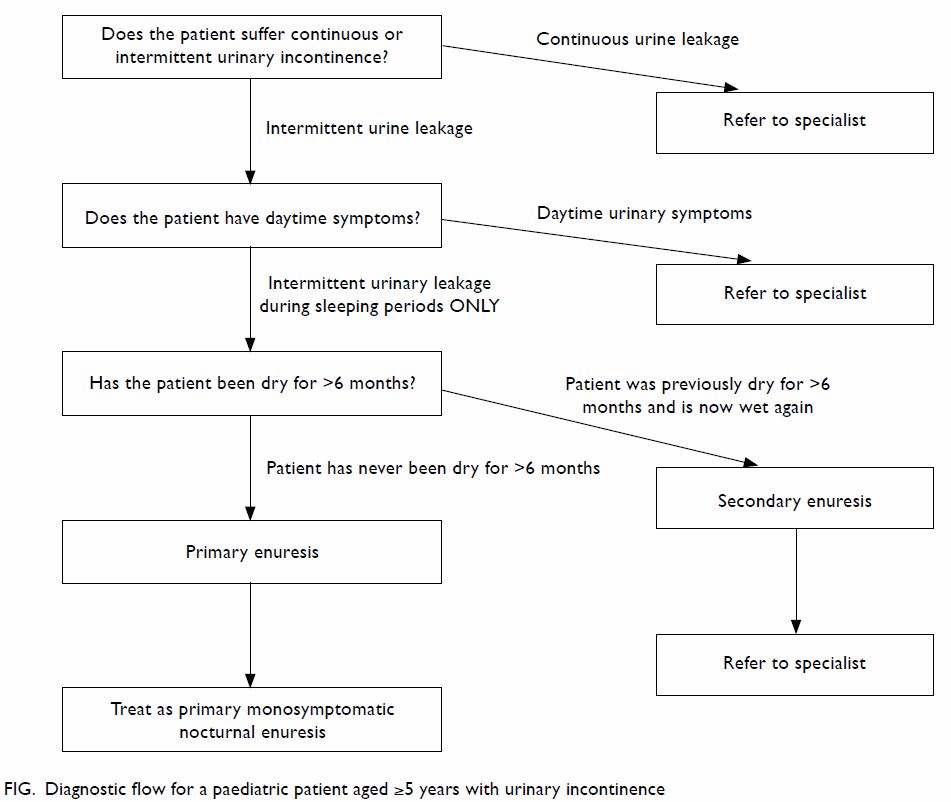
ask (Fig).
Does the patient suffer continuous or intermittent
urinary incontinence?
‘Intermittent incontinence’ is discrete leakage of
urine, whereas ‘continuous incontinence’ is constant urine leakage (both
day and night time). Patients with continuous urinary incontinence usually
have congenital anatomical anomalies, eg, ectopic ureter, exstrophy
variant, etc. These patients will need to be referred to a specialist for
further assessment.
Does the patient have daytime symptoms?
Enuresis per se is simply involuntary leakage of
urine at night time. If patients have symptoms like excessive daytime
urinary frequency (urination >8 times/day), urinary urgency (sudden and
unexpected experience of an immediate and compelling need to void),
daytime urinary incontinence (urine leakage during the awake period),
interrupted urine stream, hesitancy, weak urine stream, or other lower
urinary tract symptoms, they should be referred to a specialist for
further assessment. The list of symptoms, terminology, and definitions is
described in the Table.3
Is this ‘primary enuresis’?
Primary enuresis is defined by the ICCS as a
patient not having been dry for >6 months. If the patient has been dry
for >6 months and then has enuresis again, it is termed secondary, and
the patient should be referred to a specialist for further assessment.
History taking
The aim of history taking is to formulate a correct
diagnosis. As mentioned above, it is important to see whether the patient
has PMNE. If not, the patient may have other conditions like detrusor
overactivity or neurogenic bladder, which may need referral for further
investigation and management. The management of this group of patients is
out of the scope of this review. Questions asked during history taking
should also help to exclude underlying neurological or anatomical
anomalies. Information about bowel function and constipation should be
obtained, as should a brief assessment of psychological history. The
following aspects should be asked.
Urinary symptoms
The following symptoms of urinary storage and
emptying are important.
1. Night-time symptoms: Frequency of bedwetting (times/week), precipitating factors for wetting (eg, long holiday/sleep hours, drinking before bed). This is used to identify the severity of bedwetting.
2. Daytime symptoms: Voiding frequency in daytime (times/day), sudden urgent need to void, habit of voiding postponement, holding manoeuvres (tips of heel pressing on perineum, leg crossing), abdominal straining to void, and interrupted urine stream.
3. Urinary frequency of 3 to 8 times per day is defined as normal by the ICCS. If it is out of this range, the patient may have other problems. The presence of other daytime symptoms may signify that the patient is having non-monosymptomatic enuresis.
1. Night-time symptoms: Frequency of bedwetting (times/week), precipitating factors for wetting (eg, long holiday/sleep hours, drinking before bed). This is used to identify the severity of bedwetting.
2. Daytime symptoms: Voiding frequency in daytime (times/day), sudden urgent need to void, habit of voiding postponement, holding manoeuvres (tips of heel pressing on perineum, leg crossing), abdominal straining to void, and interrupted urine stream.
3. Urinary frequency of 3 to 8 times per day is defined as normal by the ICCS. If it is out of this range, the patient may have other problems. The presence of other daytime symptoms may signify that the patient is having non-monosymptomatic enuresis.
History taking of urinary or voiding symptoms is
often difficult in a child. Adequate time and patience in out-patient
clinics are therefore absolutely necessary. The use of a bladder diary and
enuresis chart is helpful in providing vital information, such as bladder
capacity and the presence of urinary leakage, and helps to identify
underlying bladder dysfunction.
Bowel symptoms
The frequency of bowel movements and the presence
of faecal incontinence should be asked. Bladder and bowel movements are
closely related. Constipation affects the treatment of enuresis: it is
often difficult to completely stop enuresis in a patient with chronic
constipation. Faecal incontinence can also be a symptom of constipation or
underlying spinal cord anomalies.22
Drinking habits
The quantity and quality of drinking during the day
and fluid intake in the evening are important. Evidence of diabetes
insipidus may be obtained from this element of history. With heavy
workloads and tight school schedules in Hong Kong, many patients do not
have adequate fluid intake during daytime. They prefer to drink in the
evening. It is logical to deduce that a large volume of fluid intake in
the evening increases the risk of enuresis. Patients should also be
advised to void before bed. A bladder diary is a vital instrument for the
patient and parents to monitor fluid intake and observe voiding habits.
This diary is shown to the clinician during clinic visits for progress
monitoring.
Possible underlying problems and general medical health
These symptoms include: (a) history of urinary
tract infections, (b) known urological or spinal cord problems, (c)
evidence of ADHD, autism, or other psychological problems, (d) history of
motor development or learning disability, (e) sleep habits, sleep quality
and presence of heavy snoring, and (f) family history, living environment,
or evidence of sexual abuse.
Physical examination
A general physical examination including body
weight and height can help to identify patients with growth retardation or
failure to thrive, which may be suggestive of an underlying disorder. An
abdominal examination can reveal faecal impaction. Examination of the
genitalia and inspection of underwear can identify signs of sexual abuse,
stress incontinence, or faecal incontinence. Dimples or hair tufts in the
lumbosacral area may be caused by occult spinal dysraphism.
A simple urine dipstick test may be helpful when
diabetes mellitus or urinary tract infection is suspected.
Management of primary monosymptomatic nocturnal
enuresis
Once the diagnosis of PMNE is made, advice and
treatment should be given accordingly. The patient and parents should be
educated about the prevalence of enuresis in children and the importance
of treatment. They should know that enuresis is common and that the child
should not be blamed or labelled as lazy. Yet, they should understand that
indolent psychological stress may result if the problem is not dealt with
seriously.
Discussion and general advice
The child should be advised to avoid large amounts
of fluid intake at least 1 to 2 hours before bed, avoid heavy loads of
salt and protein during dinner time, void before bed, and drink adequately
during daytime. Understanding of the patient’s drinking, voiding, and
eating habits is important to treatment adherence and hence success. It is
ideal to keep records on an enuresis chart to illustrate the frequency of
the enuresis and a bladder diary for possible daytime symptoms. Sometimes
it is difficult or unreliable for the patient or caretaker to recall the
pattern of enuresis; keeping a calendar of bed wetting nights should help
to monitor treatment progress. Rewards can also be given for dry nights.
Constipation
Urinary and bowel functions are closely related.
Children with functional constipation are 6.8 times more likely to have
lower urinary tract symptoms, and these patients have higher Voiding
Dysfunction Symptom Scores.23 The
rate of constipation is also much higher in children with urinary
incontinence. After treating the constipation, urinary incontinence
improves, especially in adolescents.24
Bowel habits should be asked, and daily passage of soft stool without
discomfort should be the aim. Principles of treatment should include a
four-step approach: Education, dis-impaction of faeces, prevention of
re-accumulation, and follow-up.25
Advice on fluid intake, fibre intake, and use of laxatives are the common
first-line treatment options. A meta-analysis from the Cochrane library
showed good results of using polyethylene glycol on paediatric patients
with functional constipation.26
Alarm therapy
Enuresis alarms are an effective measure to improve
or cure bedwetting. This is a behavioural therapy that works by
conditioning on arousal. The rationale is to wake up the child with the
alarm once the sensor attached to the child’s pants is wet. The child will
thus gradually learn to wake up before wetting the bed. This requires the
patient to wake up, cease voiding in bed, go to void in the toilet, and
reattach the alarm when they return. In a 2005 meta-analysis, about two
thirds of the children became dry during the treatment period, and nearly
half who persisted with alarm use remained dry after treatment finished.27 Its effect is similar to the use
of desmopressin.28 29 However, its long-term success rate is significantly
higher than that of desmopressin, with 68.8% of one study’s patients who
used enuresis alarms continuing to have dry nights, whereas only 46% of
the desmopressin group did.29
The ICCS recommends that the parent or caregiver
attend the child each time the alarm rings to make sure the child does not
just turn the alarm off to maximise the effect.30
As with other behavioural therapies, this is not an immediate cure. It
requires a trial of a minimum of 2 to 3 months and requires compliance
from the child and family. Despite the promising long-term success rate of
enuresis alarms, the initial dropout rate can be up to 30%.29 This is probably caused by the awkwardness of using
the alarm and the sleep disruption to the whole family. The ICCS also
recommends that the patient be reviewed on the first day of using the
alarm and 2 to 3 weeks after starting use of the alarm to improve
compliance.
Desmopressin
Desmopressin is a synthetic analogue of arginine
vasopressin antidiuretic hormone. It acts on the renal tubules to
concentrate urine. It has been hypothesised that loss of diurnal rhythm of
vasopressin is associated with enuresis in children. In the clinical
setting, desmopressin can rapidly decrease the number of wet nights.31 It has been reported that up to 70% of patients have
dry nights after treatment; yet, 50% of the “successful” patients reported
relapse after stopping the medication.32
Gradual withdrawal of desmopressin is thus advocated to help reduce the
relapse rate.33 Functional bladder
capacity has also been shown to predict the response to desmopressin.
Patients with functional bladder capacity >70% of predicted bladder
capacity were 2 times more likely to respond to desmopressin.34
Desmopressin can be prescribed as tablet or melt
(oral lyophilisate) formulation. It should be taken around 1 hour before
sleep. The melt preparation can help to further decrease fluid intake
before bed35: the patient should
be instructed not to drink any fluid after the medication until the next
morning.
According to ICCS recommendations, the effects of
desmopressin can be evaluated after 2 to 6 weeks with an enuresis diary.36 If there is significant
improvement, treatment can be continued to a maximum of 3 months. If the
patient does not respond well, re-evaluation of symptoms and diagnosis and
referral to a specialist may be needed.
Treatment resistance and other treatment options
Apart from enuresis alarms and desmopressin, there
are other second-line treatment options, but these should ideally be
initiated at the specialist level. It is important to ensure that the
patient is using the enuresis alarm correctly or taking desmopressin daily
before proceeding to second-line treatments. Inability to adhere to the
advice of limiting evening fluid or voiding before bed can also decrease
the overall treatment success rate.
For those patients who respond poorly to the
treatment, re-evaluation should be considered. Questions about bowel
habits and psychiatric conditions should be asked, and a full urine
frequency/volume chart should be recorded. Referral to specialists should
also be considered.
Second-line treatment options include combination
therapy using both enuresis alarm and desmopressin, use of tricyclic
antidepressants, indomethacin, and even daytime diuretics.
Conclusion
Primary monosymptomatic nocturnal enuresis is a
common condition that can impair a child’s psychosocial development.
Affected children and caretakers should be educated about its prevalence,
potential associated problems, associated co-morbidities, and the
necessity of treatment. It should be distinguished from
non-monosymptomatic nocturnal enuresis. Advice about drinking and voiding
habits, use of enuresis alarms, and desmopressin are common treatment
options. Patients with poor response to treatment should be referred to
specialists for re-evaluation.
Author contributions
Concept or design: All authors.
Acquisition of data: IHY Chan.
Analysis or interpretation of data: All authors.
Drafting of the article: IHY Chan.
Critical revision for important intellectual content: KKY Wong.
Acquisition of data: IHY Chan.
Analysis or interpretation of data: All authors.
Drafting of the article: IHY Chan.
Critical revision for important intellectual content: KKY Wong.
Conflicts of interest
As an editor of the journal, KKY Wong was not
involved in the peer review process. The other author has no conflicts of
interest to disclose.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
References
1. Cederblad M, Nevéus T, Åhman A,
Österlund Efraimsson E, Sarkadi A. “Nobody asked us if we needed help”:
Swedish parents experiences of enuresis. J Pediatr Urol 2014;10:74-9. Crossref
2. Goessaert AS, Schoenaers B, Opdenakker
O, Hoebeke P, Everaert K, Vande Walle J. Long-term followup of children
with nocturnal enuresis: increased frequency of nocturia in adulthood. J
Urol 2014;191:1866-70. Crossref
3. Austin PF, Bauer SB, Bower W, et al. The
standardization of terminology of lower urinary tract function in children
and adolescents: update report from the standardization committee of the
International Children’s Continence Society. Neurourol Urodyn
2016;35:471-81. Crossref
4. Franco I, von Gontard A, De Gennaro M;
International Children’s Continence Society. Evaluation and treatment of
nonmonosymptomatic nocturnal enuresis: a standardization document from the
International Children’s Continence Society. J Pediatr Urol 2013;9:234-43.
Crossref
5. Yeung CK, Sreedhar B, Sihoe JD, Sit FK,
Lau J. Differences in characteristics of nocturnal enuresis between
children and adolescents: a critical appraisal from a large
epidemiological study. BJU Int 2006;97:1069-73. Crossref
6. Yeung CK, Sihoe JD, Sit FK, Bower W,
Sreedhar B, Lau J. Characteristics of primary nocturnal enuresis in
adults: an epidemiological study. BJU Int 2004;93:341-5. Crossref
7. Hublin C, Kaprio J, Partinen M,
Koskenvuo M. Nocturnal enuresis in a nationwide twin cohort. Sleep
1998;21:579-85. Crossref
8. von Gontard A, Heron J, Joinson C.
Family history of nocturnal enuresis and urinary incontinence: results
from a large epidemiological study. J Urol 2011;185:2303-6. Crossref
9. Nørgaard JP, Pedersen EB, Djurhuus JC.
Diurnal anti-diuretic-hormone levels in enuretics. J Urol
1985;134:1029-31. Crossref
10. Rittig S, Knudsen UB, Nørgaard JP,
Pedersen EB, Djurhuus JC. Abnormal diurnal rhythm of plasma vasopressin
and urinary output in patients with enuresis. Am J Physiol 1989;256(4 Pt
2):F664-71. Crossref
11. Rittig S, Schaumburg HL, Siggaard C,
Schmidt F, Djurhuus JC. The circadian defect in plasma vasopressin and
urine output is related to desmopressin response and enuresis status in
children with nocturnal enuresis. J Urol 2008;179:2389-95. Crossref
12. Dhondt K, Raes A, Hoebeke P, Van
Laecke E, Van Herzeele C, Vande Walle J. Abnormal sleep architecture and
refractory nocturnal enuresis. J Urol 2009;182(4 Suppl):1961-5. Crossref
13. Alexopoulos EI, Malakasioti G, Varlami
V, Miligkos M, Gourgoulianis K, Kaditis AG. Nocturnal enuresis is
associated with moderate-to-severe obstructive sleep apnea in children
with snoring. Pediatr Res 2014;76:555-9. Crossref
14. Kovacevic L, Wolfe-Christensen C, Lu
H, et al. Why does adenotonsillectomy not correct enuresis in all children
with sleep disordered breathing? J Urol 2014;191(5 Suppl):1592-6. Crossref
15. Lehmann KJ, Nelson R, MacLellan D,
Anderson P, Romao RL. The role of adenotonsillectomy in the treatment of
primary nocturnal enuresis in children: a systematic review. J Pediatr
Urol 2018;14:53.e1-8. Crossref
16. Shreeram S, He JP, Kalaydjian A,
Brothers S, Merikangas KR. Prevalence of enuresis and its association with
attention-deficit/hyperactivity disorder among U.S. children: results from
a nationally representative study. J Am Acad Child Adolesc Psychiatry
2009;48:35-41. Crossref
17. Mellon MW, Natchev BE, Katusic SK, et
al. Incidence of enuresis and encopresis among children with
attention-deficit/hyperactivity disorder in a population-based birth
cohort. Acad Pediatr 2013;13:322-7. Crossref
18. Ohtomo Y. Atomoxetine ameliorates
nocturnal enuresis with subclinical attention-deficit/hyperactivity
disorder. Pediatr Int 2017;59:181-4. Crossref
19. Gulisano M, Domini C, Capelli M,
Pellico A, Rizzo R. Importance of neuropsychiatric evaluation in children
with primary monosymptomatic enuresis. J Pediatr Urol 2017;13:36.e1-6. Crossref
20. Theunis M, Van Hoecke E, Paesbrugge S,
Hoebeke P, Vande Walle J. Self-image and performance in children with
nocturnal enuresis. Eur Urol 2002;41:660-7. Crossref
21. Longstaffe S, Moffatt ME, Whalen JC.
Behavioral and self-concept changes after six months of enuresis
treatment: a randomized, controlled trial. Pediatrics 2000;105(4 Pt
2):935-40.
22. Rasquin A, Di Lorenzo C, Forbes D, et
al. Childhood functional gastrointestinal disorders: child/adolescent.
Gastroenterology 2006;130:1527-37. Crossref
23. Sampaio C, Sousa AS, Fraga LG, Veiga
ML, Bastos Netto JM, Barroso U Jr. Constipation and lower urinary tract
dysfunction in children and adolescents: a population-based study. Front
Pediatr 2016;4:101. Crossref
24. Hodges SJ, Anthony EY. Occult
megarectum—a commonly unrecognized cause of enuresis. Urology
2012;79:421-4. Crossref
25. Burgers RE, Mugie SM, Chase J, et al.
Management of functional constipation in children with lower urinary tract
symptoms: report from the Standardization Committee of the International
Children’s Continence Society. J Urol 2013;190:29-36. Crossref
26. Gordon M, MacDonald JK, Parker CE,
Akobeng AK, Thomas AG. Osmotic and stimulant laxatives for the management
of childhood constipation. Cochrane Database Syst Rev 2016;(8):CD009118. Crossref
27. Glazener CM, Evans JH, Peto RE. Alarm
interventions for nocturnal enuresis in children. Cochrane Database Syst
Rev 2005;(2):CD002911. Crossref
28. Perrin N, Sayer L, While A. The
efficacy of alarm therapy versus desmopressin therapy in the treatment of
primary mono-symptomatic nocturnal enuresis: a systematic review. Prim
Health Care Res Dev 2015;16:21-31. Crossref
29. Önol FF, Guzel R, Tahra A, Kaya C,
Boylu U. Comparison of long-term efficacy of desmopressin lyophilisate and
enuretic alarm for monosymptomatic enuresis and assessment of predictive
factors for success: a randomized prospective trial. J Urol
2015;193:655-61. Crossref
30. Neveus T, Eggert P, Evans J, et al.
Evaluation of and treatment for monosymptomatic enuresis: a
standardization document from the International Children’s Continence
Society. J Urol 2010;183:441-7. Crossref
31. Glazener CM, Evans JH. Desmopressin
for nocturnal enuresis in children. Cochrane Database Syst Rev
2002;(3):CD002112. Crossref
32. Kwak KW, Lee YS, Park KH, Baek M.
Efficacy of desmopressin and enuresis alarm as first and second line
treatment for primary monosymptomatic nocturnal enuresis: prospective
randomized crossover study. J Urol 2010;184:2521-6. Crossref
33. Dalrymple RA, Wacogne ID. Gradual
withdrawal of desmopressin in patients with enuresis leads to fewer
relapses than an abrupt withdrawal. Arch Dis Child Educ Pract Ed
2017;102:335. Crossref
34. Rushton HG, Belman AB, Zaontz MR,
Skoog SJ, Sihelnik S. The influence of small functional bladder capacity
and other predictors on the response to desmopressin in the management of
monosymptomatic nocturnal enuresis. J Urol 1996;156(2 Pt 2):651-5. Crossref
35. De Bruyne P, De Guchtenaere A, Van
Herzeele C, et al. Pharmacokinetics of desmopressin administered as tablet
and oral lyophilisate formulation in children with monosymptomatic
nocturnal enuresis. Eur J Pediatr 2014;173:223-8. Crossref
36. Vande Walle J, Rittig S, Bauer S, et
al. Practical consensus guidelines for the management of enuresis. Eur J
Pediatr 2012;171:971-83. Crossref