Hong
Kong Med J 2018 Oct;24(5):512–20 | Epub 24 Sep 2018
DOI: 10.12809/hkmj187470
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Measles: a disease often forgotten but not gone
Alexander KC Leung, FRCP (UK), FRCPCH1;
KL Hon, MD, FAAP2; KF Leong, MB, BS, MRCPCH3; CM
Sergi, FRCPC, FCAP4
1 Department of Pediatrics, The
University of Calgary, Calgary, Alberta, Canada
2 Department of Paediatrics, The Chinese
University of Hong Kong, Shatin, Hong Kong
3 Department of Pediatrics, Kuala Lumpur
General Hospital, Kuala Lumpur, Malaysia
4 Department of Pediatrics and
Department of Laboratory Medicine and Pathology, The University of
Alberta, Edmonton, Alberta, Canada
Corresponding author: Prof Alexander KC Leung (aleung@ucalgary.ca)
Abstract
Measles (rubeola) is a highly contagious
vaccine-preventable disease caused by the measles virus—a virus of the Paramyxoviridae
family. The illness typically begins with fever, runny nose, cough, and
pathognomonic enanthem (Koplik spots) followed by a characteristic
erythematous, maculopapular rash. The rash classically begins on the
face and becomes more confluent as it spreads cephalocaudally.
Laboratory confirmation of measles virus infection can be based on a
positive serological test for measles-specific immunoglobulin M
antibody, a four-fold or greater increase in measles-specific
immunoglobulin G between acute and convalescent sera, isolation of
measles virus in culture, or detection of measles virus ribonucleic acid
by reverse transcriptase-polymerase chain reaction. Complications occur
in 10% to 40% of patients, and treatment is mainly symptomatic.
Bacterial superinfections, if present, should be properly treated with
antibiotics. To eradicate measles, universal childhood immunisation and
vaccination of all susceptible individuals with measles vaccine would be
ideal. In developed countries, routine immunisation with
measles-containing vaccine is recommended, with the first and second
doses at ages 12 to 15 months and 4 to 6 years, respectively. The World
Health Organization recommends that the first and second doses of
measles-containing vaccine be given at ages 9 months and 15 to 18
months, respectively, in countries with high rates of measles
transmission.
Introduction
Measles (rubeola) is an extremely contagious, acute
febrile viral illness. The illness typically begins with fever, runny
nose, cough, and pathognomonic enanthem (Koplik spots) followed by a
characteristic erythematous, maculopapular rash. Prior to the introduction
of measles vaccine, measles was responsible for more than 2 million deaths
worldwide annually.1 2 3 The incidence
has declined dramatically over the past 20 years, and measles-associated
mortality had decreased to slightly more than 100 000 by 2015 thanks to
the increasingly widespread use of attenuated measles vaccines.2 Nevertheless, as of today, measles remains an important
cause of morbidity and mortality in young children globally, especially in
developing countries.4 As such,
physicians should familiarise themselves with this disease and be able to
recognise it early so that isolation measures can be promptly instituted
to prevent its spread. This article provides an update on current
knowledge about measles and outlines an approach to its evaluation and
management.
A PubMed search was conducted in May 2018 using
Clinical Queries with the key terms “Measles” and “Rubeola”. The search
strategy included meta-analyses, randomised controlled trials, clinical
trials, observational studies, and reviews. Discussion is based on, but
not limited to, the search results.
Aetiology
The causative organism of measles is the measles
virus, a paramyxovirus belonging to the genus Morbillivirus under
the family Paramyxoviridae of the order Mononegavirales.2 5
The measles virus is spherical, with a diameter ranging from 100 to 200
nm, and shows pleomorphism.5 6 The virus contains a single strand of ribonucleic acid
(RNA) of negative polarity enclosed within a lipid capsule.5 7 The
non-segmented genome is approximately 16 000 nucleotides in length and
contains six genes for eight viral proteins (six structural and two
non-structural proteins).2 7 8 The six
structural proteins are haemagglutinin protein, fusion protein,
nucleocapsid protein, phosphoprotein, matrix protein, and large protein.4 The haemagglutinin protein binds
to cellular receptors and enables the virus to attach to host cells.2 4 The fusion
protein enables fusion of the viral envelope with the host cell plasma
membranes, thereby allowing entry of viral ribonucleoproteins into the
cytoplasm of the host cell.2 6 The phosphoprotein maintains connection with the
nucleocapsid protein and large protein to ensure proper viral
transcription and replication.5 The
matrix protein interacts with the ribonucleoprotein complex and the
cytoplasmic tails of haemagglutinin protein and fusion protein and thus
plays a role in cell fusion.5 The
two non-structural proteins, V protein and C protein, are encoded within
the phosphoprotein gene.2 8 Although these two non-structural proteins have no role
in maintaining viral structure, they act as virulence factors,
facilitating suppression of the host’s innate immune response by
suppressing interferon production and facilitating virus replication.2 4 5
Epidemiology
Humans are the only known hosts of measles.4 5 9 The virus can be transmitted by inhalation of
virus-laden airborne droplets or small-particle aerosols that remain
suspended in the air, direct contact with infected secretions, and less
commonly, contact with contaminated fomites.2
9 10
11 Generally, the virus can
survive in the air or on fomites for up to 2 hours.1 11 As such,
disease transmission does not require direct contact with an infected
person.7 Measles is a highly contagious disease: up to 90% of susceptible
contacts develop the disease.11 12 13
Prior to the introduction of the measles vaccine,
more than 90% of children contracted measles by age 15 years.6 14 Since the
introduction of the vaccine, the disease has become increasing rare in
North America and many developed countries.11
Globally, the number of reported cases of measles decreased from 146 cases
per million in 2000 to 36 cases per million in 2015.14 Most reported cases in 2015 were from Africa. In
decreasing order of frequency, the Western Pacific and South-East Asia
regions had the next highest frequencies of measles cases.2 In the US, the
annual incidence of measles was 0.08 and 2.06 per million population in
2001 and 2015, respectively.15
Currently, measles cases in developed countries are primarily “imported”
from countries where measles is endemic and occur almost exclusively in
unvaccinated or incompletely vaccinated individuals.11 14 Outbreaks
of measles may occur because of immunity gaps in spite of high overall
vaccine coverage.2 In developed
countries, vaccine negligence or refusal is problematic and accounts for
such outbreaks.16 17 18 19 In March 2018, there was an outbreak of measles in
Okinawa, Japan. The measles virus is believed to have entered Japan via
travellers in Taiwan.
Genetic characterisation of the measles virus has
identified eight classes (A-H), which can be subdivided into 24 genotypes.20 Group A viruses circulate mainly
in China, the US, the United Kingdom, Russia, and Argentina. Group B and C
viruses circulate mainly in Japan, South Africa, and the Philippines.
Group D and E viruses circulate mainly in Western Europe. Group F viruses
circulate mainly in Africa. Group G viruses circulate mainly in Canada,
Malaysia, and Indonesia. Group H viruses circulate mainly in China, Japan,
and Korea.20
Measles affects both sexes equally,14 and young children are the most susceptible
age-group.4 12 Infants born to mothers with vaccine-induced immunity
become susceptible to measles at an earlier age than those born to mothers
with naturally acquired immunity.2
Almost all infants lose their maternal immunity by age 6 months.21 However, breastfeeding has a protective effect:
breast milk is more likely to contain measles haemagglutination antibodies
than blood samples from infants.22
Other risk factors include being an infant who is
too young to be vaccinated, being an unvaccinated or partially vaccinated
individual, travelling to endemic areas, exposure to sick individuals with
fever and respiratory symptoms from endemic areas, household exposure,
immunodeficiency, malnutrition, and vitamin A deficiency.7 14
The period of infectivity is maximal in the
prodromal phase, before the onset of rash; this coincides with peak levels
of measles virus in the respiratory tract and viraemia, which facilitate
transmission.12 21 Patients are infectious 4 days prior to through 4
days after the onset of the rash.12
21
In temperate climates, measles is most common in
late winter and early spring.2
Pathophysiology
The inhaled virus from airborne droplets or
small-particle aerosols initially infects dendritic cells, lymphocytes,
and alveolar macrophages in the susceptible host’s respiratory tract.2 10 The
haemagglutinin protein on the viral surface binds to host cell receptors
such as human membrane cofactor protein (CD46), signalling lymphocytic
activation molecule (CD150), and nectin 4 (PVRL4).2 3 4 5 6 8 10 The fusion protein on the viral surface induces
fusion of the viral envelope with the host cell’s plasma membranes and
fusion between infected host cells and neighbouring cells.8 Fusion of the viral envelope with the host cell’s
plasma membranes facilitates the release of viral ribonucleoproteins into
the cytoplasm of the host cell, while fusion between infected host cells
and neighbouring cells results in the formation of multinucleated giant
cells.2 4
6 8
The virus initially replicates locally in the epithelial cells of the
upper respiratory tract and then spreads to local lymphatic tissue.2 10 Direct
cell-to-cell transmission is responsible for dissemination of the virus
within the host.8
The virus is then disseminated to other
reticuloendothelial sites via the blood stream (primary viraemia).7 Secondary viraemia occurs several days after primary
viraemia, facilitating circulation of the virus to multiple organs such as
the skin, lymph nodes, trachea, nose, gastrointestinal tract, liver,
kidney, and bladder.2 7 10 Virus
replication in epithelial cells, endothelial cells, lymphocytes,
monocytes, and macrophages may account for the clinical features and
complications of measles virus infection.23
Infected lymphocytes and dendritic cells transfer the virus to the
epithelial cells of the respiratory tract, which are then shed through the
damaged epithelium and expelled as respiratory droplets during coughing
and sneezing, thereby enabling respiratory transmission to susceptible
individuals.2 4
Measles virus infection triggers both humoral and
cellular immune responses. Cellular immune responses to the virus are
vital for viral clearance and recovery, and individuals with T-cell
deficiencies often develop severe complications or fatal disease.2
During the acute infection and for several weeks to
months afterwards, humoral and cellular responses to new antigens are
impaired. This can persist for weeks to months, rendering the individual
more susceptible to infections caused by other pathogens.5 21 Immune
amnesia results from replacement of the established memory cell repertoire
by measles virus-specific lymphocytes.2
Delayed-type hypersensitivity is also decreased.
Histopathology
The architecture of measles-infected lymph nodes
typically shows diffuse follicular, paracortical immunoblastic hyperplasia
and diffuse effacement of the lymph node architecture. This pattern may
give the appearance of a mottled (moth-eaten) pattern on
haematoxylin-eosin staining. Warthin-Finkeldey multinucleated giant cells
occur in the prodromal phase of measles in hyperplastic lymphatic tissues.23 When antibody titres increase,
or at the time of cutaneous eruption, the Warthin-Finkeldey giant cells
disappear. Thus, they are observed only sporadically by pathologists in
nodal biopsies. Warthin-Finkeldey giant cells are syncytial cells with
diameters of 25 to 150 μm, abundant oeosinophilic cytoplasm, and 4 to 50
hyperchromatic nuclei located at the centre of the syncytia. These cells
may also be observed among buccal, conjunctival, or nasopharyngeal cells.23
Clinical manifestations
The incubation period of measles varies from 7 to
21 days, with a median of 13 days.12
18 The prodromal phase lasts 2 to
4 days.7 23
Prodromal illness caused by measles is characterised by increasing fever,
anorexia, malaise, and the classic triad of the three “C”s: coryza (runny
nose), cough, and conjunctivitis (red, watery eyes).24 Photophobia, peri-orbital oedema, and myalgias may
also be present and suggestive of influenza virus infection. One to two
days prior to the onset of the exanthem, 1- to 3-mm bluish-white papules
with the appearance of “grains of sand or rice” on an erythematous base,
which are called Koplik spots, appear on the buccal mucosa opposite the
molars; these are pathognomonic for measles infection (Fig
1).23 However, Koplik spots
are present in only 60% to 70% of patients and usually last 12 to 72
hours.7 21
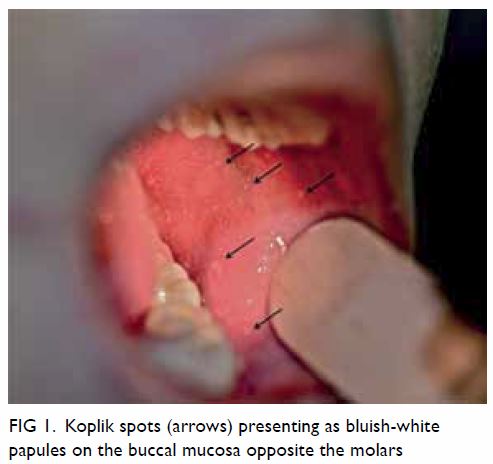
Figure 1. Koplik spots (arrows) presenting as bluish-white papules on the buccal mucosa opposite the molars
Typically, morbilliform exanthem appears 3 to 4
days after the onset of fever and peaks with the appearance of exanthem,11 which consists of blanching,
erythema, macules, and papules that classically begin on the face, around
the hairline, on the sides of neck, and behind the ears.11 25 The rash
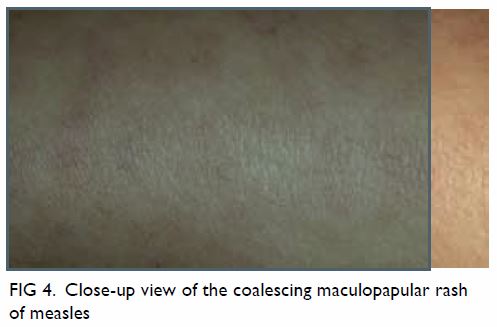
becomes more confluent as it spreads downwards to the trunk and
extremities (Figs 2 3 4).1 9 The lesions are more tense around the shoulders.7 The palms of the hands and soles of the feet are rarely
affected.26 The lesions may be
petechial or ecchymotic.7 The rash
lasts for 5 to 10 days and fades in the same directional pattern in which
it appears.7 Brownish
discolouration (especially in patients of Caucasian descent; Fig
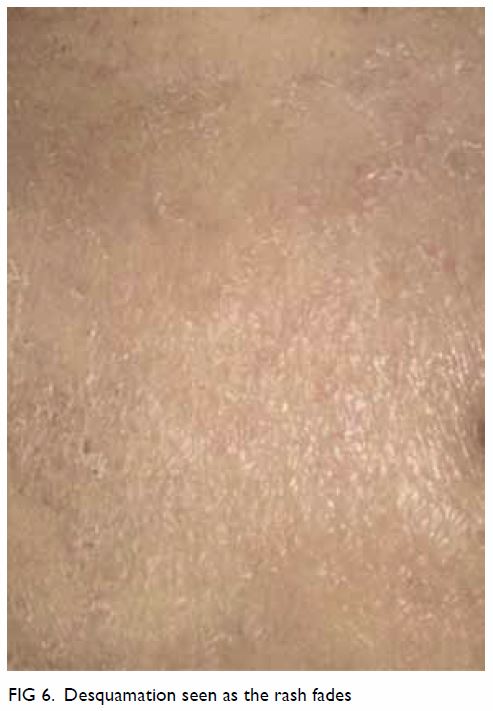
5) with fine desquamation (especially in malnourished patients; Fig 6) sometimes occurs as the rash fades.2 23 Fever
usually subsides as the rash fades.12
Persistence of fever usually indicates complications.
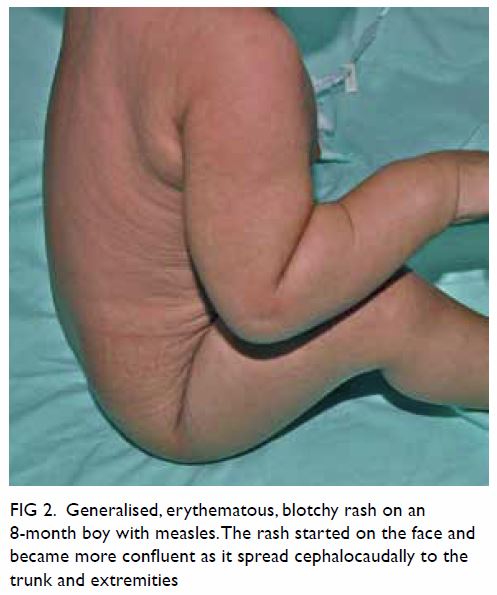
Figure 2. Generalised, erythematous, blotchy rash on an 8-month boy with measles. The rash started on the face and became more confluent as it spread cephalocaudally to the trunk and extremities
Coughing is consistently present and may persist
for weeks.7 Sore throat, abdominal
pain, cervical lymphadenopathy, and (less commonly) splenomegaly may also
be present.23
Modified measles occurs in those with pre-existing
but incompletely protective immunity to measles from either vaccination,
previous exposure to the measles virus, transplacental transfer of
anti-measles antibody, or receipt of intravenous immunoglobulin.2 23 Patients
with modified measles have a longer incubation period, milder and less
characteristic clinical manifestations, and faster resolution.23 27 These
patients might not have coryza, cough, or conjunctivitis, and they are
less contagious.23 27
Atypical measles occurs in individuals who were
vaccinated with the killed-virus measles vaccine and who are subsequently
exposed to wild-type measles virus.23
The killed-virus measles vaccine was used in the US between 1963 and 1967.23 The vaccine sensitised
individuals to measles virus antigens without providing full protection.23 Patients with atypical measles
present with headache and high, prolonged fever.23
Typically, a maculopapular rash begins on the distal extremities
(including the palms of the hands and soles of the feet) and spreads
centripetally to the trunk.23 The
rash may be vesicular, petechial, purpuric, or urticarial.23 Severe pneumonia can also occur. Bilateral pulmonary
nodules and hilar lymphadenopathy are characteristic.23 Some patients may have oedema of the hands and feet,
paraesthesia/hyperesthesia, and hepatosplenomegaly.1 12 22 Atypical measles is noncontagious.12
Complications
Complications occur in approximately 10% to 40% of
patients and are more common and severe in very young, very old, pregnant,
immunocompromised, and malnourished patients.1
11 12
21 Pneumonia accounts for 60% of
measles-associated death.6
Pneumonia can be caused by the measles virus itself (Hecht giant cell
pneumonia), or it may be caused by a secondary viral (eg, adenovirus,
herpes simplex virus) or bacterial (eg, Streptococcus pneumoniae,
Staphylococcus aureus) pathogen.4
Other respiratory tract complications include otitis media, sensorineural
hearing loss, otosclerosis, tonsillitis, sinusitis,
laryngotracheobronchitis (“measles croup”), bronchitis, and exacerbation
of tuberculosis.12 28 29
Gastrointestinal complications include gastroenteritis, gingivostomatitis,
pericoronitis, mesenteric lymphadenitis, hepatitis, pancreatitis, and
appendicitis.23 28 30
Ophthalmological complications include keratoconjunctivitis, corneal
ulceration, and blindness.18
Haematological complications include thrombocytopenia and disseminated
intravascular coagulopathy.23
Cardiac complications include pericarditis and carditis.23 Renal complications include glomerulonephritis and
acute renal failure.12
Neurological complications include febrile
seizures, primary measles encephalitis, acute post-infectious
encephalomyelitis, measles inclusion body encephalitis, and subacute
sclerosing panencephalitis.12 31 32
33 Approximately one in 1000
patients with measles develop primary measles encephalitis, typically on
day 5 of the rash (range: 1-14 days). That condition is fatal in
approximately 10% of cases. Acute post-infectious encephalomyelitis is an
autoimmune demyelinating disease that occurs approximately one in 1000
patients with measles. The condition typically manifests during the
recovery phase, within 2 weeks of the rash. Measles inclusion body
encephalitis occurs mainly in patients with impaired cellular immunity
within months of the measles infection. That condition may cause
progressive brain damage and has a high mortality rate. Subacute
sclerosing panencephalitis is a fatal, progressive degenerative central
nervous system disease that usually presents 5 to 10 years after the
measles virus infection.
Measles in pregnancy is associated with an
increased risk of spontaneous abortion, premature labour, low birth
weight, intrauterine foetal death, stillbirth, serious measles infection
in the neonate, and maternal death.4
11 21
Other problems can include absence from school by
infected children and loss of income for parents who stay at home to care
for them. Thus, the disease has an adverse effect on quality of life.2
Diagnosis and differential diagnosis
Measles should be suspected in the presence of all
the following: fever ≥101°F (38.3°C); erythematous maculopapular
(non-vesicular) rash spreading cephalocaudally from the face downwards and
lasting 3 or more days; and at least one of the three “C”s: coryza, cough,
or conjunctivitis.11 Suspicion
should be particularly strong in individuals with no measles immunity if
there is a history of exposure to measles, travel to endemic areas (eg,
Africa, Western Pacific, and South-East Asia regions), or during an
outbreak of measles. Koplik spots, if present, are pathognomonic.
Diagnosis can be difficult in areas with low measles incidence.
Differential diagnosis includes rubella, roseola,
varicella, erythema infectiosum, hand-foot-mouth disease, drug eruptions,
scarlet fever, toxic shock syndrome, infectious mononucleosis, Rocky
Mountain spotted fever, meningococcaemia, Henoch-Schönlein purpura,
systemic lupus erythematosus, Kawasaki disease, and serum sickness.
Laboratory confirmation of measles virus infection
can be based on a positive serological test for measles-specific
immunoglobulin M (IgM) antibody; a four-fold or greater increase in
measles-specific IgG titres between acute and convalescent sera; isolation
of measles virus from cultures of blood mononuclear cells, urine,
conjunctival swabs, or nasopharyngeal secretions; or detection of measles
virus RNA by reverse transcriptase-polymerase chain reaction (RT-PCR) from
blood, throat, nasal, nasopharyngeal, or urine samples.12 13 23 24
Serological testing for measles-specific IgM antibody is the most commonly
used method for confirmation of measles virus infection.2 Unfortunately, measles-specific IgM antibody might not
be detectable until 4 or more days after the onset of rash, which may
result in false negative results if the test is conducted early.2 Only approximately 75% of affected individuals will
have detectable measles-specific IgM antibody within the first 72 hours
after rash onset, but almost all affected individuals will have detectable
measles-specific IgM antibody 96 hours after rash onset.2 In addition, false positive results may rarely occur in
patients with infectious mononucleosis, rubella, parvovirus B19 infection,
and rheumatological diseases.12
Measles virus RNA testing by RT-PCR, if available, may be preferred to
serological testing, as the test is more specific, becomes positive before
measles-specific IgM antibody is detectable, and allows genotype
identification.1 2 21
Management
Treatment is mainly symptomatic and consists of the
use of antipyretics, prevention and control of dehydration, adequate
nutrition, and infection control measures.6
Bacterial infections, if present, should be properly treated with
appropriate antibiotics.6 A 2017
Cochrane systematic review showed that vitamin A supplementation is
associated with a significant reduction in mortality and morbidity in
children with measles.34 It is
recommended that vitamin A be administered to all children with acute
measles orally once daily for 2 consecutive days at age-specific doses (50
000 IU, 100 000 IU, and 200 000 IU to infants <6 months, infants aged 6
to 11 months, and children >12 months, respectively).11 23 For
children with clinical evidence of vitamin A deficiency, a third
age-specific dose is recommended 2 to 4 weeks later.11 23
There is no specific antiviral therapy for patients
with measles. Although in vitro studies have shown that the measles virus
is susceptible to ribavirin, and preliminary studies have shown the
efficacy of ribavirin in treatment of patients with measles,35 36 no
randomised controlled studies have assessed its clinical efficacy and
safety profile. Hopefully, well-designed, large-scale, randomised,
double-blind, placebo-controlled trials will provide more information on
the efficacy and safety profile of ribavirin in children with measles in
the future. Until such information is available, ribavirin cannot be
routinely recommended.
Prevention
Active immunisation
To eliminate measles, population vaccination rates
must be over 93%.4 7 Universal childhood immunisation and vaccination of all
susceptible patients with measles vaccine is recommended.37 Measles vaccines in current use contain live
attenuated measles strains that replicate within the host to induce both
humoral and cellular immunity.2 25 A single dose of measles vaccine
given at or after age 1 year is 93% to 95% effective at protecting against
measles, whereas two doses given at appropriate intervals are nearly 100%
effective.7 11 21 However,
measles vaccine given at age 9 months is only 85% protective.21
Measles vaccines can be given as a single component
(eg, in Russia and some African countries), but they are more often given
as combination vaccines, such as measles-mumps-rubella (MMR) and
measles-mumps-rubella-varicella vaccines.21
The measles-mumps-rubella-varicella vaccine has similar immunogenicity and
safety profiles to those of the MMR vaccine, except that there is a
two-fold increase in the relative risk of febrile seizures.21
In developed countries like the US and Canada,
routine immunisation with MMR vaccine is recommended, with the first and
second doses given at ages 12 to 15 months and 4 to 6 years, respectively.38 39
Measles-containing vaccine is not routinely given before age 12 months
because of the less desirable immune response before that age.7 11 The World
Health Organization recommends that the first and second doses of
measles-containing vaccine be given at 9 months and 15 to 18 months,
respectively, in countries with high rates of measles transmission.37
Measles-containing vaccine should be offered to
susceptible individuals (including children aged 6-11 months) who are at
higher risk of contracting measles: travellers to endemic areas, high
school and college students, health care personnel, and those in the
presence of a measles outbreak.12
Children who receive one dose of measles-containing vaccine prior to age
12 months should receive two additional doses, separated at least by 28
days, after age 12 months, as doses given before age 12 months should not
count as valid doses.21 38
Measles vaccines are generally safe and well
tolerated. Adverse effects usually occur 5 to 12 days post-vaccination and
consist mainly of fever, rash, and arthralgia.11
25 There is a possible association
between measles vaccine and acute disseminated encephalomyelitis, but the
excess risk is not likely to be more than 1.16 cases of acute disseminated
encephalomyelitis per million vaccines administered.40 The accusation that MMR vaccine may lead to autism
spectrum disorders is baseless. In 1998, Wakefield et al41 reported 12 children with ileal-lymphoid-nodular
hyperplasia, non-specific colitis, and pervasive developmental disorder.
The authors hypothesised that MMR vaccine could trigger bowel dysfunction
leading to gastrointestinal absorption of neurotoxic peptides, with
resulting damage to the central nervous system and autism spectrum
disorders. The article was found to be fraudulent and was retracted 12
years later. The speculative paper contained a hypothesis that was found
by ethical and misconduct committees to have been investigated
fraudulently because data were missing. Moreover, the hypothesis was not
properly investigated, raising concerns about the quality of the peer
reviewers. Unfortunately, the later retracted article was available online
for almost 12 years and promoted parental concerns about the safety of MMR
vaccine, leading to lower vaccination levels and outbreaks of measles
infection in several countries. An evidence-based meta-analysis of five
cohort studies (n=1 256 407) and five case-control studies (n=9920) found
no evidence for a link between MMR vaccination and the subsequent
development of autism or autistic spectrum disorders.42
Contra-indications for measles vaccination include
hypersensitivity to any component of the vaccine, including gelatine and
neomycin; confirmed history of an anaphylactic reaction to a previous
measles-containing vaccine; cellular immune deficiency; moderate or severe
illness; any febrile illness; and pregnancy.7
9 11
25 Measles vaccination should be
deferred in individuals who have recently used high-dose corticosteroids,
immunoglobulin, or blood products.9
Almost all states in the US require children to have two doses of
measles-containing vaccine to enrol in public school kindergartens.
Despite this requirement, the Centers for Disease Control and Prevention
(CDC) state-level analysis of kindergarten MMR vaccination rates for the
2014 to 2015 school year found that the median state-level coverage was
94%.43
Postexposure prophylaxis
Measles vaccination given to susceptible contacts
within 72 hours of exposure is effective at preventing illness or
modifying illness severity and is preferred to immunoglobulin.1 9 37 With the exception of pregnant women and
immunocompromised individuals, measles-containing vaccine should be given
to individuals who cannot provide evidence of immunity to measles as
postexposure prophylaxis.26
Passive immunisation with immunoglobulin
administered intramuscularly or intravenously within 6 days of exposure
can also be used to prevent or modify the clinical course of measles.1 33 A Cochrane
systematic review of seven studies (n=1432) showed that passive
immunisation with immunoglobulin is effective at preventing mortality from
measles, reducing the risk by 76% compared with no treatment.44 Susceptible people for whom prophylaxis with
immunoglobulin is indicated following significant exposure to measles
include pregnant women, household contacts aged <12 months, and
immunocompromised individuals.6 33 The recommended dose of
immunoglobulin administered intramuscularly is 0.25 to 0.5 mL/kg of body
weight (maximum 15 mL), and the recommended dose administered
intravenously is 400 mg/kg of body weight.9
12 37
Individuals weighing >30 kg should receive intravenously administered
immunoglobulin to achieve protective antibody levels.12 Measles vaccination should not be given within 6
months of immunoglobulin administration.12
Infection control
Suspected measles cases should be reported to local
public health authorities, and patients with suspected measles should be
seen in well-ventilated or negative pressure rooms isolated from other
patients. Health care providers and patients should wear appropriate masks
(eg, N95) or respirators.1 Only
health care providers with immunity to measles should be involved in the
care of such patients. Confirmed cases of uncomplicated measles can
usually be managed at home.12
Proper hand washing technique should be emphasised.6 Patients with measles should remain in strict airborne
isolation and should be excluded from school or work for at least 4 to 7
days after the onset of rash.1 6 23
Sick patients such as those with respiratory distress, dehydration, and
immunodeficiency should be managed in hospitals with strict airborne
transmission precautions for at least 4 days after the onset of rash in
otherwise healthy patients or for the duration of illness in
immunocompromised patients.12 23 Health care personnel should
wear appropriate masks or respirators when entering the patient’s room and
adhere to strict hand washing technique lasting several minutes. Depending
on the number of air changes per hour, the patient’s room should not be
used for up to 207 minutes after the patient’s departure.45 This is the time required for 99.9% efficient
airborne contaminant removal with 2 air changes per hour. The times
required for 99% and 99.9% efficient airborne contaminant removal with
other numbers of air changes per hour can be found in the “Airborne
contamination removal” table (Appendix B, Table B.1).45 Individuals susceptible to measles should be excluded
from school or work from the 5th through 21st day after last exposure; the
duration should be extended to 28 days if prophylactic immunoglobulin is
given.46 Such individuals should
report promptly to local public health authorities, and their care should
adhere to the same airborne transmission precautions as that of suspected
cases, should they develop any symptoms or signs of measles during that
period.12
Prognosis
The case fatality ratio ranges from less than 0.1%
to 5%, depending on the age of measles acquisition, nutritional status,
vaccine coverage, underlying conditions (eg, immunodeficiency, chronic
illness), and access to health care.8
47 Death is usually caused by
pneumonia and diarrhoea in developing countries.47
Immunity following measles virus infection is life long and is caused by
neutralising IgG antibodies to the haemagglutinin protein and creation of
memory cells.2 21 Second primary attacks are extremely rare.23
Conclusions
Measles is highly contagious and can lead to
serious and potentially fatal consequences among individuals who have not
been vaccinated. To eliminate measles from a population, universal
childhood immunisation and vaccination of all susceptible individuals with
measles vaccine is recommended. Generally, vaccination against measles is
effective and safe. Outbreaks of measles may occur because of immunity
gaps despite high overall vaccine coverage. In developed countries,
vaccine refusal is problematic and accounts for such outbreaks.
Author contributions
All authors have made substantial contributions to
the concept or design of this study; acquisition of data; analysis or
interpretation of data; drafting of the article; and critical revision for
important intellectual content.
Declaration
As an editor of the journal, KL Hon was not involved in the peer review of the article. All authors have disclosed no conflicts of interest. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
References
1. Kumar D, Sabella C. Measles: back again.
Cleve Clin J Med 2016;83:340-4. Crossref
2. Moss WJ. Measles. Lancet
2017;390:2490-502. Crossref
3. Strebel PM, Cochi SL, Hoekstra E, et al.
A world without measles. J Infect Dis 2011;204(Suppl 1):S1-3. Crossref
4. Kondamudi NP, Whitten RA. Measles.
Available from: https://www.ncbi.nlm.nih.gov/books/NBK448068/. Accessed 15
May 2018.
5. Bhattacharjee S, Yadava PK. Measles
virus: background and oncolytic virotherapy. Biochem Biophys Rep
2018;13:58-62. Crossref
6. Dardis MR. A review of measles. J Sch
Nurs 2012;28:9-12.Crossref
7. Bentley J, Rouse J, Pinfield J. Measles:
pathology, management and public health issues. Nurs Stand 2014;28:51-8. Crossref
8. Rota PA, Moss WJ, Takeda M, de Swart RL,
Thompson KM, Goodson JL. Measles. Nat Rev Dis Primers 2016;2:16049. Crossref
9. Caldararo S. Measles. Pediatr Rev
2007;28:352-4. Crossref
10. de Vries RD, Mesman AW, Geijtenbeek
TB, Duprex WP, de Swart RL. The pathogenesis of measles. Curr Opin Virol
2012;2:248-55. Crossref
11. Lindberg C, Lanzi M, Lindberg K.
Measles: still a significant health threat. MCN Am J Matern Child Nurs
2015;40:298-305. Crossref
12. Kobaidze K, Wallace G. Forgotten but
not gone: update on measles infection for hospitalists. J Hosp Med
2017;12:472-6. Crossref
13. MacFadden DR, Gold WL. Measles. CMAJ
2014;186:450. Crossref
14. Gans H, Maldonado YA. Measles:
epidemiology and transmission. Available from:
https://www.uptodate.com/contents/measles-epidemiology-and-transmission.
Accessed 25 May 2018.
15. Clemmons NS, Wallace GS, Patel M,
Gastañaduy PA. Incidence of measles in the United States, 2001-2015. JAMA
2017;318:1279-81. Crossref
16. Zipprich J, Winter K, Hacker J, et al.
Measles outbreak—California, December 2014-February 2015. MMWR Morb Mortal
Wkly Rep 2015;64:153-4.
17. Hall V, Banerjee E, Kenyon C, et al.
Measles outbreak—Minnesota April-May 2017. MMWR Morb Mortal Wkly Rep
2017;66:713-7. Crossref
18. Piccirilli G, Lazzarotto T, Chiereghin
A, Serra L, Gabrielli L, Lanari M. Spotlight on measles in Italy: why
outbreaks of a vaccine-preventable infection continue in the 21st century.
Expert Rev Anti Infect Ther 2015;13:355-62. Crossref
19. Sá Machado R, Perez Duque M, Almeida
S, et al. Measles outbreak in a tertiary level hospital, Porto, Portugal,
2018: challenges in the post-elimination era. Euro Surveill 2018;23. Crossref
20. Li S, Qian X, Yuan Z, et al. Molecular
epidemiology of measles virus infection in Shanghai in 2000-2012: the
first appearance of genotype D8. Braz J Infect Dis 2014;18:581-90. Crossref
21. Bester JC. Measles and measles
vaccination: a review. JAMA Pediatr 2016;170:1209-15. Crossref
22. Adu FD, Adeniji JA. Measles antibodies
in the breast milk of nursing mothers. Afr J Med Med Sci 1995;24:385-8.
23. Gans H, Maldonado YA. Measles:
clinical manifestations, diagnosis, treatment, and prevention. Available
from:
https://www.uptodate.com/contents/measles-clinical-manifestations-diagnosis-treatment-and-prevention.
Accessed 25 May 2018.
24. Campos-Outcalt D. Measles: why it’s
still a threat. J Fam Pract 2017;66:446-9.
25. Sood SB, Suthar K, Martin K, Mather K.
Vaccine-associated measles in an immunocompetent child. Clin Case Rep
2017;5:1765-7. Crossref
26. Gadler T, Martinez N, Ogg-Gress J.
Recognizing measles, mumps, and rubella in the emergency department. Adv
Emerg Nurs J 2018;40:110-8. Crossref
27. Roose J, Rohaert C, Jadoul A,
Fölster-Holst R, van Gysel D. Modified measles: a diagnostic challenge.
Acta Derm Venereol 2018;98:289-90. Crossref
28. Bhatt JM, Huoh KC. Otolaryngological
manifestations of measles (rubeola): a case report and brief review.
Laryngoscope 2016;126:1481-3. Crossref
29. Lai WS, Lin YY, Wang CH, Chen HC.
Measles: a missed cause of acute tonsillitis. Ear Nose Throat J
2017;96:E54-5.
30. Nobili V, Pietro S, Stefania P.
Fulminant hepatic failure following measles. Pediatr Infect Dis J
2007;26:766-7. Crossref
31. Colombo I, Forapani E, Spreafico C,
Capraro C, Santilli I. Acute myelitis as presentation of a reemerging
disease: measles. Neurol Sci 2018;39:1617-9. Crossref
32. Fisher DL, Defres S, Solomon T.
Measles-induced encephalitis. QJM 2015;108:177-82. Crossref
33. Garg RK, Malhotra HS, Rizvi I, Kumar
N, Jain A. An unusual case of acute encephalitic syndrome: is it acute
measles encephalitis or subacute sclerosing panencephalitis? Neurol India
2017;65:1333-4. Crossref
34. Imdad A, Mayo-Wilson E, Herzer K,
Bhutta ZA. Vitamin A supplementation for preventing morbidity and
mortality in children from six months to five years of age. Cochrane
Database Syst Rev 2017;(3):CD008524. Crossref
35. Bichon A, Aubry C, Benarous L, et al.
Case report: ribavirin and vitamin A in a severe case of measles. Medicine
(Baltimore) 2017;96:e9154. Crossref
36. Ortac Ersoy E, Tanriover MD, Ocal S,
Ozisik L, Inkaya C, Topeli A. Severe measles pneumonia in adults with
respiratory failure: role of ribavirin and high-dose vitamin A. Clin
Respir J 2016;10:673-5. Crossref
37. World Health Organization. Measles
vaccines: WHO position paper, April 2017—recommendations. Vaccine
2017;pii:S0264-410X(17)30974-X. Crossref
38. Drutz JE. Measles, mumps, and rubella
immunization in infants, children, and adolescents. Available from:
https://www.uptodate.com/contents/measles-mumps-and-rubella-immunization-in-infants-children-and-adolescents.
Accessed 25 May 2018.
39. McLean HQ, Fiebelkorn AP, Temte JL,
Wallace GS; Centers for Disease Control and Prevention. Prevention of
measles, rubella, congenital rubella syndrome, and mumps, 2013: summary
recommendations of the Advisory Committee on Immunization Practices
(ACIP). MMWR Recomm Rep 2013;62:1-34.
40. Baxter R, Lewis E, Goddard K, et al.
Acute demyelinating events following vaccines: a case-centered analysis.
Clin Infect Dis 2016;63:1456-62. Crossref
41. Wakefield AJ, Murch SH, Anthony A, et
al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and
pervasive developmental disorder in children. Lancet 1998;351:637-41.
Erratum in: Lancet 2004;363:750. Retraction in: Lancet 2010;375:445. Crossref
42. Taylor LE, Swerdfeger AL, Eslick GD.
Vaccines are not associated with autism: an evidence-based meta-analysis
of case-control and cohort studies. Vaccine 2014;32:3623-9. Crossref
43. Seither R, Calhoun K, Knighton CL, et
al. Vaccination coverage among children in kindergarten—United States,
2014-15 school year. MMWR Morb Mortal Wkly Rep 2015;64:897-904. Crossref
44. Young MK, Nimmo GR, Cripps AW, Jones
MA. Post-exposure passive immunisation for preventing measles. Cochrane
Database Syst Rev 2014;(4):CD010056.Crossref
45. US Centers for Disease Control and
Prevention. Guidelines for environmental infection control in health-care
facilities. Available from:
https://www.cdc.gov/infectioncontrol/pdf/guidelines/environmental-guidelines.pdf.
Accessed 20 Jul 2018.
46. US New Jersey Department of Health.
Vaccine Preventable Disease Program. Measles Exposure. January 2015.
Available from:
https://www.state.nj.us/health/cd/documents/topics/measles/measles_exposures_guidance_01_2015.pdf.
Accessed 20 Jul 2018.
47. Orenstein WA, Hinman A, Nkowane B,
Olivé JM, Reingold A. Measles and rubella global strategic plan 2012-2020
midterm review. Vaccine 2018;36(Suppl 1):A1-34. Crossref