Hong Kong Med J 2017 Jun;23(3):231–8 | Epub 10 Mar 2017
DOI: 10.12809/hkmj164942
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Outcomes after oesophageal perforation:
a retrospective cohort study of patients with
different aetiologies
TT Law, FRCSEd, FHKAM (Surgery);
Jonathan YL Chan, MB, BS;
Desmond KK Chan, FRCSEd, FHKAM (Surgery);
Daniel Tong, MS, PhD;
Ian YH Wong, FRCSEd, FHKAM (Surgery);
Fion SY Chan, FRCSEd, FHKAM (Surgery);
Simon Law, MS, FRCSEd
Division of Esophageal and Upper Gastrointestinal Surgery, Department
of Surgery, The University of Hong Kong, Queen Mary Hospital, Pokfulam,
Hong Kong
Corresponding author: Prof Simon Law (slaw@hku.hk)
Abstract
Introduction: The mortality rate after oesophageal
perforation is high despite advances in operative and
non-operative techniques. In this study, we sought
to identify risk factors for hospital mortality after oesophageal perforation treatment.
Methods: We retrospectively examined patients
treated for oesophageal perforation in a university
teaching hospital in Hong Kong between January
1997 and December 2013. Their demographic and
clinical characteristics, aetiology, management
strategies, and outcomes were recorded and
analysed.
Results: We identified a cohort of 43 patients treated
for perforation of the oesophagus (28 men; median
age, 66 years; age range, 30-98 years). Perforation
was spontaneous in 22 (51.2%) patients (15 with
Boerhaave’s syndrome and seven with malignant
perforation), iatrogenic in 15 (34.9%), and provoked
by foreign body ingestion in six (14.0%). Of the
patients, 14 (32.6%) had pre-existing oesophageal
disease. Perforation occurred in the intrathoracic
oesophagus in 30 (69.8%) patients. Emergent surgery
was undertaken in 23 patients: 16 underwent
primary repair, six surgical drainage or exclusion,
and one oesophagectomy. Twenty patients were
managed non-operatively, 13 of whom underwent
stenting. Two stented patients subsequently
required oesophagectomy. Four patients had
clinical signs of leak after primary repair: two
were treated conservatively and two required
oesophagectomy. Overall, six (14.0%) patients
required oesophagectomy, one of whom died. Nine
other patients also died in hospital; the hospital
mortality rate was 23.3%. Pre-existing pulmonary
and hepatic disease, and perforation associated
with malignancy were significantly associated with
hospital mortality (P=0.03, <0.01, and <0.01,
respectively).
Conclusions: Most oesophageal perforations
were spontaneous. Mortality was substantial
despite modern therapies. Presence of pre-existing
pulmonary disease, hepatic disease, and perforation
associated with malignancy were significantly
associated with hospital mortality. Salvage
oesophagectomy was successful in selected patients.
New knowledge added by this study
- We report the outcomes of a cohort of patients with oesophageal perforation managed in a single centre.
- Mortality rate was substantial despite advances in surgery and endoscopic therapy.
- Surgical and non-operative treatment options are available.
- The aetiology, timing of presentation, and patients’ co-morbidities should be considered carefully when managing oesophageal perforation.
- Oesophagectomy may be indicated in selected patients.
Introduction
Oesophageal perforation is uncommon, yet its
management remains a substantial challenge to
surgeons. Diagnosis and treatment are often delayed
due to lack of clinical suspicion and accurate
diagnostic tools. Hence, reported mortality rates
range from 10% to 25%.1 2 3
Oesophageal perforation can occur
spontaneously from forceful vomiting (Boerhaave’s
syndrome), or in pre-existing pathology (such as
oesophageal cancer) or can be associated with
ingestion of a foreign body. Iatrogenic perforation
usually occurs after therapeutic endoscopic
procedures such as dilatation, and is the predominant
cause of perforation reported in many studies.1 2 4 5
Diagnosis and treatment within 24 hours of
perforation are critical if favourable outcomes are to
be achieved.1 6 After diagnosis and the initial phase
of resuscitation, there is a wide range of treatment
options, which are informed by the presentation,
aetiology, location of perforation, and the extent of
mediastinal or intrathoracic contamination. Surgery
remains the mainstay of treatment; the conventional
operative approach is considered to be primary
repair of the perforation site and drainage.7 8 9 Some surgeons advocate primary repair only for those patients presented within 24 hours of perforation,10 while others would try primary repair as the initial treatment regardless of the timing of presentation.9 11
Endoscopic treatment, including stenting, is
becoming an increasingly popular means of treating
oesophageal perforation in selected patients, and
reportedly has a high technical success rate.12 13 14 15 16
Oesophageal perforation should be managed
in specialised centres. In this study, we report the
characteristics, treatment, and outcomes of a cohort
of patients with oesophageal perforation treated at a
single tertiary centre in Hong Kong over a period of
16 years.
Methods
We retrospectively identified patients treated
for perforation of the oesophagus at a university
teaching hospital in Hong Kong between January
1997 and December 2013. Patients’ demographic
characteristics, presentation, investigations,
management, and outcomes were recorded.
Diagnosis of perforation was confirmed
by one or more of the following methods:
oesophagogastroduodenoscopy (OGD), water-soluble
contrast swallow study, and contrast-enhanced
computed tomography imaging of the
neck, thorax, and abdomen. After confirmation of
the diagnosis, patients were resuscitated to address
homoeostatic and haemodynamic disturbances,
followed by definitive treatment. All patients were
kept ‘nil by mouth’, administered parenteral broad-spectrum
antibiotics and proton pump inhibitors,
and chest drain(s) was inserted if clinically indicated.
Patients with significant haemodynamic instability
or respiratory distress requiring intubation and
mechanical ventilation were admitted to the
intensive care unit (ICU) for optimisation before
definitive treatment.
Definitive treatment depended on the location
of the perforation, its aetiology, the extent of
mediastinal and intrathoracic contamination, and
the patient’s physical status. In general, patients
with malignant perforation or perforation contained
within the mediastinal pleura were treated non-operatively.
For the former, self-expanding metallic
stents were inserted under fluoroscopic guidance. In
selected patients with a benign cause of perforation
and limited contamination, a polyester oesophageal
stent (Polyflex; Boston Scientific, Natick [MA],
United States) was placed under fluoroscopic guidance. For
patients in whom the site of perforation could not
be identified, and in the absence of clinical signs
of sepsis, a conservative management strategy was
adopted. This entailed placement of a nasogastric
feeding tube under endoscopic guidance, followed
by enteral feeding for 7 days. Thereafter, a water-soluble
contrast swallow study was undertaken to
confirm the absence of a leak before oral feeding was
resumed.
When a surgical management strategy
was decided, patients with perforation of the
intra-abdominal oesophagus were treated with
laparotomy, primary repair of the perforation,
and feeding jejunostomy. For an intrathoracic
perforation with significant contamination of the
pleural cavity, thoracotomy and primary repair was
the preferred approach. A left-sided thoracotomy
was the usual approach for Boerhaave’s perforation
of the distal thoracic oesophagus. Necrotic tissue
was debrided, the edges of the perforation were
trimmed, and the defect was closed with fine sutures
in two layers. The mucosal edges of the perforation
were approximated using interrupted absorbable
sutures, and the muscular defect was approximated
using interrupted monofilament absorbable sutures.
Lung decortication was performed. One drain was
placed in close proximity to the repair, generally
accompanied by one basal and one apical large-bore
chest drain. Feeding jejunostomy was performed in
selected patients. Postoperatively patients remained
nil by mouth, and were given nutritional support and
intravenous antibiotics. A contrast swallow study
was generally performed 7 to 10 days postoperatively;
oral intake was commenced if there was no evidence
of leak. The choice of antibiotics and duration of
treatment were guided by microbiology culture
findings.
In selected patients who presented late, and
in those who developed a persistent leak after
primary repair, oesophageal exclusion (cervical
oesophagostomy and jejunostomy) followed by
second-stage oesophagectomy might be considered.
In the first stage, the oesophagus was excluded
proximally in the neck with an oesophagostomy, and
the abdominal oesophagus was stapled. A drain was
placed from the neck into the oesophageal stump for
decompression. Oesophagectomy was performed
once sepsis had subsided. A gastric tube was used
for reconstruction via the retrosternal route, and
cervical oesophagogastrostomy was performed.
The principles outlined in the Declaration of
Helsinki have been followed.
Statistical analysis
Continuous data were represented as the median
(range), unless otherwise stated. Fisher’s exact test
was used to compare categorical variables and the
Mann-Whitney U test for continuous variables.
We undertook univariate analysis to identify
factors associated with hospital mortality. P<0.05
was considered statistically significant. Data were
analysed using SPSS 20.0 (IBM Corp, Armonk [NY],
United States).
Results
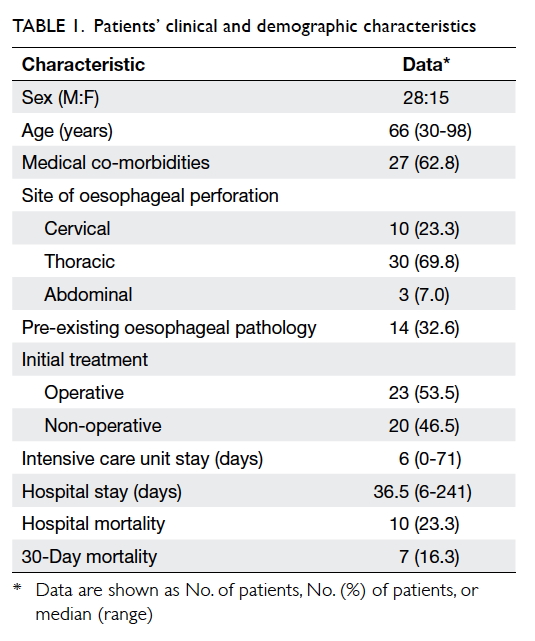
During the study period, 43 patients with
oesophageal perforation were identified. Patients’
demographic and clinical characteristics are
summarised in Table 1. The median age of the cohort was 66 years (range, 30-98 years); 28 (65.1%)
were men. Medical co-morbidities were present in
27 (62.8%) patients, and pre-existing oesophageal
pathologies were present in 14 (32.6%; of whom half
had oesophageal cancer). Spontaneous perforation
occurred in 22 (51.2%) patients: 15 occurred as a
result of Boerhaave’s syndrome and seven as a result
of malignant perforation. Fifteen (34.9%) patients
had an iatrogenic perforation: 13 occurred after
an endoscopic procedure (three after endoscopic
retrograde cholangiopancreatography and 10 after
OGD), one occurred after attempted endotracheal
intubation, and one occurred during thyroidectomy.
Of the 10 OGDs, eight had been therapeutic. Six
(14.0%) perforations were associated with ingestion
of a foreign body.
Chest pain and vomiting were the most
common presenting symptoms in patients with
spontaneous perforation, occurring in 13 and 10
patients, respectively. Surgical emphysema and
dysphagia were the least common presenting signs
and symptoms; both were only present in two
patients. Over half the patients presented and were
diagnosed within 24 hours of symptom onset. Of
the cohort of 43 patients, 29 (67.4%) underwent two
out of the three diagnostic imaging modalities. The
presenting symptoms and investigations of patients
with spontaneous perforation are shown in Table 2.
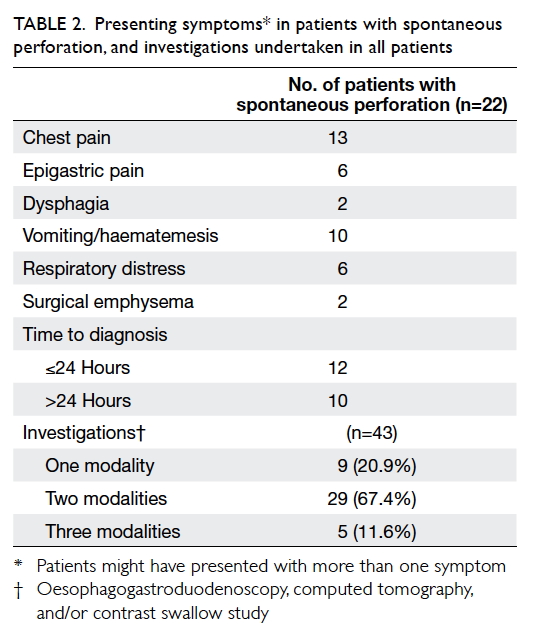
Table 2. Presenting symptoms in patients with spontaneous perforation, and investigations undertaken in all patients
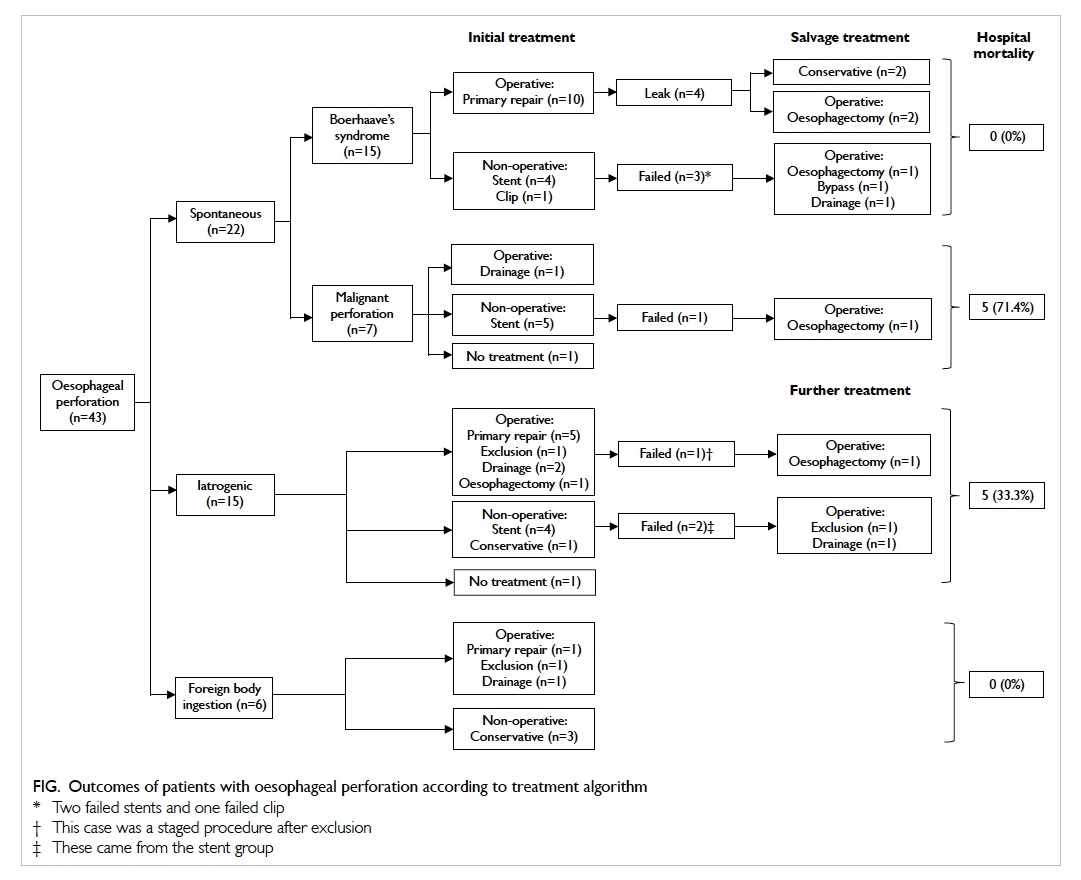
The management and outcomes of patients
are shown in the Figure. Of the 15 patients with Boerhaave’s perforation, 10 underwent primary
repair: four repairs were complicated by a leak and
two patients subsequently required oesophagectomy.
The remaining five patients were initially treated
non-operatively: four underwent endoscopic stent
placement and one endoscopic clipping of the
perforation. Three patients required subsequent
operations: one underwent oesophagectomy,
one bypass operation, and one surgical drainage.
There were no deaths in the group of patients with
Boerhaave’s syndrome.
Seven patients had malignant perforation:
five were treated with endoscopic placement of
a metallic stent. All but one of these procedures
were successful; the patient in whom stenting failed
underwent oesophagectomy. Five (71.4%) of the
seven patients with malignant perforation died
during their hospital stay.
There were 15 iatrogenic perforations (Fig).
Nine of these patients underwent early operative
treatment: five underwent primary repair, one
exclusion, two drainage, and one oesophagectomy.
There were no leaks in those who underwent primary
repair. Six patients were initially treated non-operatively,
four with stents, one with a feeding tube,
and one was judged to be unfit for treatment. Two
of the six patients initially treated non-operatively
ultimately required surgery, one underwent
exclusion, and the other surgical drainage. Five of the
15 patients with iatrogenic perforations died during
their hospital stay, with a mortality rate of 33.3%.
Six oesophageal perforations were associated
with foreign body ingestion (Fig). Three patients were treated non-operatively; of the remainder, one
underwent primary repair, one exclusion, and one
surgical drainage. None of these patients died during
hospitalisation.
Overall, 16 of the 43 patients underwent
primary repairs in the initial treatment, and four
(25%) developed clinical signs of leak subsequently.
All were from Boerhaave’s perforation. Two
required oesophagectomy while two were managed
conservatively.
Overall, six of the 43 patients underwent
oesophagectomy, generally as a salvage treatment
due to failure of other treatment modalities. Three
patients with Boerhaave’s syndrome required
oesophagectomy, two with persistent leak after
primary repair and one with a persistent leak after
stenting. All had presented >24 hours from symptom
onset. One patient with a perforated oesophageal
cancer developed a leak after stenting and required
oesophagectomy. Two patients with iatrogenic
perforation in the presence of caustic strictures
underwent oesophagectomy. Only one patient who
underwent oesophagectomy died in hospital.
Overall, 10 patients died in hospital, with a
mortality rate of 23.3%. The 30-day mortality rate
was 16.3%. The median length of hospital stay was
36.5 days (range, 6-241 days), and median ICU stay
was 6 days (range, 0-71 days).
All 10 patients who died had pre-existing
oesophageal disease; five had cancer of the
oesophagus, one caustic stricture, and four had
oesophageal varices secondary to hepatic cirrhosis.
Malignant perforation had a substantially higher
mortality rate of 71.4%. The median survival for
patients with perforated oesophageal cancer was
28.5 days (range, 13-848 days).
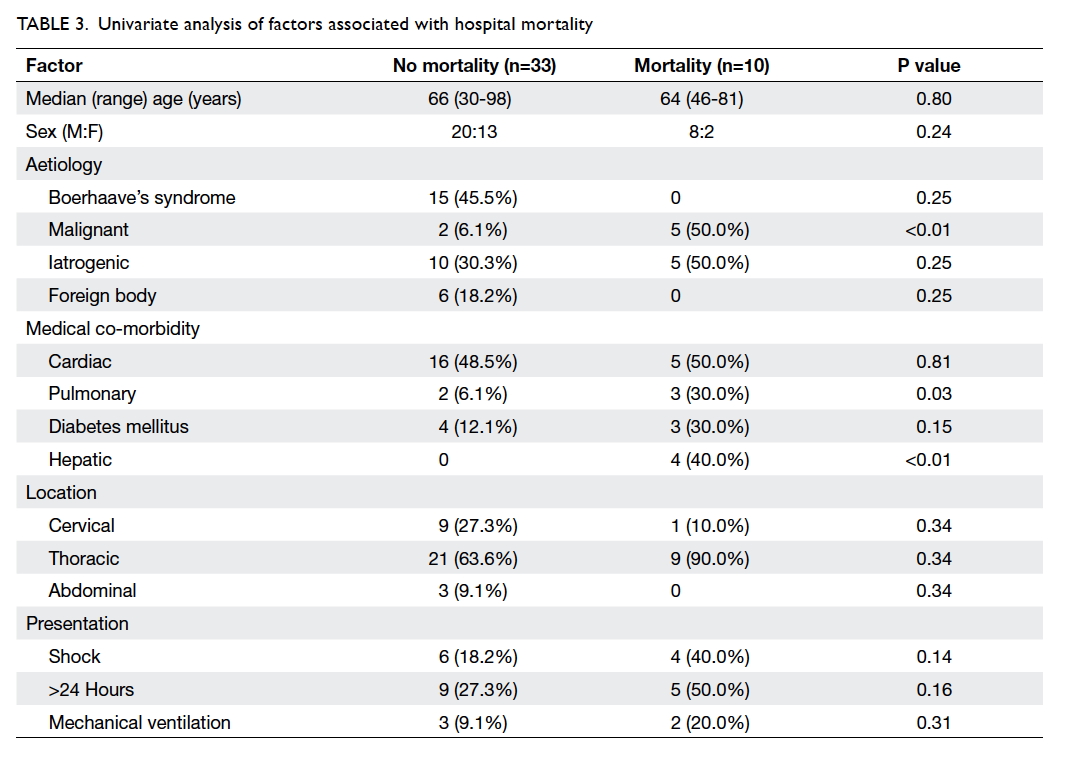
The results of univariate analysis of factors
potentially associated with hospital mortality are
shown in Table 3. The presence of pulmonary disease, hepatic disease (liver cirrhosis), and
malignant perforation were significantly associated
with hospital mortality (P=0.03, <0.01, and <0.01,
respectively), but the site of perforation and timing
of presentation were not.
Discussion
Oesophageal perforation may be difficult to diagnose.
Patients can present with a wide variety of symptoms,
which can be non-specific. It is not uncommon
for the diagnosis to be missed in the acute phase.
Computed tomography imaging (preferably with
oral contrast) should be undertaken when the index
of clinical suspicion is high, because it allows the site
of mediastinal or intra-abdominal collections to be
identified and rules out other pathologies. Of note,
OGD performed by an experienced endoscopist
using minimal insufflation is an effective means
of detecting the site and size of perforation, and is
reported to have a sensitivity and specificity of 100%
and 83% for intrathoracic perforation, respectively.17
A positive OGD therefore has a substantial influence
on clinical decision making.
Spontaneous perforation was the most
common aetiology in our cohort; around one
third was associated with underlying cancer of the
oesophagus. Squamous cell carcinoma remains
the most common malignant cell type globally,
despite the rising incidence of adenocarcinoma
in the western population. Patients often present
at an advanced stage. Of those patients with
malignant perforations, all but one had a squamous
cell carcinoma of the intrathoracic oesophagus.
Perforation either occurs spontaneously or results
from concurrent chemoradiotherapy. Ohtsu et al18 reported a perforation rate of 13.9% (five out
of 36 patients) in cases of T4-stage cancer of the
oesophagus with concurrent chemoradiotherapy.
In our cohort, perforation occurred shortly after
completion of radiotherapy in one patient.
The prognosis for patients with perforated
oesophageal cancer is poor. The disease is often
inoperable and in these circumstances treatment
is palliative.19 20 Non-operative treatment, such as
insertion of a metallic covered stent, is the usual
practice at our centre. Stenting of the intrathoracic
portion of the oesophagus is technically
straightforward and is successful in most cases.
Sealing of the perforation site can be confirmed by
a subsequent contrast study, and oral intake can be
resumed in the absence of a leak. Nevertheless, the
prognosis of this group of patients is poor despite
the successful placement of a stent, and the hospital
mortality rate remains high. Patients most often
succumb as a consequence of sepsis caused by the
perforation.
Many treatment options are available for non-malignant
perforation, and the treatment strategy
should be tailored to the individual. Factors to be
considered include the site of perforation, extent of
contamination, pre-existing oesophageal disease,
and patient co-morbidities. Operative treatment
is favoured for perforation of the intra-abdominal
oesophagus or perforation that involves the
oesophagogastric junction (OGJ). These patients
often present with abdominal pain and peritonitis.
Laparotomy, primary repair of the perforation,
and fashioning of a feeding jejunostomy allow
alimentation in the event of persistent leak. The
placement of an oesophageal stent that crosses the
OGJ has a higher chance of migration and is not
recommended.
The intrathoracic oesophagus is the most
common site of perforation. Of the three most
common causes (Boerhaave’s syndrome, iatrogenic
perforation, and foreign body ingestion), Boerhaave’s
syndrome is the most challenging. Traditionally,
Boerhaave’s syndrome is associated with a
mortality rate of up to 30%.11 Patients may present
late, the site of perforation is usually at the distal
thoracic oesophagus, and there may be extensive
contamination due to the high pressure generated
by vomiting. Contamination with food particles
is common. Operative treatment with primary
closure of the perforation and drainage is favoured
by many7 8 9; this is also our preferred approach. Many
surgeons advocate primary repair irrespective of
the timing of presentation.9 11 21 22 Leak rates after
primary repair range from 17% to 32%.9 11 21 22 23 24 Minor
leaks can be managed conservatively with drainage,
while further surgery (usually exclusion) is required
for larger leaks and in the presence of sepsis. Lin et al23 reported that the incidence of postoperative leak was 37.5% in patients in whom treatment was
delayed for more than 48 hours, compared with 0%
in those who were treated more promptly. Wright et al22 reported that three out of the four leaks in their patient cohort were repaired more than 24 hours
after perforation. The incidence of leak after primary
repair was 25.0% in our study, which is comparable
to other reports in the literature. Of the four leaks,
two patients required reoperation and ultimately
oesophagectomy; both had presented more than 24
hours after symptom onset.
Endoscopic stenting for benign perforation
has been reported in several small case series.
Freeman et al13 14 have reported the outcomes of
stent placement in patients with iatrogenic and
spontaneous perforation. They proposed a hybrid
approach, namely a combination of endoscopic
and minimally invasive surgical techniques to drain
intrathoracic and/or intra-abdominal collections.
The main advantage of this strategy is the avoidance
of thoracotomy and/or laparotomy. The incidence of
stent migration was reported to be approximately
20% in their cohort of patients with spontaneous
oesophageal perforation.14 Relative contra-indications
to stent insertion include a perforation
that crosses the OGJ and circumferential necrosis
of the oesophagus. In our experience, operative
treatment is recommended for the treatment of
Boerhaave’s syndrome unless the patient is unfit
for surgery or declines surgical treatment. Five
patients in our series initially treated with stenting
subsequently required surgery, of whom four had
benign perforations (two with Boerhaave’s syndrome
and two with iatrogenic perforations). One patient in
our cohort with oesophageal dissection complicated
by perforation underwent stenting in another
hospital before transferring to our centre; this
patient developed a persistent leak after stenting.
In that case, the placement of the stent appeared
to have aggravated the leak, and oesophagectomy
was eventually required.25 In our opinion, stent
placement in benign perforation is only suitable for
selected patients who present early and have minimal
contamination. However, stenting may allow more
time for optimisation of a patient’s condition if they
are initially judged not to be fit for surgery.
Oesophagectomy as a treatment for
perforations was first reported in the 1950s.26 Single-stage
oesophageal resection and reconstruction
was first reported by Hendren and Henderson in
1968.27 Altorjay et al28 reported a hospital mortality rate of 3.7% in a series of patients undergoing
oesophagectomy for intrathoracic perforation; in
this series iatrogenic perforation represented 55.6%
of all perforations. Some surgeons have opined that
oesophagectomy may be superior to primary repair in
the presence of pre-existing oesophageal disease and
of extensive perforation with substantial sepsis, while
the general condition of the patient should always
be taken into account.28 29 There is no consensus
about the optimum surgical approach and timing of
reconstruction after oesophagectomy. We advocate
primary repair as the initial treatment irrespective
of the timing of presentation, and oesophagectomy
is considered a salvage treatment. In our experience,
patients with persistent leak after primary repair
and sepsis should undergo oesophageal exclusion
to control sepsis before oesophagectomy is
contemplated. Oesophagectomy with primary
reconstruction can be performed safely after patient
optimisation. Oesophagectomy was undertaken in
six patients in our cohort; three of these patients had
pre-existing oesophageal disease. All patients had
a cervical oesophagogastric anastomosis fashioned
via the retrosternal route. A cervical anastomosis
distant from the infected mediastinum appears to
be a safe option.29 Thoracotomy is the most common
surgical approach, but Yeo et al30 reported using
transhiatal oesophagectomy to treat perforated
oesophageal cancer in four patients. Thoracotomy
is avoided in the transhiatal approach, but this
technique can only be considered in perforations of
the distal oesophagus and in the presence of minimal
mediastinal contamination.
Oesophageal perforation after foreign body
ingestion in adults is more common in China as a
result of its dietary culture. The foreign body is
usually a fish, chicken, or pork bone. An impacted
foreign body can usually be retrieved endoscopically;
however, oesophageal perforation can occur if there
is deep penetration of the foreign body or extensive
manipulation during retrieval. The site of perforation
is usually the cervical oesophagus, followed by the
intrathoracic oesophagus. In severe cases, operative
management is indicated; the approach is dependent
on the site of perforation, and the site and size of
any collection. The aim of management is to drain
any collection, remove any residual foreign body,
repair the perforated site, and protect the airway. In
the absence of sepsis and imaging appearances of a
peri-oesophageal collection, conservative treatment
may be warranted. Operative drainage may be
necessary if there is a sizeable collection and if there
is sepsis. Mediastinitis and sepsis are more likely
after intrathoracic perforation, and would dictate
treatment strategy.
It is essential to identify factors associated
with mortality after oesophageal perforation so
as to improve treatment and outcomes. Early
diagnosis and management (in the ‘golden 24
hours’) are reportedly associated with superior
outcomes.1 6 Malignant perforation, sepsis, the
need for mechanical ventilation on presentation,
and pulmonary co-morbidity are reported to have
a significant impact on overall survival.5 In our
cohort, pulmonary co-morbidity, hepatic disease,
and malignant perforation were associated with risk
of death. A recent meta-analysis of 75 studies that
included 2971 patients reported a pooled mortality
rate of 11.9% (95% confidence interval, 9.7%-14.3%).3
Of the different aetiologies, spontaneous perforation
had the highest mortality rate of 14.8%.3
Oesophageal perforation remains a difficult
condition to treat despite advances in surgery,
endoscopic treatment, and ICU care. The mortality
rate is still substantial with modern therapies. The
presence of pre-existing pulmonary disease, hepatic
disease, and perforation associated with malignancy
was significantly associated with hospital mortality
in our cohort. Oesophagectomy for salvage had a
reasonable success rate in selected patients.
Declaration
The authors have disclosed no conflicts of interest.
References
1. Vallböhmer D, Hölscher AH, Hölscher M, et al. Options in
the management of esophageal perforation: analysis over a
12-year period. Dis Esophagus 2010;23:185-90. Crossref
2. Søreide JA, Konradsson A, Sandvik OM, Øvrebø K, Viste
A. Esophageal perforation: clinical patterns and outcomes
from a patient cohort of Western Norway. Dig Surg
2012;29:494-502. Crossref
3. Biancari F, D’Andrea V, Paone R, et al. Current treatment
and outcome of esophageal perforations in adults:
systematic review and meta-analysis of 75 studies. World
J Surg 2013;37:1051-9. Crossref
4. Abbas G, Schuchert MJ, Pettiford BL, et al.
Contemporaneous management of esophageal perforation.
Surgery 2009;146:749-55. Crossref
5. Bhatia P, Fortin D, Inculet RI, Malthaner RA. Current
concepts in the management of esophageal perforations: a
twenty-seven year Canadian experience. Ann Thorac Surg
2011;92:209-15. CrossRef
6. Shaker H, Elsayed H, Whittle I, Hussein S, Shackcloth M.
The influence of the ‘golden 24-h rule’ on the prognosis
of oesophageal perforation in the modern era. Eur J
Cardiothorac Surg 2010;38:216-22. Crossref
7. Brinster CJ, Singhal S, Lee L, Marshall MB, Kaiser LR,
Kucharczuk JC. Evolving options in the management of
esophageal perforation. Ann Thorac Surg 2004;77:1475-83. Crossref
8. Eroglu A, Can Kürkcüogu I, Karaoganogu N, Tekinbaş C,
Yimaz O, Başog M. Esophageal perforation: the importance
of early diagnosis and primary repair. Dis Esophagus
2004;17:91-4. Crossref
9. Jougon J, Mc Bride T, Delcambre F, Minniti A, Velly JF.
Primary esophageal repair for Boerhaave’s syndrome
whatever the free interval between perforation and
treatment. Eur J Cardiothorac Surg 2004;25:475-9. Crossref
10. Flynn AE, Verrier ED, Way LW, Thomas AN, Pellegrini CA.
Esophageal perforation. Arch Surg 1989;124:1211-4. Crossref
11. Lawrence DR, Ohri SK, Moxon RE, Townsend ER,
Fountain SW. Primary esophageal repair for Boerhaave’s
syndrome. Ann Thorac Surg 1999;67:818-20. Crossref
12. Fischer A, Thomusch O, Benz S, von Dobschuetz E, Baier P,
Hopt UT. Nonoperative treatment of 15 benign esophageal
perforations with self-expandable covered metal stents.
Ann Thorac Surg 2006;81:467-72. Crossref
13. Freeman RK, Van Woerkom JM, Ascioti AJ. Esophageal
stent placement for the treatment of iatrogenic
intrathoracic esophageal perforation. Ann Thorac Surg
2007;83:2003-7. Crossref
14. Freeman RK, Van Woerkom JM, Vyverberg A, Ascioti
AJ. Esophageal stent placement for the treatment of
spontaneous esophageal perforations. Ann Thorac Surg
2009;88:194-8. Crossref
15. Kiernan PD, Khandhar SJ, Fortes DL, Sheridan MJ,
Hetrick V. Thoracic esophageal perforations. Am Surg
2010;76:1355-62.
16. Dasari BV, Neely D, Kennedy A, et al. The role of esophageal
stents in the management of esophageal anastomotic
leaks and benign esophageal perforations. Ann Surg
2014;259:852-60. Crossref
17. Horwitz B, Krevsky B, Buckman RF Jr, Fisher RS, Dabezies
MA. Endoscopic evaluation of penetrating esophageal
injuries. Am J Gastroenterol 1993;88:1249-53.
18. Ohtsu A, Boku N, Muro K, et al. Definitive
chemoradiotherapy for T4 and/or M1 lymph node
squamous cell carcinoma of the esophagus. J Clin Oncol
1999;17:2915-21.
19. Di Franco F, Lam PJ, Karat D, Hayes N, Griffin SM.
Iatrogenic perforation of localized oesophageal cancer. Br J
Surg 2008;95:837-9. Crossref
20. Jethwa P, Lala A, Powell J, McConkey CC, Gillison EW,
Spychal RT. A regional audit of iatrogenic perforation of
tumours of the oesophagus and cardia. Aliment Pharmacol
Ther 2005;21:479-84. Crossref
21. Whyte RI, Iannettoni MD, Orringer MB. Intrathoracic
esophageal perforation. The merit of primary repair. J
Thorac Cardiovasc Surg 1995;109:140-4. Crossref
22. Wright CD, Mathisen DJ, Wain JC, Moncure AC,
Hilgenberg AD, Grillo HC. Reinforced primary repair
of thoracic esophageal perforation. Ann Thorac Surg
1995;60:245-8. Crossref
23. Lin Y, Jiang G, Liu L, et al. Management of thoracic
esophageal perforation. World J Surg 2014;38:1093-9. Crossref
24. Richardson JD. Management of esophageal perforations:
the value of aggressive surgical treatment. Am J Surg
2005;190:161-5. Crossref
25. Zhu RY, Law TT, Tong D, Tam G, Law S. Spontaneous
circumferential intramural esophageal dissection
complicated with esophageal perforation and esophageal-pleural
fistula: a case report and literature review. Dis
Esophagus 2016;29:872-9. Crossref
26. Johnson J, Schwegman CW, MacVaugh H III. Early
esophagogastrectomy in the treatment of iatrogenic
perforation of the distal esophagostomy. J Thorac
Cardiovasc Surg 1956;32:827-31.
27. Hendren WH, Henderson BM. Immediate esophagectomy
for instrumental perforation of the thoracic esophagus.
Ann Surg 1968;168:997-1003. Crossref
28. Altorjay A, Kiss J, Vörös A, Szirányi E. The role of
esophagectomy in the management of esophageal
perforation. Ann Thorac Surg 1998;65:1433-6. Crossref
29. Orringer MB, Stirling MC. Esophagectomy for esophageal
disruption. Ann Thorac Surg 1990;49:35-42. Crossref
30. Yeo CJ, Killemoe KD, Klein AS, Zinner MJ. Treatment
of instrumental perforation of esophageal malignancy by
transhiatal esophagectomy. Arch Surg 1988;123:1016-8. Crossref