Hong
Kong Med J 2018 Oct;24(5):492–500 | Epub 20 Sep 2018
DOI: 10.12809/hkmj187244
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Alzheimer’s disease: insights for risk evaluation and
prevention in the Chinese population and the need for a comprehensive
programme in Hong Kong/China
Anita Yee, PhD1; Nancy BY Tsui, PhD1,2;
YN Chang1; Clarea SM Au3; Manson Fok, MB, BS, FRCS1,2,4;
LT Lau, PhD2; Teresa Chung, MPhil2; Gregory Cheng,
MD, PhD4; Rick YC Kwan, PhD5; Angela YM Leung, PhD,
FHKAN5; Johnson YN Lau, MD, FRCP1,2; David LK Dai,
FRCP, FHKAM (Medicine)6
1 Avalon Genomics (HK) Limited, Shatin,
Hong Kong
2 Department of Applied Biology and
Chemical Technology, The Hong Kong Polytechnic University, Hunghom, Hong
Kong
3 Yan Oi Tong Clarea Au Eldergarten,
Kwun Tong, Hong Kong
4 Faculty of Health Sciences, Macau
University of Science and Technology, Macau
5 Centre for Gerontological Nursing,
School of Nursing, The Hong Kong Polytechnic University, Hunghom, Hong
Kong
6 Hong Kong Alzheimermer’s Disease
Association, Hong Kong
Corresponding author: Dr David LK Dai (davidlkdai@gmail.com)
Abstract
With the ageing of the global population, China
is projected to be impacted significantly by the rising number of
patients with Alzheimer’s disease (AD). A cure for AD is not yet
available, so society should be prepared for an increasing AD-related
burden. In this review, we examine this impending problem and provide
overviews on (a) the magnitude of the problem of AD in Hong Kong/China
in the near future; (b) the genetic and lifestyle risk factors that
contribute to AD; (c) current diagnostic approaches and the potential of
newly discovered genetic biomarkers for early detection; (d)
medications, non-pharmacological interventions, and possible preventive
measures; and (e) the need for social and psychological care from the
community. In Hong Kong, primary care and AD-related support for at-risk
individuals, patients, and caregivers are inadequate. A joint effort
from the medical community, government, universities, non-governmental
organisations/charities, and industry should initiate the development of
a long-term programme for AD. Finally, we outline recommendations for
the relevant parties to consider.
Introduction
Alzheimer’s disease (AD) is the most common form of
dementia among older adults. It is an age-related chronic condition
characterised by gradual decline in memory, cognitive function, and
physical status. Patients with AD lose their self-management abilities and
require long-term care as the disease progresses. The average survival of
patients after diagnosis is approximately 8 to 10 years. Currently, no
treatment effectively reverses or halts the disease’s progression.
There are two main types of AD. Early-onset
familial AD occurs before age 60 years. It accounts for around 1% of AD
cases and typically has strong familial aggregation. Causative variants
have been identified in genes encoding amyloid precursor protein (APP),
presenilin-1, and presenilin-2. Late-onset AD (the focus of this review)
is often called sporadic AD. It is the more common type of AD, and it
usually occurs after age 60 years. Both genetic and environmental factors
contribute to the disease’s development. Before the disease manifests, a
continuum of biological and molecular changes has accumulated. Clinical
stages that precede AD, including preclinical AD and mild cognitive
impairment (MCI), have been proposed.1
By establishing biomarkers associated with the pre-symptomatic changes,
at-risk individuals can be identified for preventive interventions to
delay further cognitive decline.
Given the ageing population and the potential
impact of AD, the Hong Kong Alzheimer’s Disease Association has assembled
a number of experts to identify information critical to Hong Kong/China
and to prepare appropriate recommendations. This review aimed to provide
key information about AD, from prevention, diagnostics, and treatment to
continuous care. The review also recommended an implementable plan for the
medical community, government, universities, non-government organisations
(NGOs), charities, and industry to consider.
Prevalence and incidence
The global prevalence of dementia among people aged
≥60 years is 5% to 7%.2 In 2010,
approximately 35.6 million people lived with dementia, and this number is
expected to double every 20 years. Approximately two-thirds of dementia
cases are attributed to AD. There are regional differences in the
disease’s prevalence, with estimates of 6.9% in Western Europe, 6.5% in
North America,2 and 4.6% among the
Chinese population, including Hong Kong.3
The prevalence of dementia increases exponentially with age. For the
age-group of 60 to 64 years, the prevalence is 2% and 0.6% for Caucasian
and Chinese people, respectively; while for the age-group of 80 to 84
years, it increases to 13% and 9.4%, respectively. The annual incidence
rate (per 1000 individuals) of dementia worldwide was estimated to be 7.5,
with regional rates of 8.0 in China, 8.8 in Western Europe, and 10.5 in
North America.4
China has one of the largest elderly populations.
Between 2015 and 2030, the proportion of the population aged ≥60 years is
estimated to increase from 15% to 25% in China and Macau and from 22% to
34% in Hong Kong.5 With this
population ageing, the number of patients with AD is expected to increase
substantially in the near future.
Progression from asymptomatic to disease manifestation
Pathophysiology
A notable pathological feature of AD is the
aggregation of amyloid-β (Aβ) peptides in the brain. Amyloid-β peptides
are derived from proteolytic cleavage of APP by β- and γ-secretases. This
process produces diverse types of Aβ peptides, among which the Aβ42
peptide is strongly self-aggregating. When the clearance mechanism is
impaired, the level of Aβ peptides in the brain rises, and the peptides
assemble into insoluble extracellular amyloid plaques, which are
neurotoxic.6 Other pathological
features include the formation of intracellular neurofibrillary tangles
from abnormally hyperphosphorylated and cleaved tau proteins,
neuroinflammatory responses triggered by the presence of aggregated Aβ,
and oxidative stress induced by reactive oxygen species generated in
Aβ-altered cells.6 7 Although the specific sequences of pathological events
remain uncertain, the consequences of synaptic/neuronal dysfunction,
neuronal degeneration, reduced neural network connectivity, and brain
atrophy are well established and commonly found in the hippocampus, which
is the key functional location of memory.
Diagnostic criteria
The criteria for diagnosing AD include significant
decline in at least two cognitive domains, one of which is learning and
memory, and the deficits’ interference with daily abilities and
independence.8 The characteristics
of MCI include concern about change in cognition and evidence of lower
performance in one or more cognitive domains, while the abilities of
independent living are preserved.9
Validated assessment tools, such as the Mini-Mental State Examination,
Montreal Cognitive Assessment, Clinical Dementia Rating, and Alzheimer
Disease Assessment Scale–Cognitive Subscale (ADAS-Cog), are commonly used.
In the preclinical AD stage, elderly people are asymptomatic with normal
cognitive performance, but adverse molecular changes in the brain may have
accumulated significantly, which may lead to subsequent disease
development.1
Conversion from mild cognitive impairment to
Alzheimer’s disease
Not all subjects with MCI convert to AD. A
meta-analysis revealed that the cumulative proportion of subjects with MCI
who progressed to AD was 33.6% in studies conducted in specialist clinical
settings and 28.9% in population studies.10
The adjusted annual conversion rates from MCI to AD were 8.1% and 6.8% in
specialist settings and population studies, respectively. In Hong Kong,
15.9% of subjects with MCI had developed dementia at the end of a 2-year
follow-up, according to a prospective study.11
Subjects with MCI were more prone to AD progression if they had the risk
factors of apolipoprotein E (APOE) ε4 allele, abnormal
cerebrospinal fluid (CSF) tau level, hippocampal and medial temporal lobe
atrophy, entorhinal atrophy, depression, diabetes, hypertension, older
age, female sex, lower Mini-Mental State Examination score, or higher
ADAS-Cog score.12 Some of these
factors will be further elaborated in the next section.
Risk and protective factors in Alzheimer’s disease
Late-onset AD has contributions from both genetic
and environmental factors. Genetics predisposes individuals to
susceptibility to AD before birth. Environmental exposure modifies the
risk of disease development. In addition, co-morbidity of AD with other
diseases appears to occur frequently, probably due to interactions between
disease pathways.
Genetic factors
The estimated heritability of AD ranges from 60% to
80%.13 Among the reported
AD-associated genetic variants, the APOE ε4 allele is most
prominent. In Caucasians, the odds ratios (ORs) of developing AD for
individuals carrying one and two copies of ε4 allele have been reported as
2.8 and 11.8, respectively.14
Comparable results have also been obtained in Chinese people, with ORs of
3.1 and 11.7, respectively.15
The population frequency of the ε4 allele is
relatively low in Chinese people (Fig a), particularly Southern Chinese people (Fig
b). Among patients with AD, the allele frequencies of ε4 vary
geographically, with the lowest frequency observed in Chinese patients
(heterozygous: 32.8%; homozygous: 5.5%) [Fig c].16
This observation indicates potential variation in the genetic makeup of
patients with AD in different ethnic groups. The relatively low ε4 allele
frequency in Chinese patients with AD also suggests the existence of
genetic factors in addition to ε4 allele in the Chinese population.
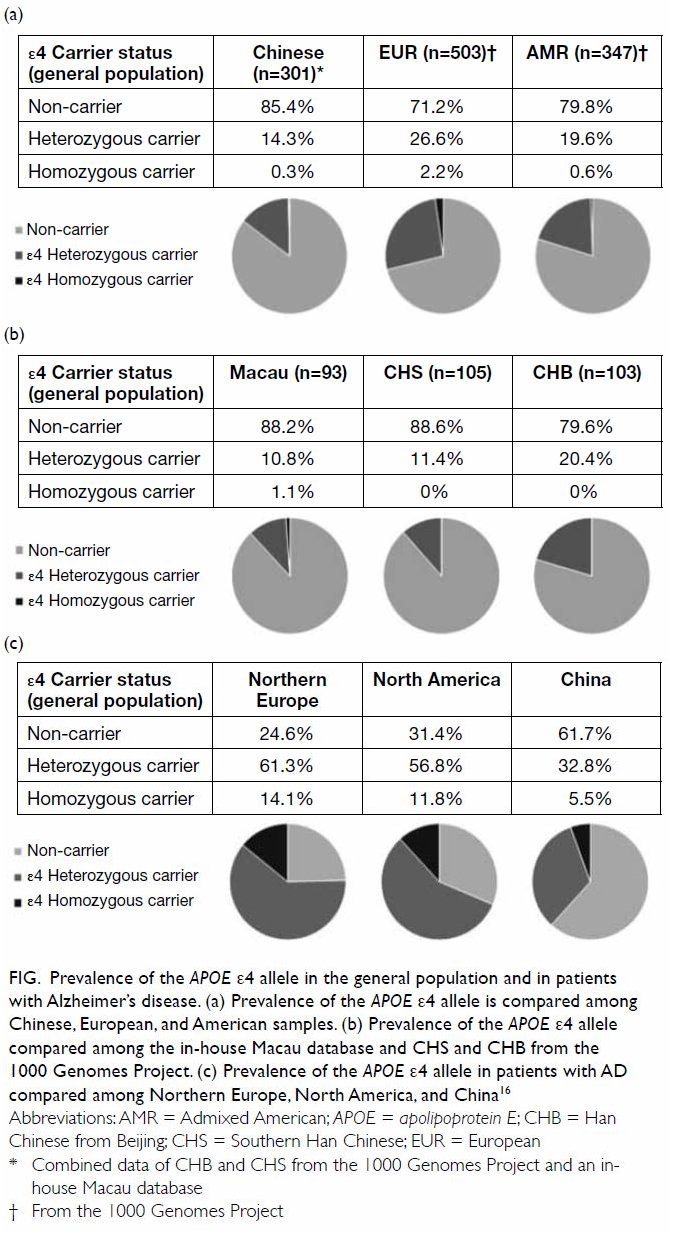
Figure. Prevalence of the APOE ε4 allele in the general population and in patients with Alzheimer’s disease. (a) Prevalence of the APOE ε4 allele is compared among Chinese, European, and American samples. (b) Prevalence of the APOE ε4 allele compared among the in-house Macau database and CHS and CHB from the 1000 Genomes Project. (c) Prevalence of the APOE ε4 allele in patients with AD compared among Northern Europe, North America, and China16
Genome-wide association studies (GWASs) allow the
identification of disease-associated variants without a priori
hypotheses about potential candidates. More than 10 novel genetic markers
for AD have been identified by GWASs thus far. They have modest effect
sizes (ORs around 1.2, or 0.83 for alleles with protective effects).
Meta-analyses of validation studies have confirmed the contributions of CR1
(rs6656401)17 and CD33
(rs3865444)18 to AD susceptibility
in Chinese people. We have also retrieved relevant articles from PubMed
about each of the GWAS-identified single nucleotide polymorphisms (SNPs)
(using the keywords “Alzheimer’s disease”, “Chinese”, and the gene name or
SNP identifier) and conducted a meta-analysis (methodology is shown in the
online supplementary Appendix). We found a significant association of
rs610932 in the MS4A gene cluster in Chinese patients with AD (Fig S1a). Negative results were obtained for other
reported SNPs (Fig S1b-f).
Recent advances in next-generation sequencing have
allowed investigation of rare variants on a genome-wide scale with
single-base resolution. Using this technology, a rare missense mutation in
TREM2 (rs75932628), which has an allelic frequency of 0.64%, was
found to confer a significant risk of AD in Caucasians (OR: 2.9).19
In the future, large-scale GWASs focusing on
Chinese patients would be worthwhile, to facilitate comprehensive
identification of novel Chinese-specific SNP markers. This represents an
important medical research area for Hong Kong/China.
Environmental factors
Diet
The traditional Mediterranean diet is widely
accepted as optimal for health. Higher adherence to this diet is
associated with reduced total mortality and incidence of cardiovascular,
neoplastic, and neurodegenerative diseases. The Mediterranean diet is
characterised by high intake of vegetables, legumes, fruits, nuts, and
cereals; moderate consumption of fish; low to moderate intake of dairy
products; olive oil as the major source of fat; low consumption of meat;
and regular but moderate intake of wine during meals. With regard to
cognitive health, greater adherence to the Mediterranean diet has been
related to better cognitive function and lower risk of AD.20 Among older Chinese women, the “vegetables-fruits”
dietary pattern has been associated with reduced risk of cognitive
impairment. It includes frequent intake of vegetables, fruits, soy, and
soy products and low consumption of fats and oils.21 This shares some food items with the Mediterranean
diet and may suggest the potential importance of foods with anti-oxidant
and anti-inflammatory properties for improvement of cognitive health.
Sleep
Sleep disturbances are common in patients with
AD—difficulty falling asleep, more disrupted nocturnal sleep, and
increased wakefulness after sleep onset. These lead to reduction of total
sleep time and excessive daytime sleepiness.22
Sleep disturbances can result from neurodegenerative changes and emergent
AD, and conversely, they can increase the risk of AD. Increased sleep
fragmentation has been related to lower baseline cognitive performance and
a more rapid rate of cognitive decline.23
Animal studies have revealed a potential mechanism of the influence of
sleep on AD. During sleep, there is more convective exchange of CSF and
interstitial fluid in the brain due to increased interstitial space
surrounding brain cells.24 This
convective flux increases clearance of Aβ peptides and other toxic
compounds compared with that during wakefulness. Poor-quality or
insufficient sleep may slow down the removal of neurotoxic substances from
the brain, leading to increased susceptibility to AD. This reciprocal
relationship between sleep and AD forms a vicious cycle that may cause
further pathological changes in patients.
Physical activity
Physical activity attenuates the risk of
cerebrovascular diseases and improves attention, processing speed,
executive function, and memory. Moreover, aerobic exercise reversed
age-related volume loss of the hippocampus in older adults without
dementia.25 Any frequency of
moderate exercise performed in midlife and late life has been shown to
reduce the risk of MCI (ORs: 0.61 and 0.68, respectively).26 In a randomised controlled trial on older adults with
memory problems, subjects assigned to an intervention group that performed
moderate-intensity physical activity over a 6-month period showed
improvement in ADAS-Cog scores, and such effect was sustained even after
18 months.27 Taken together,
studies have indicated a positive impact of physical activity on cognitive
function, probably via improving cerebral metabolism, circulation, and
endurance towards oxidative stress. All of these are important in brain
plasticity and thus potentially prevent AD.
Cognitive reserve
This hypothesis proposes that individuals with
greater cognitive reserve can tolerate more pathological changes, thus
delaying the onset of AD. However, at the time of onset, these individuals
may show more rapid cognitive decline because more pathological changes
have been accumulated.28 With
greater cognitive reserve, the brain may be more resilient to cognitive
damage or use compensatory networks more effectively when coping with
pathology. Education is a major contributor to cognitive reserve. Higher
education has been shown to reduce the risk of dementia and protect
against further cognitive decline for an additional 7 years after the
first signs appear, as compared with less-educated counterparts.29 Occupational attainment and engaging in leisure
activities also reduce the risk of developing dementia.28
Co-morbidity with other diseases
Diabetes mellitus
The incidence of AD is 50% to 100% higher in people
with type 2 diabetes mellitus (T2DM) than that in those without.30 Higher blood glucose level has been associated with
increased risk of dementia, even among people without T2DM.31 The pathological features of T2DM and AD are similar
in many ways. While T2DM is characterised by aggregation of islet amyloid
polypeptide in the pancreas and loss of β-cells, Aβ plaques and neuronal
loss occur in the brains of patients with AD. Impairment of insulin
signalling may be an underlying pathological process common to both
diseases. Chronic hyperglycaemia, activation of inflammatory pathways,
oxidative stress, and accumulation of advanced glycation end products
could alter insulin receptor sensitivity and lead to peripheral insulin
resistance in T2DM.32 A similar
disturbance in the brain could account for the abnormalities in AD.
Depression
A meta-analysis revealed that late-life depression
was associated with an increased risk of AD (OR: 1.65).33 People with MCI were also depressed more often than
normal controls. Reciprocally, patients with depression had higher
deficiencies of executive function, memory, and attention.34 Molecular mechanisms proposed to link depression with
AD include activation of the hypothalamic-pituitary-adrenal axis and
elevation of glucocorticoid production levels in depression. Prolonged
dysregulation of these pathways can cause damage to the hippocampus.
Biomarkers for early detection of Alzheimer’s disease
Imaging
Brain imaging enables characterisation of
pathological progression. Hippocampal atrophy is a relevant marker of
memory loss and can be assessed by structural magnetic resonance imaging
(MRI). Reduction in hippocampal volume by 10% to 15% and 15% to 30% were
found in people with amnestic MCI and AD, respectively, relative to
healthy controls.35 Longitudinal
analysis has also shown a higher rate of atrophy in patients with AD and
MCI-to-AD converters.36 Amyloid
imaging can be conducted using positron emission tomography (PET) with
Pittsburgh compound B (PIB) tracer. Retention of 11C-PIB correlates with
brain amyloid level and can differentiate patients with AD from normal
individuals. Among patients with MCI, those who exhibited higher 11C-PIB
retention were more likely to convert to AD than those with lower
retention.37 Brain glucose
metabolism can be assessed using 18F-fluorodeoxyglucose (FDG)
PET scanning. Reduction in glucose metabolism is associated with decline
in cognitive ability. Glucose metabolic reduction has been shown to be
particularly prominent in the medial temporal lobe of patients with MCI,
while such reduction has been observed in the parietotemporal, frontal,
and posterior cingulate cortices in patients with AD.38 Imaging techniques to detect tau deposition,
inflammation, and neurotransmitter alterations have also been developed
and may serve as biomarkers after careful evaluation.
Cerebrospinal fluid characterisation
Cerebrospinal fluid is considered as a highly
relevant sampling source for AD biomarkers because it directly interacts
with the brain. Reduced levels of Aβ42 peptide in CSF have been
detected in patients with AD, probably due to deposition into amyloid
plaques in the brain.39 Elevated
levels of phosphorylated tau in CSF may reflect neurofibrillary tangles in
the brain, while total tau level is linked to cognitive decline.
Integrating various biomarkers may further enhance the identification of
elderly people who are at risk of developing AD. For example, a study in
Hong Kong has shown that the AD-CSF Indices, an approach that combines Aβ42,
total tau, and phosphorylated tau, were able to differentiate patients
with AD from controls without dementia with high sensitivity and
specificity.40 Another example
utilised data extracted from whole-brain structural MRI and 18F-FDG
PET scans, CSF biomarkers, and clinical variables including age,
education, APOE genotype, and ADAS-Cog score; these were combined
into a model that greatly reduced the misclassification rate of MCI-to-AD
converters than that using clinical variables alone.41
Despite advances in brain imaging and CSF
biomarkers for early detection, variability in measurement methods, the
availability and cost of imaging, and the invasiveness of the lumbar
puncture procedure impose limitations on their widespread use. Increasing
efforts are being made to search for surrogate markers that serve similar
functions.
Circulating biomarkers in blood
Blood samples can be obtained easily with
standardised and minimally invasive methods. One of the earliest proposed
blood biomarkers was homocysteine, the level of which is increased in AD.42 Recent studies have taken
advantage of “omics” approaches to derive a signature of biomarkers. DNA
methylation profiling of blood samples has revealed several AD-associated
differential methylation sites43
that may represent blood-specific epigenetic changes due to AD. The blood
transcriptome approach revealed a blood RNA signature of 170
oligonucleotide probe sets that can differentiate patients with AD from
controls with high sensitivity and specificity.44
In another study, an RNA signature involving 48 genes was derived and
applied to subjects with MCI to predict their cognitive changes.45 Biomarkers may also be developed from the blood
proteome, such as a panel of 18 out of 120 signalling proteins that serves
as a classifier of AD.46 Although
further evaluations on the utility of proposed signatures are needed,
these studies have demonstrated the feasibility of using blood as a
sampling source for identification of surrogate AD biomarkers for early
detection.
Current treatment
Medications
Drugs approved for AD treatment include
acetylcholinesterase inhibitors (donepezil, rivastigmine, galantamine) and
an N-methyl-D-aspartate receptor antagonist (memantine). They slow down
cognitive decline by targeting cholinergic transmission and glutamate
release in the brain, respectively. Antidepressant, antipsychotic, and
anti-anxiety drugs can be prescribed for behavioural symptoms.
Non-pharmacological intervention
Cognitive stimulation improves both general
cognition and specific cognitive domains, such as attention and memory.
Cognitive interventions are also beneficial to elderly people with MCI,
with improvements to memory and delay of cognitive decline. Among the
cognitive approaches of cognitive stimulation, cognitive training, and
cognitive rehabilitation, clinical guidelines have recommended cognitive
stimulation for all people with mild dementia47
because of its efficacy (standardised mean difference of 0.41 for
cognition), which is similar to that of cholinesterase inhibitor
medication.48 Cultural
appropriateness should be considered when applying evidence-based
non-pharmacological interventions, particularly to older adults.
Multimodal activities can be mapped against domains within the Chinese
culture. For instance, Six Arts, a core set of Confucian philosophical
teachings comprising six disciplines (rites, music, archery,
charioteering, literacy, and numeracy), corresponds to the major mind-body
functional domains of social functioning, music and rhythm, visuospatial
skills, and fine motor skills.49
Social and psychological management
Patients with AD require continuous, integrated
health care after diagnosis. Families are the major care providers outside
clinical or institutional settings. Caring for a patient with AD is
associated with significant risks to the caregiver’s health and
well-being.50 Stress and anxiety
may arise when caregivers perceive that caregiving demands exceed
available resources. Promoting help-seeking through increasing awareness,
scaling up the supply of diagnostic and care services, and reducing
barriers to access resources can enhance both social and psychological
support to the patients and their caregivers. In addition, public
education about dementia can reduce stigmatising attitudes and alleviate
any hindrances to early help-seeking and intervention.51
In view of public expectation and demand, the
Dementia Community Support Scheme, a pilot scheme funded by the Community
Care Fund of Hong Kong, was launched in February 2017 to provide
dementia-related community support services in District Elderly Community
Centres.52 The scheme provides
elderly people with health care, training, and support services based on
their individual care plans to enhance their cognitive function, knowledge
of home safety, self-care ability, physical functioning, social skills,
and adherence to medication instructions. It also provides caregivers with
training and support services, such as stress management, knowledge about
taking care of elderly people with dementia, counselling services, and
formation of carer support groups to alleviate their burden.
Our recommendations
The ageing society of Hong Kong and the upcoming
ageing of the population have motivated the preparation of a
dementia-friendly community. Alzheimer’s Disease International has defined
a framework for dementia-friendly communities that includes the four
components of people, organisations, partnerships, and communities and
advocates timely diagnosis and post-diagnostic support by primary health
care and appropriate professionals.53
The World Alzheimer Report 2016 criticised the over-specialisation of
overall dementia care and emphasised the role of primary care in early
detection, diagnosis, disclosure, treatment, collaboration with social
care, and continuing support to patients’ families.54 We propose that the medical community, government,
universities, NGOs/charities, and industry in Hong Kong/China should
collaborate closely to develop measures to cope with the medical and
social impacts of AD.
Increase public awareness
The general public should be educated about the
symptoms of dementia, including AD, to increase their awareness. Mass
media, such as soap operas and television programmes, could be used for
public education, while health care professionals will be key supportive
information providers.
Early detection
Health education allows people to be familiarised
with the symptoms of AD and initiate assessment when they suspect that
their relatives have the disease, which facilitates early detection.
Training for medical professionals needs to be focused on enhancing the
perceptions of suitability and ability to arrive at a diagnosis and the
value of doing so in a timely manner.54
Prevention
The benefits of healthy diet, regular physical
activity, sufficient sleep, and cognitive stimulation for cognitive health
should be promoted. The public should also be aware that diseases such as
T2DM and depression can increase the risk of developing AD. The Department
of Health and NGOs could collaborate on promotion of preventive measures.
Diagnosis with genetic biomarker consideration
Identification of blood-based biomarkers may
provide insight into the development of AD. Systematic and large-scale
research is worthy of support, especially that on the identification of
biomarkers that are unique to the Chinese population. Collaborations
between academic institutions and industry will speed up research
progress. A carefully designed plan should also be developed to ensure the
appropriate utilisation of these biomarkers and environmental factors for
early detection. Governmental support is essential to push forward the
utilisation of research findings on AD diagnosis.
Potential therapeutics
Compounds targeting Aβ pathology, tau pathology,
mitochondrial dysfunction, and neuroinflammation have entered clinical
trials.55 Keeping track of these
trials can help to bring the latest information to the local community.
Identification of genetic markers, aberrant gene expression patterns, and
epigenetic profiles could provide insight on the aetiology of AD and thus
may provide novel therapeutic targets. For example, a recent study using
an AD mouse model revealed that interleukin-33 treatment could reverse
synaptic plasticity and cognitive deficits.56
Treatment with interleukin-33 reduced soluble Aβ and amyloid plaque
deposition and decreased proinflammatory response in the brain. It may
serve as a therapeutic candidate.
Continuous integrated care
A system of continuing care in the context of
function preservation and living support must be easily accessible to
patients with AD. It should guide the standards of patient care at
different phases of the disease condition. A comprehensive integrated care
programme can assist patients with AD in slowing down cognitive decline
and preservation of function. It can also reduce unnecessary
hospitalisation. Non-government organisations can help to moderate
continual care pathways, such as caregiver training and support, day care,
and residential services. Support and care for families and caregivers
should also be readily available. Mutual help among patients, their
families, and caregivers can enhance their coping ability with situations
related to the condition.
An example: “Project Sunrise”
The myth of the inadequacy of primary care for
dementia care in Hong Kong surrounds the accuracy of diagnosis, missing
secondary diagnoses, accessibility of medicine, investigations, caseload,
time, and remuneration. In the past 3 years, the Hong Kong Alzheimer’s
Disease Association has spearheaded a project named “Project Sunrise” at
Tsuen Wan and Kwun Tong. The project trains family physicians in diagnosis
of early AD and alerts them about unusual presentations that warrant
further investigation and referrals. The resulting shortening of the
period from seeking medical attention to diagnosis and initiation of
treatment was demonstrated to be 2.1 months. The crux of the project lies
in a pre-diagnostic assessment protocol and capacity building of primary
care doctors and related health care professionals. Community awareness
and training and service industries to be alerted about the needs of
persons with dementia are other important elements of the project, which
aspires to the dementia-friendly community concept. A Mental Health Review
led by the Food and Health Bureau has investigated dementia-related needs
and provided 10 recommendations in its 2016 report.57 Moving forward, the task is shifting from secondary
to primary care, and when a broad-based primary foundation is built, we
will enter the era of task sharing, when early diagnosis and treatments
are initiated promptly in the community. Longer-term case management for
people with dementia and care paths to facilitate appropriate care can
then be provided.
Author contributions
Concept and design of study: CSM Au, JYN Lau, DLK
Dai.
Acquisition of data: YN Chang, T Chung.
Analysis and interpretation of data: A Yee, NBY Tsui, LT Lau.
Drafting of the article: A Yee.
Critical revision of important intellectual content: M Fok, LT Lau, G Cheng, RYC Kwan, AYM Leung, JYN Lau, DLK Dai.
Acquisition of data: YN Chang, T Chung.
Analysis and interpretation of data: A Yee, NBY Tsui, LT Lau.
Drafting of the article: A Yee.
Critical revision of important intellectual content: M Fok, LT Lau, G Cheng, RYC Kwan, AYM Leung, JYN Lau, DLK Dai.
Acknowledgements
We thank Maggie Lee of Hong Kong Alzheimer’s
Disease Association for discussion input; Prof Claudia Lai of the School
of Nursing, The Hong Kong Polytechnic University for research
coordination; Prof Terry Lum and Dr Gloria Wong of the Sau Po Centre on
Ageing, The University of Hong Kong for discussion input about
non-pharmacological interventions for Alzheimer’s disease; and Ms Polly
Chan, Ms Connie Leung, Mr Nelson Li, and Ms Rebecca Shiu of Yan Oi Tong
for valuable discussion about the potential roles of non-governmental
organisations/charities in Alzheimer’s disease research.
Declaration
JYN Lau is the managing director of Avalon Genomics
and a shareholder of its parent company, Avalon Biomedical Management. He
is also an executive of Athenex Corporation and a board member of C-Mer
Eye Care Holdings Limited, Porton Fine Chemicals, Aiviva, and Avagenex.
All other authors have disclosed no conflicts of interest. All authors had
full access to the data, contributed to the study, approved the final
version for publication, and take responsibility for its accuracy and
integrity.
Funding/support
This work was supported by the Moonchu Foundation,
a private donation from CSM Au, and The Hong Kong Polytechnic University
(grant account number: 5.ZJL7).
Ethical approval
The Macau database was established with ethics
approval from the Clinical Research Ethics Committee of the University
Hospital, Macau University of Science and Technology. All subjects were
recruited with written informed consent. All experiments were performed in
accordance with the relevant guidelines and regulations.
References
1. Langbaum JB, Fleisher AS, Chen K, et al.
Ushering in the study and treatment of preclinical Alzheimer disease. Nat
Rev Neurol 2013;9:371-81. Crossref
2. Prince M, Bryce R, Albanese E, Wimo A,
Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic
review and metaanalysis. Alzheimers Dement 2013;9:63-75.e2. Crossref
3. Wu YT, Lee HY, Norton S, et al.
Prevalence studies of dementia in Mainland China, Hong Kong and Taiwan: a
systematic review and meta-analysis. PLoS One 2013;8:e66252. Crossref
4. Ferri CP, Prince M, Brayne C, et al.
Global prevalence of dementia: a Delphi consensus study. Lancet
2005;366:2112-7. Crossref
5. World Population Ageing, 2015.
Population Division, Department of Economic and Social Affairs, United
Nations; 2015.
6. Huang Y, Mucke L. Alzheimer mechanisms
and therapeutic strategies. Cell 2012;148:1204-22. Crossref
7. Heneka MT, Carson MJ, El Khoury J, et
al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol
2015;14:388-405. Crossref
8. McKhann GM, Knopman DS, Chertkow H, et
al. The diagnosis of dementia due to Alzheimer’s disease: recommendations
from the National Institute on Aging-Alzheimer’s Association workgroups on
diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement
2011;7:263-9. Crossref
9. Albert MS, DeKosky ST, Dickson D, et al.
The diagnosis of mild cognitive impairment due to Alzheimer’s disease:
recommendations from the National Institute on Aging-Alzheimer’s
Association workgroups on diagnostic guidelines for Alzheimer’s disease.
Alzheimers Dement 2011;7:270-9. Crossref
10. Mitchell AJ, Shiri-Feshki M. Rate of
progression of mild cognitive impairment to dementia—meta-analysis of 41
robust inception cohort studies. Acta Psychiatr Scand 2009;119:252-65. Crossref
11. Chan WC, Lam LC, Tam CW, et al.
Neuropsychiatric symptoms are associated with increased risks of
progression to dementia: a 2-year prospective study of 321 Chinese older
persons with mild cognitive impairment. Age Ageing 2011;40:30-5. Crossref
12. Li JQ, Tan L, Wang HF, et al. Risk
factors for predicting progression from mild cognitive impairment to
Alzheimer’s disease: a systematic review and meta-analysis of cohort
studies. J Neurol Neurosurg Psychiatry 2016;87:476-84. Crossref
13. Gatz M, Reynolds CA, Fratiglioni L, et
al. Role of genes and environments for explaining Alzheimer disease. Arch
Gen Psychiatry 2006;63:168-74. Crossref
14. Bertram L, McQueen MB, Mullin K,
Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic
association studies: the AlzGene database. Nat Genet 2007;39:17-23. Crossref
15. Liu M, Bian C, Zhang J, Wen F.
Apolipoprotein E gene polymorphism and Alzheimer’s disease in Chinese
population: a meta-analysis. Sci Rep 2014;4:4383. Crossref
16. Ward A, Crean S, Mercaldi CJ, et al.
Prevalence of apolipoprotein E4 genotype and homozygotes (APOE
e4/4) among patients diagnosed with Alzheimer’s disease: a systematic
review and meta-analysis. Neuroepidemiology 2012;38:1-17. Crossref
17. Jin C, Li W, Yuan J, Xu W, Cheng Z.
Association of the CR1 polymorphism with late-onset Alzheimer’s
disease in Chinese Han populations: a meta-analysis. Neurosci Lett
2012;527:46-9. Crossref
18. Li X, Shen N, Zhang S, et al. CD33
rs3865444 polymorphism contributes to Alzheimer’s Disease susceptibility
in Chinese, European, and North American populations. Mol Neurobiol
2015;52:414-21. Crossref
19. Jonsson T, Stefansson H, Steinberg S,
et al. Variant of TREM2 associated with the risk of Alzheimer’s
disease. N Engl J Med 2013;368:107-16. Crossref
20. Lourida I, Soni M, Thompson-Coon J, et
al. Mediterranean diet, cognitive function, and dementia: a systematic
review. Epidemiology 2013;24:479-89. Crossref
21. Chan R, Chan D, Woo J. A cross
sectional study to examine the association between dietary patterns and
cognitive impairment in older Chinese people in Hong Kong. J Nutr Health
Aging 2013;17:757-65. Crossref
22. Peter-Derex L, Yammine P, Bastuji H,
Croisile B. Sleep and Alzheimer’s disease. Sleep Med Rev 2015;19:29-38. Crossref
23. Lim AS, Kowgier M, Yu L, Buchman AS,
Bennett DA. Sleep fragmentation and the risk of incident Alzheimer’s
disease and cognitive decline in older persons. Sleep 2013;36:1027-32. Crossref
24. Xie L, Kang H, Xu Q, et al. Sleep
drives metabolite clearance from the adult brain. Science 2013;342:373-7.
Crossref
25. Erickson KI, Voss MW, Prakash RS, et
al. Exercise training increases size of hippocampus and improves memory.
Proc Natl Acad Sci U S A 2011;108:3017-22. Crossref
26. Geda YE, Roberts RO, Knopman DS, et
al. Physical exercise, aging, and mild cognitive impairment: a
population-based study. Arch Neurol 2010;67:80-6. Crossref
27. Lautenschlager NT, Cox KL, Flicker L,
et al. Effect of physical activity on cognitive function in older adults
at risk for Alzheimer disease: a randomized trial. JAMA 2008;300:1027-37.
Crossref
28. Stern Y. Cognitive reserve in ageing
and Alzheimer’s disease. Lancet Neurol. 2012;11:1006-12. Crossref
29. Amieva H, Mokri H, Le Goff M, et al.
Compensatory mechanisms in higher-educated subjects with Alzheimer’s
disease: a study of 20 years of cognitive decline. Brain 2014;137:1167-75.
Crossref
30. Biessels GJ, Staekenborg S, Brunner E,
Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic
review. Lancet Neurol 2006;5:64-74. Crossref
31. Crane PK, Walker R, Hubbard RA, et al.
Glucose levels and risk of dementia. N Engl J Med 2013;369:540-8. Crossref
32. De Felice FG, Ferreira ST.
Inflammation, defective insulin signaling, and mitochondrial dysfunction
as common molecular denominators connecting type 2 diabetes to Alzheimer
disease. Diabetes 2014;63:2262-72. Crossref
33. Diniz BS, Butters MA, Albert SM, Dew
MA, Reynolds CF 3rd. Late-life depression and risk of vascular dementia
and Alzheimer’s disease: systematic review and meta-analysis of
community-based cohort studies. Br J Psychiatry 2013;202:329-35. Crossref
34. Rock PL, Roiser JP, Riedel WJ,
Blackwell AD. Cognitive impairment in depression: a systematic review and
meta-analysis. Psychol Med 2014;44:2029-40. Crossref
35. Frisoni GB, Fox NC, Jack CR Jr,
Scheltens P, Thompson PM. The clinical use of structural MRI in Alzheimer
disease. Nat Rev Neurol 2010;6:67-77. Crossref
36. Risacher SL, Shen L, West JD, et al.
Longitudinal MRI atrophy biomarkers: relationship to conversion in the
ADNI cohort. Neurobiol Aging 2010;31:1401-18. Crossref
37. Okello A, Koivunen J, Edison P, et al.
Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB
PET study. Neurology 2009;73:754-60. Crossref
38. Mosconi L. Brain glucose metabolism in
the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies
in MCI and AD. Eur J Nucl Med Mol Imaging 2005;32:486-510.Crossref
39. Blennow K, Dubois B, Fagan AM, Lewczuk
P, de Leon MJ, Hampel H. Clinical utility of cerebrospinal fluid
biomarkers in the diagnosis of early Alzheimer’s disease. Alzheimers
Dement 2015;11:58-69. Crossref
40. Chu LW, Shea YF, Ha J. Validation of
AD-CSF-Index in Chinese patients with Alzheimer’s disease and nondemented
controls. Am J Alzheimers Dis Other Demen 2015;30:522-6. Crossref
41. Shaffer JL, Petrella JR, Sheldon FC,
et al. Predicting cognitive decline in subjects at risk for Alzheimer
disease by using combined cerebrospinal fluid, MR imaging, and PET
biomarkers. Radiology 2013;266:583-91. Crossref
42. Seshadri S, Beiser A, Selhub J, et al.
Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease.
N Engl J Med 2002;346:476-83. Crossref
43. Lunnon K, Smith R, Hannon E, et al.
Methylomic profiling implicates cortical deregulation of ANK1 in
Alzheimer’s disease. Nat Neurosci 2014;17:1164-70. Crossref
44. Fehlbaum-Beurdeley P, Jarrige-Le Prado
AC, Pallares D, et al. Toward an Alzheimer’s disease diagnosis via
highresolution blood gene expression. Alzheimers Dement 2010;6:25-38. Crossref
45. Lunnon K, Sattlecker M, Furney SJ, et
al. A blood gene expression marker of early Alzheimer’s disease. J
Alzheimers Dis 2013;33:737-53. Crossref
46. Ray S, Britschgi M, Herbert C, et al.
Classification and prediction of clinical Alzheimer’s diagnosis based on
plasma signaling proteins. Nat Med 2007;13:1359-62. Crossref
47. National Collaborating Centre for
Mental Health (UK). Dementia: A NICE-SCIE guideline on supporting people
with dementia and their carers in health and social care. In: National
Institute for Health and Clinical Excellence: Guidance. Leicester, UK:
British Psychological Society; 2007.
48. Woods B, Aguirre E, Spector AE, Orrell
M. Cognitive stimulation to improve cognitive functioning in people with
dementia. Cochrane Database Syst Rev 2012;(2):CD005562. Crossref
49. Wong GH, Ng CK, Lai CK, et al.
Development of Six Arts, a culturally appropriate multimodal
nonpharmacological intervention in dementia. Gerontologist 2015;55:865-74.
Crossref
50. Richardson TJ, Lee SJ, Berg-Weger M,
Grossberg GT. Caregiver health: health of caregivers of Alzheimer’s and
other dementia patients. Curr Psychiatry Rep 2013;15:367. Crossref
51. Cheng ST, Lam LC, Chan LC, et al. The
effects of exposure to scenarios about dementia on stigma and attitudes
toward dementia care in a Chinese community. Int Psychogeriatr
2011;23:1433-41. Crossref
52. Food and Health Bureau, Hong Kong SAR
Government. Dementia Community Support Scheme. Available from:
http://www.hpdo.gov.hk/en/dcss_index.html. Accessed 10 Apr 2018.
53. Alzheimer’s Disease International.
Dementia friendly communities: key principles. 2016. Available from:
https://www.alz.co.uk/adi/pdf/dfc-principles.pdf. Accessed 1 Feb 2018.
54. Prince M, Comas-Herrera A, Knapp M,
Guerchet M, Karagiannidou M. World Alzheimer Report 2016. Improving
Healthcare for People Living with Dementia: Coverage, Quality and Costs
Now and in the Future. London, Alzheimer’s Disease International; 2016.
55. Mangialasche F, Solomon A, Winblad B,
Mecocci P, Kivipelto M. Alzheimer’s disease: clinical trials and drug
development. Lancet Neurol 2010;9:702-16. Crossref
56. Fu AK, Hung KW, Yuen MY, et al. IL-33
ameliorates Alzheimer’s disease-like pathology and cognitive decline. Proc
Natl Acad Sci U S A 2016;113:E2705-13. Crossref
57. Food and Health Bureau, Hong Kong SAR
Government. Mental Health Review Report. 2017. Available from:
https://www.fhb.gov.hk/download/press_and_publications/otherinfo/180500_mhr/e_mhr_full_report.pdf.
Accessed 1 Feb 2018.

