Hong Kong Med J 2018 Apr;24(2):166–74 | Epub 6 Apr 2018
DOI: 10.12809/hkmj177123
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE CME
Understanding breast cancer screening—past, present,
and future
Jacqueline CM Sitt, MB, BS, FHKCR; CY Lui, MB,
ChB, FHKCR; Lorraine HY Sinn, MB, BS, FHKCR; Julian CY Fong, MB, BS, FHKCR
Hong Kong Women’s Imaging Limited, Suite 319, 3/F,
Central Building, Central, Hong Kong
Corresponding author: Dr Jacqueline CM Sitt (jacquelinesitt@gmail.com)
Abstract
This article provides an up-to-date overview of
breast cancer mammography screening and briefly discusses its history,
controversies, current guidelines, practices across Asia, and future
directions. An emphasis is made on shared decision-making—instead of
giving just a ‘yes’ or ‘no’ answer to patients, the focus should be on
providing sufficient information about the pros and cons of screening to
help women make a personal, informed choice. Frontline experts, including
breast surgeons, oncologists, breast radiologists, and their
representative professional associations should all participate in
guideline panels, with the goal of improving cancer detection, reducing
mortality, and improving patient outcome.
Introduction
This article provides an up-to-date overview of
breast cancer mammography screening and briefly discusses its history,
controversies, current guidelines, practices across Asia, and future
directions. An emphasis is made on shared decision-making—instead of
giving just a ‘yes’ or ‘no’ answer to patients, the focus should be on
providing sufficient information about the pros and cons of screening to
help women make a personal, informed choice.
Goals and advantages of breast cancer screening
The goal of mammographic screening (and other
breast-cancer screening tests) is to detect breast cancer earlier than it
would otherwise manifest clinically, when it is less likely to have
spread. Data clearly show that detection of breast cancers at smaller
sizes and lower (earlier) stages is associated with better patient
outcomes, lower morbidity, and reduced breast cancer deaths.1 Reduced morbidity is likely to be related to
feasibility of breast conservation and hence less extensive surgery, fewer
associated complications such as lymphoedema, less chemotherapy, and hence
fewer adverse effects.2 Other
benefits of diagnosing screen-detected cancers at an earlier stage also
include a lower cost of treatment and consequent reduced financial burden
on health care resources.3
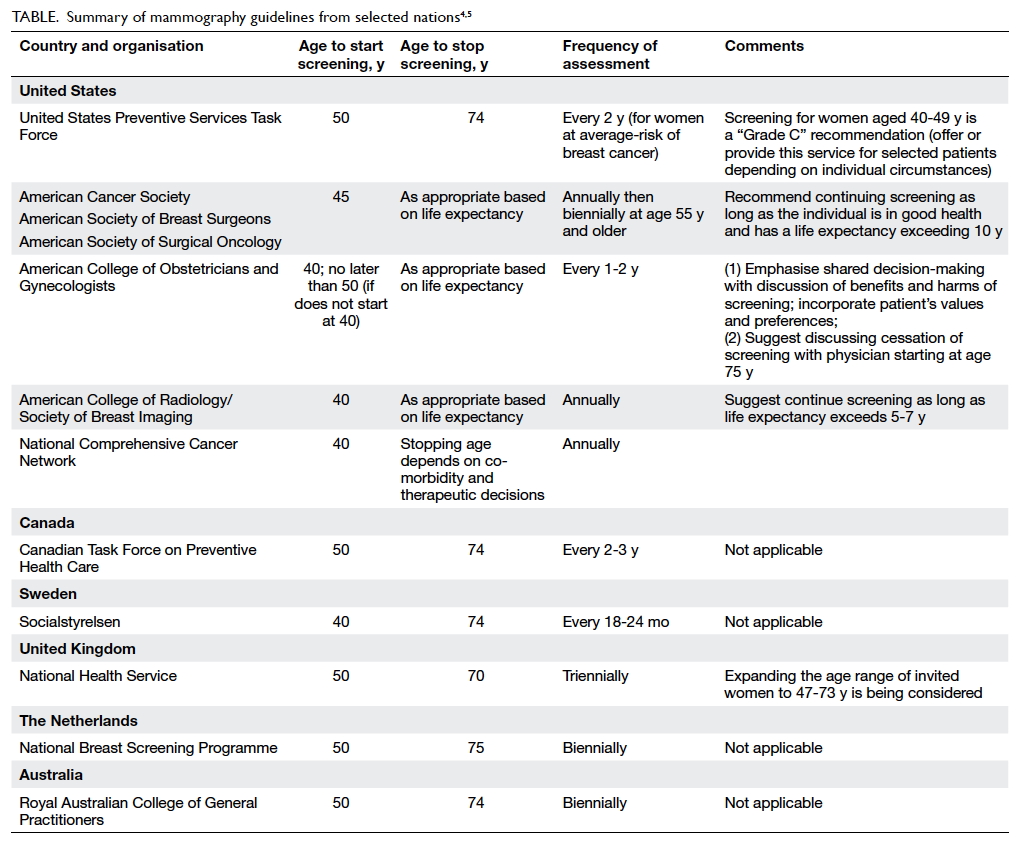
Current guidelines
The Table summarises the mammography guidelines from
selected nations.4 5 In common, all organisations emphasise that the
benefits of screening outweigh the harm at all ages.3 6 They all
endorse informed decision-making and the importance of informing women
about both benefits and limitations of screening. However, there remain
legitimate concerns about guideline differences, including the complexity
of the guidelines; weak adherence to creating opportunities for informed
decision-making; unreadiness of referring clinicians to discuss benefits,
limitations, and harm associated with screening; and the lack of reminder
systems, which results in weaker adherence to recommended screening
intervals. Despite these concerns, it is widely accepted that high
adherence to even the least aggressive guidelines will save more lives
than the current weak adherence to regular screening programmes.4
Current scientific evidence to support screening
Randomised controlled trials (RCTs) have been the
gold standard for proving that early detection with mammography decreases
mortality from breast cancer. Since the very first screening RCT performed
in New York in the 1960s, there have been eight prospective RCTs and
numerous subsequent meta-analyses published. Most well-executed RCTs
demonstrated a 20% to 30% decrease in mortality from breast cancer when
women were invited for screening. These results laid a solid foundation
for population-based screening programmes worldwide.1 7 8
Subsequent studies that generated data from
population-based screening programmes have provided further evidence of
the benefits of screening mammography. The true benefit reported (in terms
of mortality reduction) ranged from 38% to 49%, even higher than that
shown by RCTs. This difference demonstrates that service screening studies
measure the direct effect of screening on women who actually underwent
mammography, and not just those who were invited to undergo mammography
(as opposed to the methodology of RCTs). Service screening studies also
tend to measure the effect of more recent screening practices that have
benefited from improved mammography technology, better breast positioning
techniques, and improved interpretive skills.1
9
Understanding screening controversy and ‘mammographic
wars’
The Canadian National Breast Screening Study: root of
all controversies
One exception to the RCTs that reported
unfavourable results of mammographic screening was the Canadian National
Breast Screening Study (CNBSS). It was conducted between 1980 and 1985,
and was divided into two parts. The first CNBSS included approximately 50
000 volunteer women aged 40 to 49 years, and determined the mortality
benefit in the experimental group, who were assigned to annual screening
mammography plus clinical breast examination (CBE) versus the control
group who received usual care.10
The second CNBSS had almost 40 000 volunteer women aged 50 to 59 years,
and compared the benefit of annual mammography plus CBE with that of
yearly CBE alone.11
From the time the results were first published in
1992 and again after follow-up in 2000, 2002, and 2014, the CNBSS has been
controversial, because it is the only RCT to have reported no decrease in
mortality associated with an invitation to screening. The study also
claimed a 22% overdiagnosis of screen-detected invasive cancer, increasing
to up to 35% when cases of ductal carcinoma in situ (DCIS) were included.12 13
14 However, the credibility and
scientific value of the CNBSS study have been repeatedly questioned in
peer-reviewed publications.15 16 17
18 19
Most criticisms of this study are related to vulnerabilities and
shortcomings in its execution, including flaws in the randomisation
process, lack of statistical power, non-generalisable results, poor
quality imaging, suboptimal mammographic image acquisition and
interpretation by untrained personnel, and inconsistent thresholds for
interpretation.
The flaws in the randomisation process principally
arose from three areas. First, unlike all other RCTs, potential
participants in the Canadian trials initially underwent a careful physical
examination. Second, women with positive findings on physical examination,
including palpable lumps, skin or nipple retraction, and even palpable
axillary adenopathy, were not excluded from this ‘screening’ trial.18 Finally, randomisation was unblinded and
decentralised. Because almost 80% of women with advanced palpable cancers
were assigned to the screening arm in the first round of the study, there
has been speculation that concerned clinicians did not follow the
randomisation process, but rather assigned some symptomatic women to the
study group so that they would undergo mammography.19 Whether the imbalance was due to intentional
tampering or occurred by chance alone, the net effect was the same—namely,
a failure to produce two equal cohorts of patients for comparison.
The CNBSS was also criticised at the time of the
trial for poor quality mammography, even compared with mammographic
imaging of that era.15 20 To reduce radiation dose, mammography for the trial
was performed without the benefit of scatter-reducing grids despite their
routine use and availability. Standard imaging for much of the trial used
a straight lateral view, not a mediolateral-oblique view, which images
more tissue. The combination of poor quality imaging and the
investigators’ resistance to taking corrective action led two advisors’
resignation in protest. In addition, technologists who participated in the
trial received no special training in performing mammography. Radiologists
new to mammography also received no training in interpretation.18 There was also a lack of immediate follow-up after
recommendations for biopsy had been made. Overall, about 25% of the
recommended biopsies were ultimately not performed.18
The CNBSS trials are an excellent example of the
need to carefully consider all facets of a large-scale screening trial
before accepting its results as scientifically valid. The numerous design
and execution flaws described above explain in large part why the results
of the CNBSS are dramatically different from those of all other RCTs.
Ultimately, on the basis of the methodology of the CNBSS, the World Health
Organization excluded those results when analysing the breast-screening
data in the International Agency for Research on Cancer report.21
Controversial meta-analysis results from the Nordic
Cochrane Centre
The greatest debate on the value of breast
screening arose after the publication of a highly controversial but
frequently quoted meta-analysis by Gotzsche (a medical statistician and
director of the Nordic Cochrane Centre) and Olsen in The Lancet in
2000. Their study concluded that there was no benefit of mortality
reduction by screening, after discarding six of eight RCTs because they
deemed the randomisation to be “inadequate”. The only two RCTs included in
their analysis showed no benefit, including the Malmo trial and the
notorious CNBSS.7 22
Gotzsche and Olsen’s critique and methodology have
caused much controversy and, in turn, have been criticised heavily by
leading expert breast imagers, public health clinicians, and professional
bodies such as the Society of Breast Imaging.7
8 23
24 25
26 27
Gotzsche and Olsen’s use of quoted figures from cancer registries rather
than actual patient data, their selective approach to studies, and in
particular the ignoring of the flaws of the CNBSS, have received the
harshest criticism. Many experts have commented that Gotzsche and Olsen
overstated the limitations of most of the well-executed RCTs, thereby
reflecting a “context-free” application of guidelines in a way that did
not address the real issues relevant to the effectiveness of mammographic
screening. Moreover, Gotzsche and Olsen’s recommendation to abandon
screening altogether has hampered collaborative efforts to improve breast
cancer detection and control.27
Swiss Medical Board’s decision to stop population-based
screening in 2014
In February 2014, the Swiss Medical Board attempted
to overturn the widespread practice of mammography screening in
Switzerland by stating that new systematic mammography screening
programmes should not be introduced, irrespective of women’s age, and
recommended that existing programmes should be discontinued. Their main
argument was that the absolute risk reduction in breast cancer mortality
was low and that the adverse consequences of screening (false-positive
test results, overdiagnosis, overtreatment, and high costs and expense of
follow-up tests and procedures) were substantial.28
29
The Swiss Medical Board’s attempt initiated a new
phase of heated arguments and debate about the benefits of screening.
Expert breast cancer clinicians in both the United States and Europe
(including leading cancer associations in Switzerland) rejected their
report. One criticism was that the Swiss Medical Board relied heavily on
the controversial work by Gotzsche and Olsen and again quoted data from
the flawed CNBSS. Another criticism that attracted great attention was the
questionable “expert panels” of the board: they included a medical
ethicist, a clinical epidemiologist, a clinical pharmacologist, an
oncology surgeon, a nurse scientist, a lawyer, and a health economist.
Frontline breast imagers, with expertise in diagnosing breast diseases,
were excluded from the review panels because of a “conflict of interest”.28 29
The Swiss Medical Board did not adequately consider
the fact that assessment of the balance between benefit and harm involves
a value judgement that each woman should make only after she is fully
informed about the strengths and weaknesses of screening mammography. They
also disregarded the extensive literature in support of screening
mammography (RCTs and population service screening studies), making their
attempt at stopping national mammography screening unjustified.
Potential risks of screening overstated
Commonly mentioned potential harms of screening
include false-positive mammograms, recall for additional imaging, a
false-positive biopsy, missed breast cancer, radiation dose, patient
anxiety, and, above all, overdiagnosis.
Overdiagnosis is defined as the detection (and
subsequent actions taken) of a cancer by screening that would not have
progressed to become symptomatic in a woman’s lifetime.1 The estimation of overdiagnosis is complex, highly
debated, and very difficult to measure.3
Reported figures range widely, from 0% to 50%, vary greatly in terms of
methodological rigour, and testify to the inexact nature of most
mathematical models.30 31 32 33 34 When
appropriate adjustments for temporal trends, risk factors, and lead time
are considered, the level of overdiagnosis should be low, within the range
of 0% to 10%.32 Importantly, a
recent study of over 5 million women (aged 50-64 years) screened by the
United Kingdom’s National Health Service showed that there was a
significant negative association between the detection of DCIS at
screening and invasive interval cancers. In that study, Duffy and
colleagues analysed the data from four consecutive screen years and the
36-month outcome after each relevant screen. For every three
screen-detected cases of DCIS, there was one less interval case of
invasive cancer over the next 3 years. They agreed that the policy on
detection and treatment of DCIS is worthwhile and can prevent subsequent
invasive cancers.35
The effect of screening on heightening a patient’s
anxiety has also been long questioned by critics, but the magnitude of the
effect may have been over-exaggerated. In a survey of over 1200 women with
a 6-question anxiety scale to understand the short-term and long-term
impact of a recall examination, women involved in the digital mammographic
imaging screening trial demonstrated only a transient, limited increase in
anxiety after a false-positive mammogram compared with those with a
negative mammogram, and there was no difference between the two groups’
intention to undergo mammography again in the subsequent 2 years.36 Schwartz et al reported that 96% of American women
who received a false-positive mammography report were glad that they
underwent the test and remained supportive of screening.37 Most women agreed that the anxiety, inconvenience,
and the few image-guided needle biopsies using local anaesthesia
associated with a recall from screening, were minor compared with dying of
breast cancer.38
To summarise, papers citing a high rate of
overdiagnosis in screening (in the magnitude of 20% or higher) and
claiming that false-positives are a significant cause of patient anxiety
are believed by most experts to be overstating the case.
Harms of not screening underestimated
Although it is important to discuss all aspects of
screening asymptomatic women (including potential harm), the harm of not
attending screening is underestimated and not discussed. For instance,
women who do not attend screening have significantly larger tumours, a
higher stage at diagnosis, poorer overall and disease-specific survival,
and higher costs of treatment.39
It has been estimated that the cost of treating advanced metastatic breast
cancer exceeds US$ 250 000 per patient, and the average cost of treating
advanced cancer in the first year after diagnosis is almost double that of
early cancers, mainly owing to the difference in costs of chemotherapy.3 40
The cost of treatment and lost productivity each year will far exceed the
cost of annual screening and, additionally, do not include the indirect
value of the lives saved (as a productive member of workforce).1
Situation in Asia
Rising breast cancer incidence: a universal phenomenon
among Asian women
The incidence of breast cancer continues to
increase worldwide. It remains highest in the United States and Europe,
but has been increasing substantially in Asian countries over the past
three decades.41 Studies that
compare invasive breast cancer data from Asia with those from the United
States over a 20-year period have shown that female breast cancer
incidence among Asian and Western populations is more similar than
expected.42 The incidence of
female breast cancer in China will continue to rise, and is expected to
exceed 100 per 100 000 women by 2021, giving a total of 2.5 million cases.43
According to GLOBOCAN 2012 of the International
Agency for Research on Cancer, the specialised cancer research agency of
the World Health Organization, almost a quarter (24%) of all breast
cancers were diagnosed within the Asia-Pacific region, with the greatest
number occurring in China (46%).44
The age-standardised incidence rate was highest among Taiwanese (65.9 per
100 000), followed by Singaporeans, South Koreans, and Japanese.44 In a
multiracial country such as Singapore, Chinese women have been noted to
have a significantly higher risk of developing breast cancer than Malays
and Indians.45
The disease burden in Hong Kong is no different.
Locally, the age-standardised incidence rate was 58.8 per 100 000 in 2015,
with over 3900 new cases per year.46
A study of the local trend in female breast cancer incidence from 1973 to
1999 by the University of Hong Kong showed a significant yearly increase
of an average of 3.6%; the increase was most marked and continued to
accelerate in the younger age-groups. It was speculated that such trend
changes were related to Westernisation of lifestyle.47 All these data indicate that the disease burden in
Hong Kong is increasing and comparable to that of all other civilised
Asian countries and cities.
Breast screening programmes in Asia
Breast screening services in Asian countries and
cities are highly variable: some have advanced nationwide screening
programmes and others have less developed programmes.48 South Korea and Taiwan are both well recognised for
their experience in running such programmes, the former having the highest
intake rate and the latter being the most well-structured.
South Korea places a very strong emphasis on
screening for cancer control in general. Its national health service
offers mammography and CBE every 2 years to women aged 40 or older, and at
no cost to the 50% of people with the lowest incomes. Their programme is
popular and widely accepted by the general public, and achieved an uptake
of as high as 66% in 2014. Benefits of downstaging from screening were
also observed. However, South Korea encountered a problem of potential
overdiagnosis, with a noticeably higher false-positive rate when compared
with other places.
Taiwan’s health authorities have been recognised
for rolling-out well-organised and well-resourced screening programmes,
with good support from a local randomised controlled trial showing a
reduction in mortality by 40% with mammography screening.49 Since 2004, their health service has provided free
breast screening to women aged 50 to 69 years, expanded in 2010 to those
aged 40 to 49 years. By 2015, about 40% of the target population
participated in screening. It is believed that the cause of the suboptimal
participation rate was not due to capacity or outreach, but rather the
Taiwanese public’s values and attitude. Nonetheless, with more resources
being directed to public education and motivation, Taiwan’s health
authorities are pushing their goal to 60% by 2018.
The experience of screening programmes in Singapore
and Japan is more equivocal. Despite having sufficient scientific evidence
to support their role in reducing mortality and reducing invasive cancer
incidence, the participation rate has remained lower than expected, mostly
owing to cultural barriers and paradigms, or a lack of central governing.
Singapore established its national, population-wide screening programme
(BreastScreen Singapore) in 2002 and now covers women aged 40 to 69 years.
The participation rate has been noted to plateau at 40% since 2010, short
of the target of 70%. The health promotion board believes that apart from
cultural issues, costs (as screening is paid by an individual’s medical
insurance account) constitute the greatest barrier to uptake.
The study of population-based screening in Japan
has been complex, with scattered data owing to the lack of a single
national organisation for monitoring. The participation rate remains lower
than in other comparable Asian countries in the past century, again likely
because of cultural paradigms. Despite these barriers, in the past decade,
Japanese health officials have started designing their own methods and
protocols for screening, particularly targeting the higher incidence of
cancer among younger women (aged 40-49 years) and the large proportion of
patients with dense breasts. After the launch of government-funded
screening programmes, a clinical trial that started in 2007 (Japan
Strategic Anti-cancer Randomised Trial, J-START) of over 70 000 women
undergoing adjunctive ultrasonography to supplement mammography for
screening showed an increased sensitivity and detection rate for early
preclinical cancers.41
In China, there is no nationwide screening
programme for breast cancer. A mammographic screening programme was
attempted in 2005 but was abandoned because of lack of funding and
concerns about false-positive diagnoses. Despite these barriers, national
guidelines established in 2007 recommend annual mammography for women aged
40 to 49 years, and every 1 to 2 years for those aged 50 to 69 years. In a
Beijing study of 1.46 million women (aged 35 to 59 years) who underwent
screening by ultrasonography from 2009 to 2011, the cancer detection rate
was 48.0 per 100 000, including 440 cases at early stage that constituted
69.7% of cases detected. The detection rate was lower than anticipated,
maybe in part owing to the young age of the screened group and omission of
mammography as a screening tool. Subsequently, a second-generation
screening programme was initiated in 2012, after modification of the
screening methods, cohort size (6 million), and target population that
included women aged 35 to 64 years. The new screening procedures include
parallel CBE and breast ultrasonography; women with suspicious findings
from either examination are recommended to undergo mammographic imaging.50 Although the design of this
screening protocol deviates from the standard practice of other countries,
we believe that the programme will bring more research data and
experience, and eventually lead to more comprehensive guidelines and
consensus on a screening approach in China.
Breast-screening programmes in Hong Kong: room for
development
The awareness of breast cancer and acceptance of
screening in Hong Kong is growing, but is still inadequate. According to
the latest Breast Cancer Registry Report No 8 (2016), which covers 13 453
breast cancer patients diagnosed from 2006 onwards, the mean and median
age of patients at diagnosis was 52.6 and 51.3 years, respectively, and
about two-thirds of patients were aged 40 to 59 years. The screening
habits among these patients were poor, with over 60% never having
undergone mammography screening before their cancer diagnosis.51
Although to date there has been no population-based
screening for women in Hong Kong, opportunistic screening has long been
practised in the private sector. The largest voluntary self-financed and
self-referred opportunistic screening programme is run by the Tung Wah
Group of Hospitals. In a retrospective review of their performance from
1998 to 2002 involving over 46 600 screening mammograms, a breast cancer
detection rate of five cases per 1000 population was noted, which was
comparable to the detection rate of Western screening programmes at that
time.52
Regarding the input of expertise and quality
assurance, the Hong Kong College of Radiologists issued their mammographic
statement in 2006 (latest revision in 2015).53
Quoting desirable goals recommended by the United Kingdom and United
States as a reference the statement sets specific benchmarks for standards
of mammographic machines, quality of screening mammograms, radiation dose
limits, and accreditation requirements of reporting radiologists.53 Given these guidelines, together with recent advances
in mammographic technology, we believe that there should be room for
further local development of large-scale quality breast-screening
programmes.
Designing a screening programme for Hong Kong: can
there be a protocol tailor-made for Chinese women?
When planning a breast-screening programme, it is
necessary to decide whom to screen (ie, at what age and the target
screening population) and how to screen (ie, screening method).
For the decision of whom to screen, we should note
that the mean age at diagnosis of breast cancer in Chinese women is 45 to
55 years, considerably younger than for western women.43 Starting screening at age 40 or 45 years would likely
be a better fit for Chinese women than starting at age 50 years, as
recommended by some western guidelines. As for the target screening
population, current data favour universal screening over risk-based
screening (pre-selecting patients according to risk profile). First, one
should note that 80% of women with newly diagnosed breast cancer have no
family history (ie, first-degree relative) or other significant previous
risk factors, and therefore risk-based screening will miss a majority of
screen-detected breast cancers.3 54 Second, a recent 10-year
population-based cohort study of over 1.4 million asymptomatic Taiwanese
women undergoing various breast-cancer screening regimens showed that
universal mammography screening based only on age and sex was more
effective than other screening regimens (risk-based biennial mammography
screening or annual CBE alone).49
In that study, universal biennial mammography screening was associated
with a 41% reduction in mortality and a rate of overdiagnosis of only 13%.
In contrast, risk-based biennial mammography (pre-selecting patients
according to risk profile or risk score) did not lead to any statistically
significant reduction in mortality. Moreover, among all screening
regimens, only universal biennial screening was associated with a clear
downstaging shift in tumours (30% reduction of stage 2+ cancers), a
crucial factor that can improve patient outcome.49
Regarding methods of screening, conventional
screening uses standard two-view full-field digital (two-dimensional; 2D)
mammography. Multiple studies have proven that screening by digital breast
tomosynthesis (DBT; also called three-dimensional mammography) can
increase cancer detection rates compared with 2D mammography alone, and
can reduce the recall rate for benign findings (false-positives). 1 55 A
retrospective analysis of over 454 000 screens showed that use of DBT was
associated with relative increases of 41% in invasive cancer detection,
49% in positive predictive value (PPV) for recall, and 21% in PPV for
biopsy, in addition to a 15% reduction in the overall number of recalls.56 A recent meta-analysis by a
Korean group also showed that screening with DBT increased detection of
early invasive cancers of <2 cm.57
The American College of Radiology Commission on Breast Imaging now
recommends that mammography and DBT are “usually appropriate” for
screening of average-risk women, noting that DBT addresses some
limitations of standard digital mammography.58
In Hong Kong, DBT has been increasingly adopted to replace or serve as an
adjunct to 2D mammography in opportunistic screening. We anticipate that
the shift to DBT screening will become a global trend.
The use of whole-breast ultrasonography to screen
dense breasts is also commonly adopted in Asia, including for
opportunistic screening in Hong Kong. In Japan, this practice was
reinforced by a government-funded RCT (J-START) that studied the use of
adjunctive ultrasonography to supplement mammography in screening over 70
000 women. The J-START study showed favourable results of increased
sensitivity and detection rate for early, preclinical cancers.41
Screening for high-risk women is often considered a
separate entity. According to the American College of Radiology’s
Appropriateness Criteria, women at high risk due to prior mantle radiation
between the ages of 10 and 30 years should start mammography 8 years after
radiation therapy, but not before age 25. For women with a genetic
predisposition, annual screening mammography is recommended to begin 10
years earlier than the age that an affected relative had been diagnosed,
but not before age 30. Annual screening by magnetic resonance imaging is
recommended in high-risk women as an adjunct to mammography.59
Future directions for Hong Kong
We believe that health care in Hong Kong should
have the capability and expertise to roll out quality, large-scale
population-screening programmes that are comparable to those in other
developed Asian countries and cities. When we examine the common themes
among available guidelines, literature, and expert reviews worldwide, the
global trend is to provide women with an informed choice.
In the discussion of whether breast-cancer
screening is feasible, one should bear in mind that this is an emotive
issue. Apart from the critical appraisal of scientific evidence, the
interpretation of literature and subsequent formulation of recommendations
should always account for the socioeconomic, historical, and contextual
realities. The value judgement of women should also be respected.
Frontline experts, including breast surgeons,
oncologists, breast radiologists, and their representative professional
associations should all participate in guideline panels, with a will to
end the ‘mammography wars’. Our Holy Grail should always be focused on
improving cancer detection, reducing mortality, and improving patient
outcome.
References
1. Eby PR. Evidence to support screening
women annually. Radiol Clin North Am 2017;55:441-56.
2. Society of Breast Imaging Breast
Screening Leadership Group. Screening in the 40-49 Age Group. Available
from:
https://www.sbi-online.org/RESOURCES/BreastScreeningLeadershipGroupResources.aspx.
Accessed 19 Nov 2017.
3. Ray KM, Price ER, Joe BN. Evidence to
support screening women in their 40s. Radiol Clin North Am 2017;55:429-39.
4. The American College of Radiology.
Guidelines for Breast Cancer Screening—An Update. SBI Breast Imaging
Symposium 2016. Available from:
https://www.sbi-online.org/Portals/0/Breast%20Imaging%20Symposium%202016/Final%20Presentations/4-7%20800am%20Smith%20-%20Guidelines%20for%20Breast%20Cancer%20Screening.pdf.
Accessed 19 Nov 2017.
5. Shieh Y, Eklund M, Sawaya GF, et al.
Population-based screening for cancer: hope and hype. Nat Rev Clin Oncol
2016;13:550-65. Crossref
6. Byrne SK. What's the Buzz: Tell me
what’s happening in breast cancer screening. Asia Pac J Oncol Nurs
2017;4:122-6. Crossref
7. Freer P, Moy L, DeMartini W; the
Screening Leadership Group. Breast Cancer Screening: Understanding the
Randomised Controlled Trials. Available from:
https://www.sbi-online.org/Portals/0/Screening%20Leaders/Breast%20Cancer%20Screening_Understanding%20the%20Randomized%20Controlled%20Trials.pdf.
Accessed
19 Nov 2017.
8. Duffy SW, Chen TH, Smith RA, Yen AM,
Tabar L. Real and artificial controversies in breast cancer screening.
Breast Cancer Manage 2013;2:519-28. Crossref
9. Newell M, Eby PR; the Breast Screening
Leadership Group. Benefits of Screening Mammography: Data from Population
Service Screening. Available from:
https://www.sbi-online.org/Portals/0/Screening Leaders/Benefitsof
Screening Mammography_Data from PopulationService Screening.pdf. Accessed
19 Nov 2017.
10. Miller AB, Baines CJ, To T, et al.
Canadian National Breast Screening Study: 2. Breast cancer detection and
death rates among women aged 50 to 59 years. CMAJ 1992;147:1477-88.
11. Miller AB, Baines CJ, To T, et al.
Canadian National Breast Screening Study: 1. Breast cancer detection and
death rates among women aged 40 to 49 years. CMAJ 1992;147:1459-76.
12. Miller AB, To T, Baines CJ, et al.
Canadian National Breast Screening Study-2: 13-year results of a
randomized trial in women aged 50-59 years. J Natl Cancer Inst
2000;92:1490-9. Crossref
13. Miller AB, To T, Baines CJ, et al. The
Canadian National Breast Screening Study-1: breast cancer mortality after
11 to 16 years of follow-up. A randomized screening trial of mammography
in women age 40 to 49 years. Ann Intern Med 2002;137:305-12. Crossref
14. Miller AB, Wall C, Baines CJ, et al.
Twenty five year follow-up for breast cancer incidence and mortality of
the Canadian National Breast Screening Study: randomised screening trial.
BMJ 2014;348:g366. Crossref
15. Kopans DB, Feig SA. The Canadian
National Breast Screening Study: a critical review. AJR Am J Roentgenol
1993;161:755-60. Crossref
16. Burhenne LJ, Burhenne HJ. The Canadian
National Breast Screening Study: a Canadian critique. AJR Am J Roentgenol
1993;161:761-3. Crossref
17. Kopans D. Breast Imaging. 3rd ed.
Lippincott Williams & Wilkins: Philadelphia; 2007.
18. Heywang-Köbrunner SH, Schreer I,
Hacker A, et al. Conclusions for mammography screening after 25-year
follow-up of the Canadian National Breast Cancer Screening Study (CNBSS).
Eur Radiol 2016;26:342-50. Crossref
19. Boyd NF. The review of randomization
in the Canadian National Breast Screening Study. Is the debate over? CMAJ
1997;156:207-9.
20. Baines CJ, Miller AB, Kopans DB, et
al. Canadian National Breast Screening Study: assessment of technical
quality by external review. AJR Am J Roentgenol 1990;155:743-7; discussion
8-9. Crossref
21. International Agency for Cancer on
Research (IARC), World Health Organization. IARC Handbooks of Cancer
Prevention. Volume 7: Breast Cancer Screening. IARC Press; 2002. Available
from:
http://www.iarc.fr/en/publications/pdfs-online/prev/handbook7/Handbook7_Breast-4.pdf.
Accessed 15 Mar 2018.
22. Gotzsche PC, Olsen O. Is screening for
breast cancer with mammography justifiable? Lancet 2000;355:129-34. Crossref
23. Tabár L, Dean PB, Cen TH, et al. The
impact of mammography screening on the diagnosis and management of
early-phase breast cancer. In: Francescatti D, Silverstein M, editors.
Breast Cancer: A New Era in Management. Springer New York; 2014: 31-78. Crossref
24. Bock K, Borisch B, Cawson J, et al.
Effect of population-based screening on breast cancer mortality. Lancet
2011;378:1775-6. Crossref
25. Patnick J, Perry N, de Wolf C. Effect
of population-based screening on breast cancer mortality—Authors’ reply.
Lancet 2012;379:1298.
26. Mcneil DG. Confronting cancer:
scientist at work—Peter Gotzsche; A career that bristles with
against-the-grain conclusions. Available from:
http://www.nytimes.com/2002/04/09/science/confronting-cancer-scientist-work-peter-gotzsche-career-that-bristles-with.html. Accessed
19 Nov 2017.
27. Cuzick J. Breast cancer screening—time
to move forward. Lancet 2012;379:1289-90. Crossref
28. Chiolero A, Rodondi N. Lessons from
the Swiss Medical Board recommendation against mammography screening
programs. JAMA Intern Med 2014;174:1541-2. Crossref
29. Biller-Andorno N, Juni P. Abolishing
mammography screening programs? A view from the Swiss Medical Board. N
Engl J Med 2014;370:1965-7. Crossref
30. Helvie MA, Chang JT, Hendrick RE, et
al. Reduction in late-stage breast cancer incidence in the mammography
era: Implications for overdiagnosis of invasive cancer. Cancer
2014;120:2649-56. Crossref
31. Kopans DB, Smith RA, Duffy SW.
Mammographic screening and “overdiagnosis”. Radiology 2011;260:616-20. Crossref
32. Puliti D, Duffy SW, Miccinesi G, et
al. Overdiagnosis in mammographic screening for breast cancer in Europe: a
literature review. J Med Screen 2012;19 Suppl 1:42-56. Crossref
33. Duffy SW, Agbaje O, Tabar L, et al.
Overdiagnosis and overtreatment of breast cancer: estimates of
overdiagnosis from two trials of mammographic screening for breast cancer.
Breast Cancer Res 2005;7:258-65. Crossref
34. Bleyer A, Welch HG. Effect of three
decades of screening mammography on breast-cancer incidence. N Engl J Med
2012;367:1998-2005. Crossref
35. Duffy SW, Dibden A, Michalopoulos D,
et al. Screen detection of ductal carcinoma in situ and subsequent
incidence of invasive interval breast cancers: a retrospective
population-based study. Lancet Oncol 2016;17:109-14. Crossref
36. Tosteson AN, Fryback DG, Hammond CS,
et al. Consequences of false-positive screening mammograms. JAMA Intern
Med 2014;174:954-61. Crossref
37. Schwartz LM, Woloshin S, Fowler FJ,
Jr, et al. Enthusiasm for cancer screening in the United States. JAMA
2004;291:71-8. Crossref
38. Kopans DB. An open letter to panels
that are deciding guidelines for breast cancer screening. Breast Cancer
Res Treat 2015;151:19-25. Crossref
39. Duffy SW, Chen TH, Smith RA, Yen AM,
Tabar L. Real and artificial controversies in breast cancer screening.
Breast Cancer Manage 2013;2:519-28. Crossref
40. Montero AJ, Eapen S, Gorin B, et al.
The economic burden of metastatic breast cancer: a U.S. managed care
perspective. Breast Cancer Res Treat 2012;134:815-22. Crossref
41. Ohuchi N, Ishida T, Kawai M, et al.
Randomized controlled trial on effectiveness of ultrasonography screening
for breast cancer in women aged 40-49 (J-START): research design. Jpn J
Clin Oncol 2011;41:275-7. Crossref
42. Sung H, Rosenberg PS, Chen WQ, et al.
Female breast cancer incidence among Asian and Western populations: more
similar than expected. J Natl Cancer Inst 2015;107. Crossref
43. Fan L, Strasser-Weippl K, Li JJ, et
al. Breast cancer in China. Lancet Oncol 2014;15:e279-89. Crossref
44. Youlden DR, Cramb SM, Yip CH, et al.
Incidence and mortality of female breast cancer in the Asia-Pacific
region. Cancer Biol Med 2014;11:101-15.
45. Singapore Health Promotion Board.
National Registry of Diseases Office. Singapore Cancer Registry Interim
Annual Report: Trends in cancer incidence in Singapore 2010-2014.
Available from:
https://www.nrdo.gov.sg/docs/librariesprovider3/default-document-library/cancer-trends-2010-2014_interim-annual-report_final-%28public%29.pdf?sfvrsn=0.
Accessed 19 Nov 2017.
46. Hospital Authority, Hong Kong. Hong
Kong Cancer Registry. Available from: http://www3.ha.org.hk/cancereg/.
Accessed 19 Mar 2018.
47. Leung GM, Thach TQ, Lam TH, et al.
Trends in breast cancer incidence in Hong Kong between 1973 and 1999: an
age-period-cohort analysis. Br J Cancer 2002;87:982-8. Crossref
48. Breast cancer in Asia—The Challenge
and Response. A Report from the Economist Intelligence Unit. Available
from: https://www.eiuperspectives.economist.com/sites/default/files/EIU
Breast Cancer in Asia_Final.pdf. Accessed 19 Nov 2017.
49. Yen AM, Tsau HS, Fann JC, et al.
Population-based breast cancer screening with risk-based and universal
mammography screening compared with clinical breast examination: a
propensity score analysis of 1 429 890 Taiwanese women. JAMA Oncol
2016;2:915-21. Crossref
50. Song QK, Wang XL, Zhou XN, et al.
Breast cancer challenges and screening in China: lessons from current
registry data and population screening studies. Oncologist 2015;20:773-9.
Crossref
51. A Hong Kong Breast Cancer Foundation
Initiative. Hong Kong Breast Cancer Registry Report No. 8, 2016. Available
from:
http://www.hkbcf.org/download/bcr_report8/hkbcf_report_2016_full_report.pdf.
Accessed 19 Nov 2017.
52. Lui CY, Lam HS, Chan LK, et al.
Opportunistic breast cancer screening in Hong Kong; a revisit of the Kwong
Wah Hospital experience. Hong Kong Med J 2007;13:106-13.
53. Hong Kong College of Radiologists
Mammography Statement. Available from:
https://www.hkcr.org/templates/OS03C00336/case/lop/HKCR
MammographyStatement_rev20150825.pdf. Accessed 19 Nov 2017.
54. Destounis SV, Arieno AL, Morgan RC, et
al. Comparison of breast cancers diagnosed in screening patients in their
40s with and without family history of breast cancer in a community
outpatient facility. AJR Am J Roentgenol 2014;202:928-32. Crossref
55. Rafferty EA, Rose SL, Miller DP, et
al. Effect of age on breast cancer screening using tomosynthesis in
combination with digital mammography. Breast Cancer Res Treat
2017;164:659-66. Crossref
56. Friedewald SM, Rafferty EA, Rose SL,
et al. Breast cancer screening using tomosynthesis in combination with
digital mammography. JAMA 2014;311:2499-507. Crossref
57. Yun SJ, Ryu CW, Rhee SJ, et al.
Benefit of adding digital breast tomosynthesis to digital mammography for
breast cancer screening focused on cancer characteristics: a
meta-analysis. Breast Cancer Res Treat 2017;164:557-69. Crossref
58. Monticciolo DL, Newell MS, Hendrick
RE, et al. Breast cancer screening for average-risk women: recommendations
from the ACR commission on breast imaging. J Am Coll Radiol
2017;14:1137-43. Crossref
59. Expert Panel on Breast Imaging;
Mainiero MB, Moy L, Baron P, et al. ACR Appropriateness Criteria Breast
Cancer Screening. J Am Coll Radiol 2017;14:S383-90. Crossref