Hong
Kong Med J 2018 Aug;24(4):350–60 | Epub 30 Jul 2018
DOI: 10.12809/hkmj176949
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Multidrug-resistant organism carriage among residents
from residential care homes for the
elderly in Hong Kong: a prevalence survey with stratified cluster sampling
H Chen, MB, BS, FHKAM (Community Medicine)1;
KM Au, MB, ChB1;
KE Hsu, BSc, MSc1; Christopher KC Lai, MB, ChB, FHKAM
(Pathology)2; Jennifer Myint, MB, BS, FHKAM (Medicine)3;
YF Mak, MB, BS, FHKAM (Medicine)4; SY Lee, BSc, MSc5;
TY Wong, MB, BS, FHKAM (Medicine)5; NC Tsang, MB, BS, FHKAM
(Pathology)2
1 Infection Control Branch, Centre
for Health Protection, Department of Health, Hong Kong
2 Department of Pathology, Queen
Elizabeth Hospital, Jordan, Hong Kong
3 Department of Rehabilitation,
Kowloon Hospital, Homantin, Hong Kong
4 Department of Medicine, Queen
Elizabeth Hospital, Jordan, Hong Kong
5 Infection Control Team, Queen
Elizabeth Hospital, Jordan, Hong Kong
Corresponding author: Dr H Chen (ch459@ha.org.hk)
Abstract
Introduction: A point
prevalence survey was
conducted to study the epidemiology of and risk
factors associated with multidrug-resistant organism
carriage among residents in residential care homes
for the elderly (RCHEs).
Methods: A total of 20 RCHEs in
Hong Kong were
selected by stratified single-stage cluster sampling.
All consenting residents aged ≥65 years from the
selected RCHEs were surveyed by collection of nasal
swab, axillary swab, rectal swab or stool on one single
day for each home. Specimens were cultured and
analysed for methicillin-resistant Staphylococcus
aureus (MRSA), multidrug-resistant Acinetobacter
(MDRA, defined as concomitant resistance to
fluoroquinolones, carbapenems, aminoglycosides,
cephalosporins and beta-lactam with or without
beta-lactamase inhibitors), vancomycin-resistant
Enterococcus (VRE), and carbapenemase-producing
Enterobacteriaceae (CPE). One third of the MRSA-positive
samples were selected at random for
molecular typing; all positive MDRA, VRE and
CPE samples were tested for molecular typing.
Demographic and health information of residents
including medical history, history of hospitalisation,
antimicrobial usage, and use of indwelling catheters
were collected to determine any associated risk
factors.
Results: Samples of 1028
residents from 20 RCHEs
were collected. Prevalence of MRSA was estimated as 30.1% (95%
confidence interval [CI]=25.1%-35.6%)
and MDRA 0.6% (95% CI=0.1%-4.1%). No residents
carried VRE nor CPE. Residents living in privately
run RCHEs were associated with MRSA carriage.
Non-Chinese residents were associated with MRSA
carriage with borderline significance.
Conclusions: This survey
provided information
about multidrug-resistant organism carriage among
RCHE residents. This information will enable us
to formulate targeted surveillance and control
strategies for multidrug-resistant organisms.
New knowledge added by this study
- Prevalence of methicillin-resistant Staphylococcus aureus among residents in residential care homes for the elderly (RCHE) was higher (30.1%, 95% confidence interval=25.1%-35.6%) than that of multidrug-resistant Acinetobacter (0.6%, 95% confidence interval=0.1%-4.1%).
- No residents were detected to be carriers of vancomycin-resistant Enterococcus (VRE) and carbapenemase-producing Enterobacteriaceae (CPE) in participating RCHEs, despite of the fact that these RCHEs had a history of receiving discharged VRE or CPE carriers from the hospitals.
- Such information is useful for hospitals in formulation of targeted admission surveillance and infection control strategy to prevent the spread of multidrug-resistant organisms.
Introduction
Multidrug-resistant organisms (MDROs) are
micro-organisms
that are resistant to one or more classes of
antimicrobial agent.1 Infections
caused by MDROs
often fail to respond to standard therapy and
require treatment with “big gun” antibiotics, which
may be associated with higher toxicity and cost.
Infection with MDROs leads to prolonged illness
and higher mortality than more common infections.
Discharging asymptomatic colonisers from hospital
to the community, especially to long-term care
facilities, may increase the risk of transmission
among community residents.2
In Hong Kong, residential care homes for
the elderly (RCHEs) are a heterogeneous group of
institutions providing different levels of care for
elderly people, who, for personal, social, health
or other reasons, can no longer live alone or with
their families. Around 9% of the elderly population
in Hong Kong requires residential care. As of
March 2015, there were approximately 750 RCHEs
providing over 79 000 residential places for elderly
people.3
Long-term care facilities are an important
reservoir for MDROs.4 Risk factors
from reported
cases of MDRO infection and colonisation include
use of indwelling medical devices, frequent antibiotic
usage and prolonged hospitalisations, all of which
are common among residents of long-term care
facilities.5
Methicillin-resistant Staphylococcus aureus
(MRSA) is defined as S aureus being resistant to
penicillinase-resistant penicillins (eg, methicillin,
oxacillin or cloxacillin) and cephalosporins. As a
common pathogen causing health care–associated
infections, MRSA has placed a substantial burden
on health care resources.6 In Hong
Kong, MRSA
is endemic.7 More than 40% of S
aureus isolated
in public hospitals are MRSA. Half of the MRSA
carriers among hospitalised patients aged ≥65 years
were admitted from RCHEs.8
Prevalence of MRSA
among long-term care residents in Europe ranged
from 8% to 25%.9 10
Multidrug-resistant Acinetobacter (MDRA)
is defined as a pathogen showing concomitant
resistance to fluoroquinolones, carbapenems,
aminoglycosides, cephalosporins, and beta-lactam
with or without beta-lactamase inhibitors. Among
hospitalised patients,11
especially in intensive care
units,12 13 MDRA is an important pathogen. It can cause
pneumonia, blood stream infection, skin and
soft tissue infection, and urinary tract infection.14 15
Data on MDRA prevalence among RCHE residents
in Hong Kong are limited.
Vancomycin-resistant Enterococcus (VRE)
is defined as Enterococcus faecalis or Enterococcus
faecium which is resistant to vancomycin.
Carbapenemase-producing Enterobacteriaceae
(CPE) is Enterobacteriaceae resistant to the
carbapenem class of antibiotics. Compared with
Western countries, incidence of infection with
emerging MDROs such as VRE and CPE is relatively
low in Hong Kong16; however, in
2013, there were
outbreaks of VRE among geriatric patients in public
hospitals in Kowloon. These outbreaks raised
concerns about the discharge of asymptomatic
carriers back to RCHEs that may lead to further
outbreaks, particularly if there is a lapse in
infection control practice in RCHEs.17
There have been few local studies on the
prevalence of MDRO colonisation among RCHE
residents.18 19 A better understanding of local MDRO
epidemiology in RCHE settings is important for
planning surveillance and control strategies to
prevent increases in MDRO prevalence among
RCHE residents.
The present survey aimed to estimate the
prevalence of MDROs with public health impacts
such as MRSA, MDRA, VRE and CPE among RCHE
residents in Kowloon City District, Hong Kong,
and to examine risk factors associated with MDRO
colonisation.
Methods
Population and setting
A point prevalence survey was conducted to estimate
the MDRO burden among residents in participating
RCHEs and associated factors of MDRO carriage.
All RCHEs with a capacity of 30 residents or more in
the catchment area of Queen Elizabeth Hospital and
Kowloon Hospital in Kowloon City District were
included. All residents aged ≥65 years who were in
the RCHE at 9 am (the reference time) on the survey
day, and consented to participate were included.
Sampling strategy
A list of all 60 RCHEs in the target area was
retrieved. The RCHEs were stratified by home type:
‘non-private’ for government-subsidised homes and
‘privately run’ for profit-making homes. Stratified
single-stage cluster sampling was applied to select
a representative sample of residents from RCHEs
at the ratio of 1:8, which was similar to the ratio of
residential beds provided by non-private to privately
run homes.
Sample size planning
Sample size estimation was based on the primary
objective of the study, which was to determine the
prevalence of MDROs (MRSA, MDRA, VRE and
CPE) in RCHEs. Prevalence of MRSA colonisation
was estimated to be 18.7% based on a local study in
RCHEs in 2011.20 Since no prior
information on the
design effect and intraclass correlation coefficient
was available, a conservative approach was taken.
The intraclass correlation coefficient was set at
0.025 and the design effect was set at 2, based on
estimates from a previous local study on infections
in RCHEs.21 Assuming the 95%
confidence interval
(CI) of MRSA prevalence estimated from current
study to be ±3.74% (relative precision [ie, margin
of error] was 3.74/18.7(%) = 0.2), the sample size
required was 836.22
Sample size was not estimated for the
prevalence of MDRA, VRE, or CPE. For MDRA,
local prevalence in RCHEs was not available. From
experience in hospitals, it was expected that the
prevalence of MDRA would be lower than that of
MRSA and higher than that of VRE.
From experience in hospitals and from
admission screening data for VRE and CPE, VRE
was expected to be very uncommon and CPE was
expected to be even rarer in RCHEs. Based on
information from the Infection Control Branch,
Centre for Health Protection, which keeps statistics
on patients discharged from hospital to RCHEs,
there were a total of 40 VRE carriers discharged from
hospitals to RCHEs in Kowloon City District from
January to September 2013. The RCHE bed capacity
in Kowloon City District was 7796 at the end of
September 2013; therefore, a rough estimation was
made for the prevalence of VRE in these RCHEs
of 0.51% (40/7796). On the basis of the estimated
sample size for measuring MRSA prevalence in
RCHE (ie, 836) the study has the power to detect
VRE prevalence with point estimate of 0.51% (95%
CI=0%-1.20%), with a relative precision of 1.34.
As the median bed capacity in RCHEs in
Kowloon City District is 74, assuming 60% of RCHE
residents would agree to be surveyed, a total of 1400
residents from 19 RCHEs needed to be recruited.
Assuming a response rate from RCHEs of 60%,
at least 32 RCHEs needed to be invited to join the
study.
Data and specimen collection
Invitation letters were sent to RCHEs to introduce
the survey and invite them to join. For RCHEs that
agreed to participate, the survey team visited the
RCHE twice. The first visit was to obtain consent
from residents (consent day). The second visit was to
collect information and specimen from consenting
residents on a single day between September and
December 2015 (survey day). The RCHEs were
allowed to select the survey day freely.
Residents who consented but were absent
on the survey day were excluded from the survey.
Potential additional residents (including those
absent on the consent day but present on the survey
day) were invited to join on the survey day.
A survey form was used to collect RCHE
information including home type and resident
information including demographics, medical
history, use of indwelling catheter, history of
hospitalisation, and history of antimicrobial use
over the previous year. Resident information
was extracted from medical records stored in
RCHEs. Nearly all residents were under the care
of the Community Geriatric Team of the Hospital
Authority. The Community Geriatric Team records
were comprehensive, including medical history,
hospitalisation to public hospitals, and medication
prescribed by public hospitals. Occasionally,
residents would seek help from private doctors.
The RCHEs keep records of private consultations,
including date of medical consultation, name of
doctor consulted, and medication prescribed by
private doctors. We extracted the best available
data from these two sources. Functional status of
residents was assessed by the survey team using the
Katz index.23 The Katz index
assesses independence
in activities of daily living on a 7-point Likert
scale from 0 to 6, where 6 points implies total
independence. The survey team consisted of doctors
and nurses who had experience working in infection
control for at least 1 year. Inter-rater reliability for
the Katz index among members of the team during
the pilot survey was assessed using the Fleiss kappa
coefficient.
For each consenting resident, the survey team
took the following samples: nasal swab for MRSA,
axillary swab for MRSA and MDRA, stool (or rectal
swab in cases when stool could not be collected) for
VRE, CPE, and MDRA. A standard survey protocol
on swab taking was developed and survey team
members were trained for specimen collection. For
the rectal swab, faeces should be evident on the
swab. All specimens were sent to the Microbiology
Laboratory of Queen Elizabeth Hospital for culture.
One third of the MRSA-positive samples were
selected at random for molecular typing. All MDRA,
VRE and CPE samples were subjected to molecular
typing.
For missing data identified in the survey forms,
the relevant RCHE was contacted shortly after the
survey for remedial work. Double data entry by two
different staff members was adopted to minimise
data entry error. To ensure data quality, 5% of the
data were selected from the cleansed dataset to
check against the hard copies.
Microbiological methods
The nasal, axillary, and rectal swab specimens
collected were directly inoculated onto agar plates.
Rectal swabs were visually inspected for presence of
faecal materials. For faecal samples, sterile swab was
used to swab a viscous portion of specimens and to
inoculate onto agar plates.
Screening for MRSA was performed using
chromID MRSA agar (bioMérieux, Marcy-l‘Étoile,
France). The chromID MRSA agars were incubated
at 35 ± 2°C for 24 hours. Green colonies were
picked for further characterisation by Gram stain,
coagulase and Staphaurex latex agglutination test
(Thermo Fisher Scientific, Waltham [MA], US).
Methicillin susceptibility was confirmed by cefoxitin
disk diffusion test.
Typically, MDRA is characterised by
Gram stain, biochemical reactions, and Vitek
2 (bioMérieux) with Gram-negative ID cards.
Selective cultivation of MDRA was performed using
CHROMagar Acinetobacter agars with multiple-drug
resistant selective supplement (CHROMagar,
Paris, France) which were incubated at 35 ± 2°C
for 48 hours. Resistance to fluoroquinolones,
carbapenems, aminoglycosides, cephalosporins, and
beta-lactams was confirmed by disk diffusion test.
Surveillance for VRE was performed using
chromID VRE (bioMérieux) agar, which were
incubated at 35 ± 2°C for 48 hours. Suspected
colonies were characterised by Gram stain,
biochemical tests, and Vitek 2 with Gram-positive
ID cards. Vancomycin susceptibility was confirmed
by disk diffusion test and E-test.
chromID CARBA (bioMérieux) was used
to selectively recover CPE. The chromID CARBA
agars were incubated at 35 ± 2°C for 24 hours.
Gram stain, biochemical tests, and Vitek 2 with
Gram-negative ID cards were used for identification
of Enterobacteriaceae. Non-susceptibility to
meropenem, imipenem and ertapenem were
confirmed using E-tests. Presence of carbapenemase
production was screened for using a modified
Hodge test with meropenem and ertapenem
and a combined-disc test with boronic acid and
ethylenediaminetetraacetic acid. Results were
confirmed with GeneXpert (Cepheid, Sunnyvale
[CA], US) Carba-R assay. All disk diffusion tests were
performed according to the Clinical and Laboratory
Standards Institute.24
Molecular typing was performed using
DiversiLab version 3.6.1 (bioMérieux). Typing
procedures were performed according to the
manufacturer’s instructions. The cluster analysis was
performed according to the guidelines provided by
the manufacturer using Pearson’s correlation and the
Kullback-Leibler method. Isolates were categorised
as indistinguishable, similar, or different.
Data analysis
R software (ver. 3.2.0; https://www.r-project.org)
was
used for statistical analysis. For all analyses, statistical
significance was defined as P<0.05. Descriptive
statistics were computed using all data collected.
The “survey” package (version 3.30-3) in R was
used to calculate the prevalence of MDRO carriage
adjusted for cluster sampling. The prevalence of
MDRO carriage among all surveyed RCHEs was
calculated using the “svyciprop” function from the
“survey” package, which calculates the prevalence as
the sample-weighted estimator of the proportion.25
The CI was calculated by a procedure closely related
to that proposed by Breeze for use in the United
Kingdom General Household Survey26
which is
calculated as a binomial probability using the Wilson
interval method,27 followed by a
logit transform.25
Prevalence of MDRO carriage among individual
RCHEs was calculated by dividing the number of
residents positive for MDRO culture by the total
number of residents surveyed in that particular
RCHE. Percentages for other study variables
were calculated similarly. Logistic regression with
adjustments for cluster sampling was performed
using “svyglm” function from the “survey” package
to identify risk factors for MRSA carriage. Variables
were included for multivariate analysis if P<0.25
in univariate analysis; or if variables had been
considered as risk factors of infection in previous
studies, such as mobility status,28
use of medical
devices,29 presence of wound,29 home size,29
sex,30 and
recipient of Governmental Allowance (as a surrogate
measurement of socio-economic status).31
Selected
variables were incorporated into the multivariate
regression model in descending order of effect size
estimated from the univariate regression. Variables
were not included to multivariate regression model
if the model with additional variable showed no
statistical significance in the residual sum of squares
reduction.
Grouping of quantitative variables for
regression modelling was based on following criteria:
(i) RCHE capacity was stratified into two groups by
median RCHE capacity; (ii) resident age was grouped
for every 10 years; (iii) Katz index was grouped into
the reference group (6 points), low dependence (3-5
points) and high dependence (0-2 points); (iv) RCHE
length of stay stratified into two groups by median
RCHE length of stay among surveyed residents; and
(v) hospital length of stay stratified into two groups
by mean length of stay reported by the Hospital
Authority for 2014-2015.32
The survey was conducted in a linked and
anonymous manner to avoid unnecessary anxiety
or stigmatisation due to positive MDRO carriage
status.33 Measures were taken
during the process
of preparation, specimen collection, and data
processing and storage to ensure protection of
participants’ anonymity.
Results
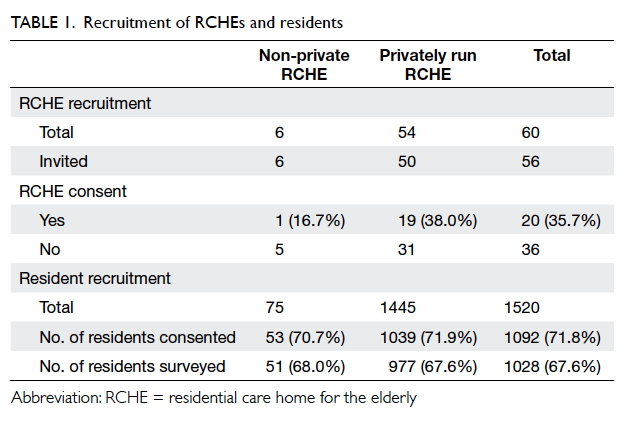
We invited 56 RCHEs (50 privately run and 6
non-private)
among the 60 RCHEs in Kowloon City
District to participate in the study. Of these, 20
RCHEs joined the study (Table 1). The number of
residents of the recruited RCHEs ranged from 25 to
265.
A pilot survey was conducted in one RCHE
from which 45 residents joined. The Fleiss kappa
coefficient of the total Katz index was 0.977, and
scores for individual items ranged from 0.972 to
1, suggesting good inter-rater reliability among all
members of the survey team.
Including those who participated in the
pilot, 1520 eligible residents were invited and 1092
consented to participate in this survey (consent
rate, 71.8%). Consent could not be obtained from
the remaining 428 residents, either because they
refused or their relatives or guardians could not be
contacted.
On the survey days for selected RCHEs,
10 residents who had previously given consent
refused to participate, 27 left the RCHE for personal
business, 24 were hospitalised, and three were
attending medical appointments. The remaining
1028 residents completed the survey.
Swabs were taken from 1028 residents on
a single day (survey day) for each RCHE during
the 3-month period from mid-September to mid-December 2015 (1026 nasal
swabs, 1027 axillary
swabs, 373 stool and 654 rectal swabs), achieving a
survey rate of 67.6%.
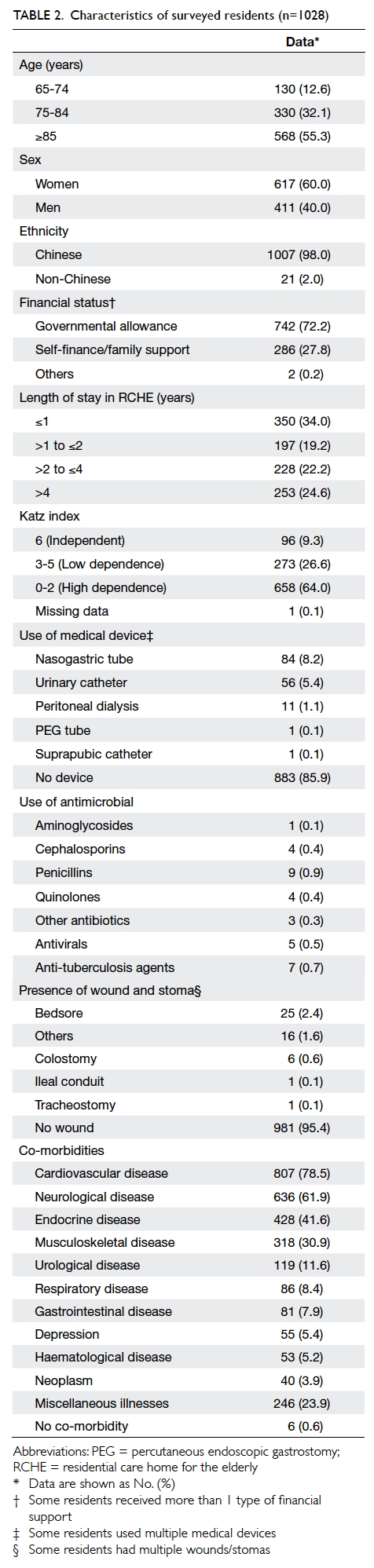
Demographics and underlying co-morbidity
of residents
Among the 1028 respondents, 411 (40.0%) were men
and 617 (60.0%) were women. The median age was
85 years (range, 65-104 years) and more than half
(55.3%) were aged ≥85 years. The majority were of
Chinese ethnicity (98.0%). The median length of stay
in RCHE was 1.8 years (range, 1 day to 23.4 years).
Table 2 shows the majority did not regularly use any
medical devices (85.9%) or have any wounds (95.4%).
Almost all respondents (99.8%) had underlying
chronic diseases. The most common disease was
hypertension (72.8%) followed by dementia (38.3%),
stroke (31.3%), diabetes (26.8%), and ischaemic
heart disease (22.0%). Over half of respondents
(58.6%) had a history of hospitalisation in the past
12 months with a mean of 2.9 episodes of hospital
admission (range, 1-16 episodes). More than half of
respondents (60.7%) had used antibiotics in the past
12 months. The most commonly used antibiotics
were amoxicillin/clavulanate (50.4%) followed by
levofloxacin (12.9%) and piperacillin/tazobactam
(7.2%). Most respondents (90.6%) were partially or
totally dependent in activities of daily living, with a
Katz index of <6. Of the respondents, 1.36% had a
history of known MDRO in the past 12 months.
Prevalence of multidrug-resistant organisms
Out of 1028 residents, 1027 were tested for MRSA
with 282 positive results (prevalence adjusted for
cluster sampling: 30.1%; 95% CI=25.1%-35.6%). All
1028 residents were tested for MDRA and three
carried MDRA (prevalence adjusted for cluster
sampling: 0.6%; 95% CI=0.1%-4.1%). A total of 1027
residents were tested for VRE and CPE; all tested
negative. Culture positive rates of MRSA for nasal
swab and axillary swab were 22.1% and 10.3%,
respectively. Culture positive rates for MDRA for
axillary swab, rectal swab, and stool were 0.1%, 0.2%,
and 0.5%, respectively.
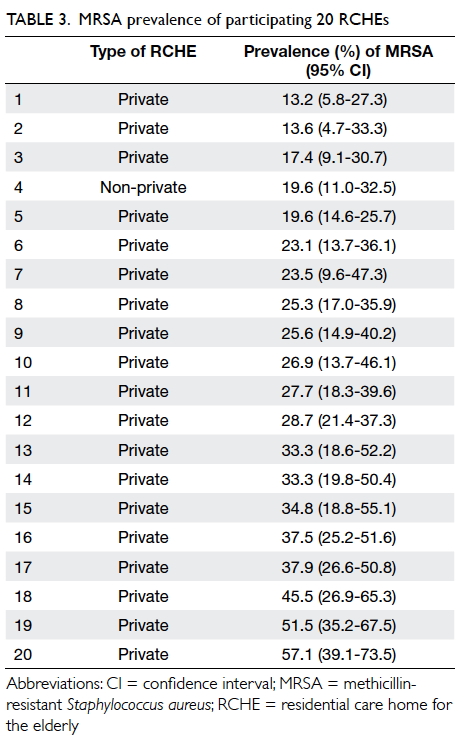
All participating RCHEs (n=20) had MRSA
carriers with MRSA prevalence ranging from 13.2%
to 57.1% (Table 3). There were no common MRSA
sources revealed by the diversified molecular typing
of 54 patterns (no band difference between strains
within a pattern) and 12 groups (1 band difference
between strains within group).
Three residents living in the same RCHE
carried MDRA. The prevalence of MDRA at this
RCHE was 11.5% (95% CI=4.00%-28.98%). Strain
typing revealed that all three likely belonged to
the same MDRA strain, as the band patterns were
identical.
Risk factors of multidrug-resistant organism
colonisation
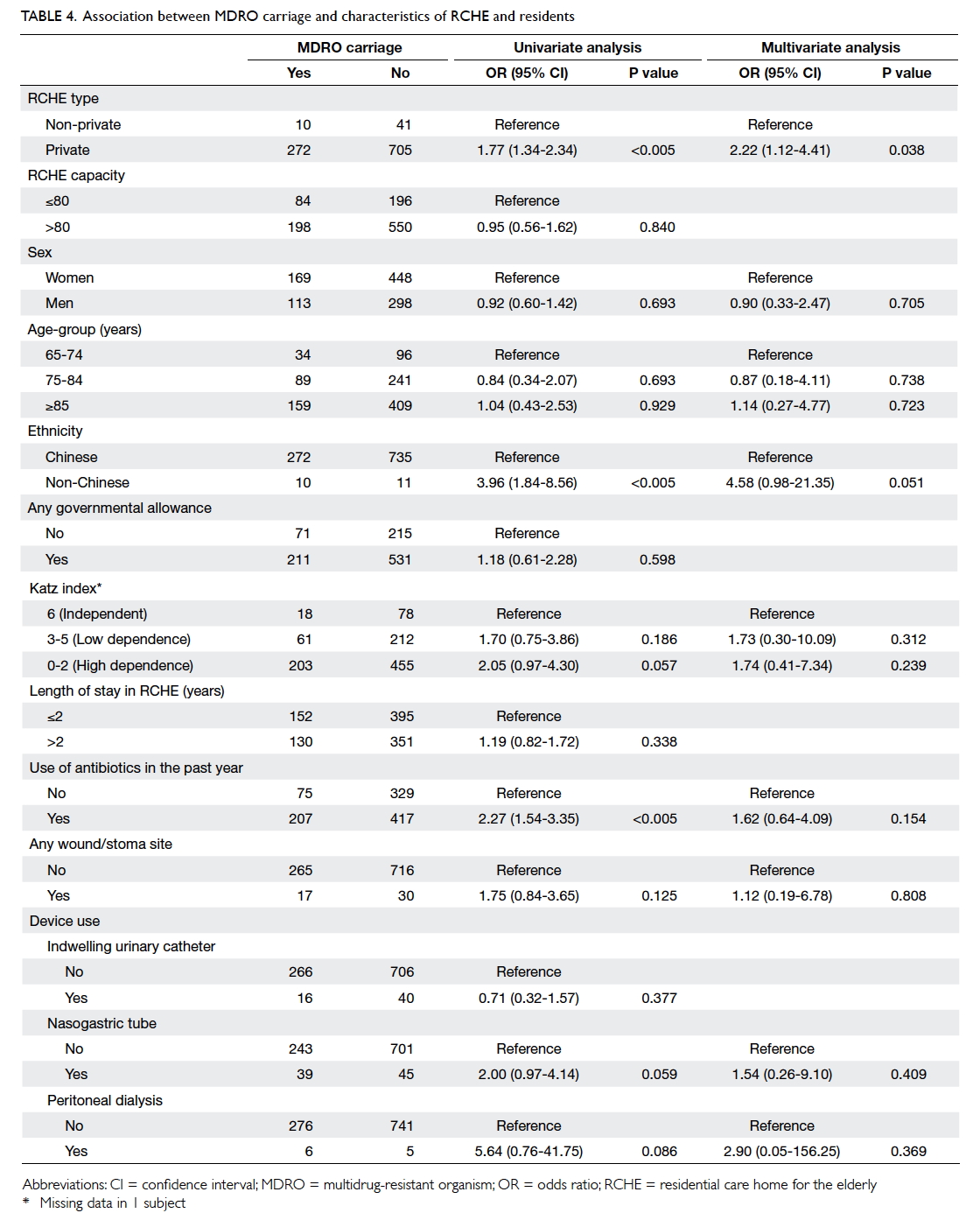
Compared with the 742 MDRO non-carriers,
univariate analysis revealed several factors
associated with MDRO positivity (Table 4). Inclusion
of RCHE capacity, governmental allowance, and
indwelling urinary catheter in the multivariate
logistic regression model did not provide statistically
significant decrease in residual sum of squares when
compared with the simpler model; therefore, the
simpler model was used. This model revealed that
residents from privately run RCHEs were associated
with MRSA colonisation and non-Chinese residents
were associated with MRSA carriage with borderline
significance.
Owing to the low participation rate of non-private
RCHEs, an additional regression model was
developed with residents from only privately run
RCHEs, to explore the association of different risk
factors with MRSA colonisation. After comparison,
no differences in terms of direction, effect size, or
statistical significance were observed between the
two models.
Discussion
In the present study, the survey revealed a high
prevalence of MRSA among RCHE residents in Hong
Kong. The prevalence of MDRA, however, remained
low in the same population, and VRE or CPE was not
found among surveyed residents.
All RCHEs surveyed had MRSA carriers. The
adjusted prevalence of MRSA colonisation was
30.1%, which is similar to that of another survey
conducted in RCHEs in Hong Kong Island during
the same period of time (32.2%).34
Prevalence of
MRSA was much higher than that found in previous
studies in 2005 (2.8%)19 and in
2011 (21.6%).35 Internationally,
MRSA prevalence in Hong Kong
is similar to that in the US (31%),36
but higher than
that in nursing home studies in the United Kingdom
(4.7%)37 and in Shanghai, China
(10.6%).38
The adjusted prevalence of MDRA was 0.6%.
This is similar to a local hospital study conducted
in 2014, which recorded a prevalence of multidrug-resistant
Acinetobacter baumannii of 0.57%.39
As all
three cases of MDRA were found in the same RCHE
with identical molecular typing, we suspected a
common source for the three carriers. We visited
the RCHE and encouraged staff to implement better
infection control practices. There were no subsequent
outbreaks reported. Internationally, the prevalence
of MDRA is much lower than that reported in studies
from the US (prevalence of multidrug-resistant A
baumannii was 15.0%)40 and
Australia (prevalence of
multidrug-resistant A baumannii was 5.2%).41
In RCHEs, the prevalence of MRSA is rising
rapidly, and that of MDRA has the potential to rise.
Thus, infection control practice in RCHEs should be
enhanced. Early identification of residents carrying
MDRO enables RCHE staff to implement enhanced
infection control practices such as early isolation
or cohorting. Hand hygiene protocols should
be followed carefully by health care workers in
RCHEs, especially when handling patients’ food or
medication; after napkin rounds; and before and after
nursing care processes.42
Environmental hygiene
measures, such as regular cleansing and disinfection
of residents’ immediate environment and frequently
touched areas, are of similar importance.43
The present study identified no VRE or CPE
carriers from 373 stool and 654 rectal swabs
of the residents screened. This echoes an earlier
study of 28 RCHEs in Hong Kong Island from July
to August 2015.34 Among 1408
subjects screened in
that study, a single resident had CPE and VRE was
not detected in any screened specimens.
To contain the spread of VRE and CPE among
residents in RCHEs, current practice is to inform the
RCHE before a VRE or CPE carrier is planned to be
discharged from hospital. The RCHE staff members
are recommended to enhance infection control
practices, to use designated equipment with the
carrier, and to adopt modified contact precaution
when providing care to the carrier. This strategy has
been successful; no outbreaks have been detected
among RCHEs receiving VRE or CPE carriers, and
the prevalence of VRE and CPE remains low in
these RCHEs. Extra resources are needed if a similar
strategy is adopted to control further increases in the
prevalence of MRSA and MDRA.
In the present study, residents of privately
run RCHEs were more likely than residents of non-private
RCHEs to be carriers of MRSA. This could
be due to privately run RCHEs being more resource-limited,
as reflected by the typically lower staff-to-residents
ratio.44
The present study also found that MRSA
colonisation was more common in non-Chinese
residents than in Chinese residents. This is consistent
with previously published research.45
To mitigate
this, future infection control training should raise
awareness among RCHE staff of this issue and to
adopt adequate infection control measures for
Chinese and non-Chinese residents alike.
Increased age, use of medical device, and
previous MRSA colonisation or infection are risk
factors that have been previously reported to be
associated with MRSA colonisation.46
However,
the present study did not show any statistically
significance differences between MRSA carriers and
non-carriers by multivariate analysis. This could be
due to the small sample size or selection bias in this
study. A larger study is required to identify other risk
factors.
There are some potential limitations to the
present study. We conducted the survey in RCHEs
in Kowloon City District. This may affect the
generalisation of the results to RCHEs in the rest
of Hong Kong. Among 56 RCHEs invited, 19 out of
50 privately run RCHEs and 1 out of 6 non-private
RCHEs agreed to join the survey; 67.6% of residents
from these RCHEs participated. The low participation
rate of RCHEs may reduce the representativeness
of study sample to the Hong Kong population of
RCHE residents. We had no information on non-participating
residents for baseline characteristics
comparison. Self-selection bias cannot be excluded.
The sample size required to accurately assess MRSA
prevalence was estimated. The actual sample size may
be insufficient for risk factor identification and effect
size estimation. We extracted residents’ information
from medical records kept by participating RCHEs;
therefore, information bias due to measurement
error cannot be eliminated, and missing data in
the medical records may lead to bias. Prevalence of
MRSA or MDRA may be underestimated as only
nasal and axillary swabs were taken. Other sites such
as wounds, catheter sites, groins or perianal region
were not sampled. The MDRA detection sensitivity
would be improved by using sterile sponges to
sample multiple body sites.47
Conclusions
Emergence of MDROs is a global health threat and
Hong Kong is not exempt. Residents of RCHEs are
particularly vulnerable to MDRO colonisation or
infection. Enhanced infection control is important
to mitigate further increases in MDRO prevalence in
RCHEs. The present study provides an understanding
of the situation of MDROs in RCHEs. Further
larger-scale studies on MDROs in Hong Kong are
required to formulate a targeted infection control
programme to prevent further spread of MDROs in
the community.
Author contributions
Concept and design of study: All authors.
Acquisition of data: H Chen, KM Au, KE Hsu, CKC Lai, J Myint, YF Mak.
Analysis and interpretation of data: H Chen, KE Hsu.
Drafting of the article: H Chen, KE Hsu.
Critical revision of important intellectual content: H Chen, CKC Lai, J Myint, YF Mak, SY Lee, TY Wong, NC Tsang.
Acquisition of data: H Chen, KM Au, KE Hsu, CKC Lai, J Myint, YF Mak.
Analysis and interpretation of data: H Chen, KE Hsu.
Drafting of the article: H Chen, KE Hsu.
Critical revision of important intellectual content: H Chen, CKC Lai, J Myint, YF Mak, SY Lee, TY Wong, NC Tsang.
Acknowledgement
The authors thank colleagues of the Community
Geriatric
Assessment Team of Queen Elizabeth Hospital and Kowloon
Hospital for their dedication and support. The authors also
thank the health care workers of all participating RCHEs.
Funding/support
This research received no specific grant from any
funding
agency in the public, commercial, or not-for-profit sectors.
Declaration
All authors have disclosed no conflicts of
interest. All authors
had full access to the data, contributed to the study, approved
the final version for publication, and take responsibility for its
accuracy and integrity.
Ethical approval
The survey was approved by the Ethics Committee of
Kowloon
Central Cluster, the Hospital Authority, and the Department
of Health. Written informed consent was obtained from all
residents or from their relatives or guardians.
References
1. Institute of Medicine (US) Forum on
Emerging Infections.
Antimicrobial resistance: issues and options—workshop
report. Washington: National Academies Press (US);
1998. Available from: http://www.ncbi.nlm.nih.gov/books/NBK100885/.
Accessed 13 May 2015.
2. Institute of Medicine (US) Forum on
Emerging
Infections. The resistance phenomenon in microbes and
infectious disease vectors: implications for human health
and strategies for containment: workshop summary.
Washington: National Academies Press (US); 2003.
Available from: http://www.ncbi.nlm.nih.gov/books/NBK97138/. Accessed 13
May 2015.
3. Social Welfare Department, Hong Kong SAR
Government. List
of residential care homes. Available from:
http://www.swd.gov.hk/en/index/site_pubsvc/page_elderly/sub_residentia/id_listofresi/.
Accessed 31 Mar 2015.
4. Strausbaugh LJ, Crossley KB, Nurse BA,
Thrupp LD.
Antimicrobial resistance in long-term-care facilities. Infect
Control Hosp Epidemiol 1996;17:129-40. Crossref
5. Safdar N, Maki DG. The commonality of
risk factors for
nosocomial colonization and infection with antimicrobial-resistant
Staphylococcus aureus, enterococcus, Gram-negative
bacilli, Clostridium difficile, and Candida. Ann
Intern Med 2002;136:834-44. Crossref
6. Boyce JM, Cookson B, Christiansen K, et
al. Meticillin-resistant
Staphylococcus aureus. Lancet Infect Dis
2005;5:653-63. Crossref
7. Ho P, Yuen K, Yam W, Wong S, Luk W.
Changing patterns of
susceptibilities of blood, urinary and respiratory pathogens
in Hong Kong. J Hosp Infect 1995;31:305-17. Crossref
8. Chuang V, Tsang I, Wong T.
Methicillin-resistant
Staphylococcus aureus (MRSA) in public hospitals in Hong
Kong. Commun Dis Watch 2010;7:49-50.
9. Brugnaro P, Fedeli U, Pellizzer G, et
al. Clustering and
risk factors of methicillin-resistant Staphylococcus aureus
carriage in two Italian long-term care facilities. Infection
2009;37:216-21. Crossref
10. Talon DR, Bertrand X.
Methicillin-resistant Staphylococcus
aureus in geriatric patients: usefulness of screening in
a chronic-care setting. Infect Control Hosp Epidemiol
2001;22:505-9. Crossref
11. Abbo A, Navon-Venezia S, Hammer-Muntz
O, Krichali
T, Siegman-Igra Y, Carmeli Y. Multidrug-resistant
Acinetobacter baumannii. Emerg Infect Dis 2005;11:22-9. Crossref
12. Bergogne-Bérézin E, Towner KJ.
Acinetobacter spp. as
nosocomial pathogens: microbiological, clinical, and
epidemiological features. Clin Microbiol Rev 1996;9:148-65.
13. Dijkshoorn L, Nemec A, Seifert H. An
increasing threat in
hospitals: multidrug-resistant Acinetobacter baumannii.
Nat Rev Microbiol 2007;5:939-51. Crossref
14. Gales AC, Jones RN, Forward KR,
Liñares J, Sader HS,
Verhoef J. Emerging importance of multidrug-resistant
Acinetobacter species and Stenotrophomonas maltophilia
as pathogens in seriously ill patients: geographic patterns,
epidemiological features, and trends in the SENTRY
Antimicrobial Surveillance Program (1997-1999). Clin
Infect Dis 2001;32 Suppl 2:S104-13. Crossref
15. Gaynes R, Edwards JR, National
Nosocomial Infections
Surveillance System. Overview of nosocomial infections
caused by Gram-negative bacilli. Clin Infect Dis
2005;41:848-54. Crossref
16. Lo J, Wong T. Update on surveillance
of multi-antimicrobial
resistance. Commun Dis Watch 2011;8:98-9.
17. Choi K, Chen H, Wong T. Vancomycin
resistant
enterococcus (VRE) in Hong Kong. Commun Dis Watch
2011;8:102-3.
18. Ho PL, Lai EL, Chow KH, Chow LS, Yuen
KY, Yung
RW. Molecular epidemiology of methicillin-resistant
Staphylococcus aureus in residential care homes for
the elderly in Hong Kong. Diagn Microbiol Infect Dis
2008;61:135-42. Crossref
19. Ho PL, Wang TK, Ching P, et al.
Epidemiology and genetic
diversity of methicillin-resistant Staphylococcus aureus
strains in residential care homes for elderly persons in
Hong Kong. Infect Control Hosp Epidemiol 2007;28:671-8. Crossref
20. Chen H, Yau C, Leung L, Hsu E, Ng H,
Wong TY. Prevalence
of methicillin resistant Staphylococcus aureus (MRSA)
carriage among residents of Residential Care Homes for
Elderly in Hong Kong. Proceedings of the Hong Kong
Society for Infectious Diseases 16th Annual Scientific
Meeting; 2012 Mar 10; Hong Kong. Hong Kong: HKSID;
2012: 11.
21. Chen H, Chiu AP, Lam PS, et al.
Prevalence of infections in
residential care homes for the elderly in Hong Kong. Hong
Kong Med J 2008;14:444-50.
22. World Health Organization.
Tuberculosis prevalence
surveys: a handbook. Available from:
http://www.who.int/tb/advisory_bodies/impact_measurement_taskforce/resources_documents/thelimebook/en/.
Accessed 20 Jan 2015.
23. Katz S, Downs TD, Cash HR, Grotz RC.
Progress
in development of the index of ADL. Gerontologist
1970;10:20-30. Crossref
24. Clinical and Laboratory Standards
Institute. Performance
Standards for Antimicrobial Susceptibility Testing;
Twenty-fifth Informational Supplement. CLSI document
M100-S25. Wayne, PA: Clinical and Laboratory Standards
Institute; 2015.
25. Graubard BI, Korn EL. Confidence
intervals for proportions
with small expected number of positive counts estimated
from survey data. Surv Methodol 1998;24:193-201.
26. Breeze E. General household survey,
report on sampling
error (based on 1985 and 1986 data). London: HMSO; 1990. Available from:
https://library.herts.ac.uk/cgi-bin/koha/opac-detail.pl?biblionumber=9939.
Accessed 3 Jan
2018.
27. Brown LD, Cai TT, DasGupta A. Interval
estimation for a
binomial proportion. Stat Sci 2001;16:101-17. Crossref
28. Bradley SF, Terpenning MS, Ramsey MA,
et al. Methicillin-resistant
Staphylococcus aureus: colonization and infection
in a long-term care facility. Ann Intern Med 1991;115:417-22. Crossref
29. Manzur A, Gavalda L, Ruiz de Gopegui
E, et al. Prevalence
of methicillin-resistant Staphylococcus aureus and
factors associated with colonization among residents
in community long-term-care facilities in Spain. Clin
Microbiol Infect 2008;14:867-72. Crossref
30. O’Sullivan NP, Keane CT. Risk factors
for colonization
with methicillin-resistant Staphylococcus aureus among
nursing home residents. J Hosp Infect 2000;45:206-10. Crossref
31. Grundmann H, Tami A, Hori S, Halwani
M, Slack R.
Nottingham Staphylococcus aureus population study:
prevalence of MRSA among elderly people in the
community. BMJ 2002;324:1365-6. Crossref
32. Hospital Authority, Hong Kong SAR
Government. 2014-2015 Hospital Authority Statistical Report. May 2016.
Available from: http://www.ha.org.hk/haho/ho/stat/HASR1415_1.pdf. Accessed
9 Sep 2016.
33. World Health Organization. Guidelines
for measuring
national HIV prevalence in population-based surveys.
2005. Available from:
http://www.who.int/hiv/pub/surveillance/measuring/en/. Accessed 21 May
2015.
34. Cheng VC, Chen JH, Ng WC, et al.
Emergence of
carbapenem-resistant Acinetobacter baumannii in nursing
homes with high background rates of MRSA colonization.
Infect Control Amp Hosp Epidemiol 2016;37:983-6. Crossref
35. Cheng VC, Tai JW, Wong ZS, et al.
Transmission of
methicillin-resistant Staphylococcus aureus in the long
term care facilities in Hong Kong. BMC Infect Dis
2013;13:205. Crossref
36. Reynolds C, Quan V, Kim D, et al.
Methicillin-resistant
Staphylococcus aureus (MRSA) carriage in 10 nursing
homes in Orange County, California. Infect Control Hosp
Epidemiol 2011;32:91-3. Crossref
37. Cox RA, Bowie PE.
Methicillin-resistant Staphylococcus
aureus colonization in nursing home residents: a prevalence
study in Northamptonshire. J Hosp Infect 1999;43:115-22. Crossref
38. Gu FF, Zhang J, Zhao SY, et al. Risk
factors for methicillin-resistant
Staphylococcus aureus carriage among residents
in 7 nursing homes in Shanghai, China. Am J Infect Control
2016;44:805-8. Crossref
39. Cheng VC, Chen JH, So SY, et al. Use
of fluoroquinolones is
the single most important risk factor for the high bacterial
load in patients with nasal and gastrointestinal colonization
by multidrug-resistant Acinetobacter baumannii. Eur J
Clin Microbiol Infect Dis 2015;34:2359-66. Crossref
40. Mody L, Gibson KE, Horcher A, et al.
Prevalence of
and risk factors for multidrug-resistant Acinetobacter
baumannii colonization among high-risk nursing home
residents. Infect Control Hosp Epidemiol 2015;36:1155-62. Crossref
41. Lim CJ, Cheng AC, Kennon J, et al.
Prevalence of
multidrug-resistant organisms and risk factors for carriage
in long-term care facilities: a nested case-control study. J
Antimicrob Chemother 2014;69:1972-80. Crossref
42. Cheng VC, Chen JH, Poon RW, et al.
Control of hospital
endemicity of multiple-drug-resistant Acinetobacter
baumannii ST457 with directly observed hand hygiene.
Eur J Clin Microbiol Infect Dis 2015;34:713-8. Crossref
43. Barnes SL, Morgan DJ, Harris AD,
Carling PC, Thom
KA. Preventing the transmission of multidrug-resistant
organisms: modeling the relative importance of hand
hygiene and environmental cleaning interventions. Infect
Control Hosp Epidemiol 2014;35:1156-62. Crossref
44. Ngai YH. Institutional risk factors
for influenza
outbreaks in Hong Kong elderly homes: a retrospective
cohort study. 2016. Available from: http://hub.hku.hk/handle/10722/237233.
Accessed 5 Dec 2017.
45. Leung YH, Lai RW, Chan AC, et al. Risk
factors
for community-associated methicillin-resistant
Staphylococcus aureus infection in Hong Kong. J Infect
2012;64:494-9. Crossref
46. Forster AJ, Oake N, Roth V, et al.
Patient-level factors
associated with methicillin-resistant Staphylococcus
aureus carriage at hospital admission: a systematic review.
Am J Infect Control 2013;41:214-20. Crossref
47. Doi Y, Onuoha EO, Adams-Haduch JM, et
al. Screening for
Acinetobacter baumannii colonization by use of sponges. J
Clin Microbiol 2011;49:154-8. Crossref