DOI: 10.12809/hkmj154524
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Neurocysticercosis: diagnostic dilemma
Joyce HM Cheng, MB, ChB; Eric MW Man, MB, ChB, FRCR; SY Luk, MB, BS, FRCR; Wendy WC Wong, MB, BS, FRCR
Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong
Corresponding author: Dr Joyce HM Cheng (chm915@ha.org.hk)
Case report
A 7-year-old Nepalese boy with unremarkable past
health was admitted in July 2013 with a 2-week
history of headache, dizziness, and vomiting that
subsided spontaneously. On admission, he was
afebrile with unremarkable neurological and fundal
examination.
He had been living in Nepal until the age of
5 years and then immigrated to Hong Kong. He
recently travelled to Nepal but had otherwise no
contact history with pigs or febrile persons.
Blood tests were unremarkable except mildly
elevated erythrocyte sedimentation rate of 26 mm/h.
Lumbar puncture yielded normal cerebrospinal fluid
(CSF) white cell count, and protein and glucose level.
Culture of blood and CSF was negative.
Initial contrast computed tomographic
brain showed two subcentimetre (7 mm and 9
mm) left parieto-occipital adjacent rim-enhancing
lesions (Fig a) with T1-weighted hypointense and T2-weighted/fluid-attenuated
inversion recovery hyperintense signal on contrast
magnetic resonance imaging (MRI) [Fig b]. Significant perifocal oedema and mass effect
with compression on the left occipital horn was
noted. Magnetic resonance spectroscopy showed a
significant lipid-lactate peak (Fig c), decreased N-acetylaspartate,
and no elevated choline. No amino acid peak was identified. Perfusion imaging with
arterial spine labelling showed no elevated regional
cerebral blood flow (Fig d). Contrast MRI of the spine showed no leptomeningeal enhancement,
intradural nor extradural spinal mass lesions.
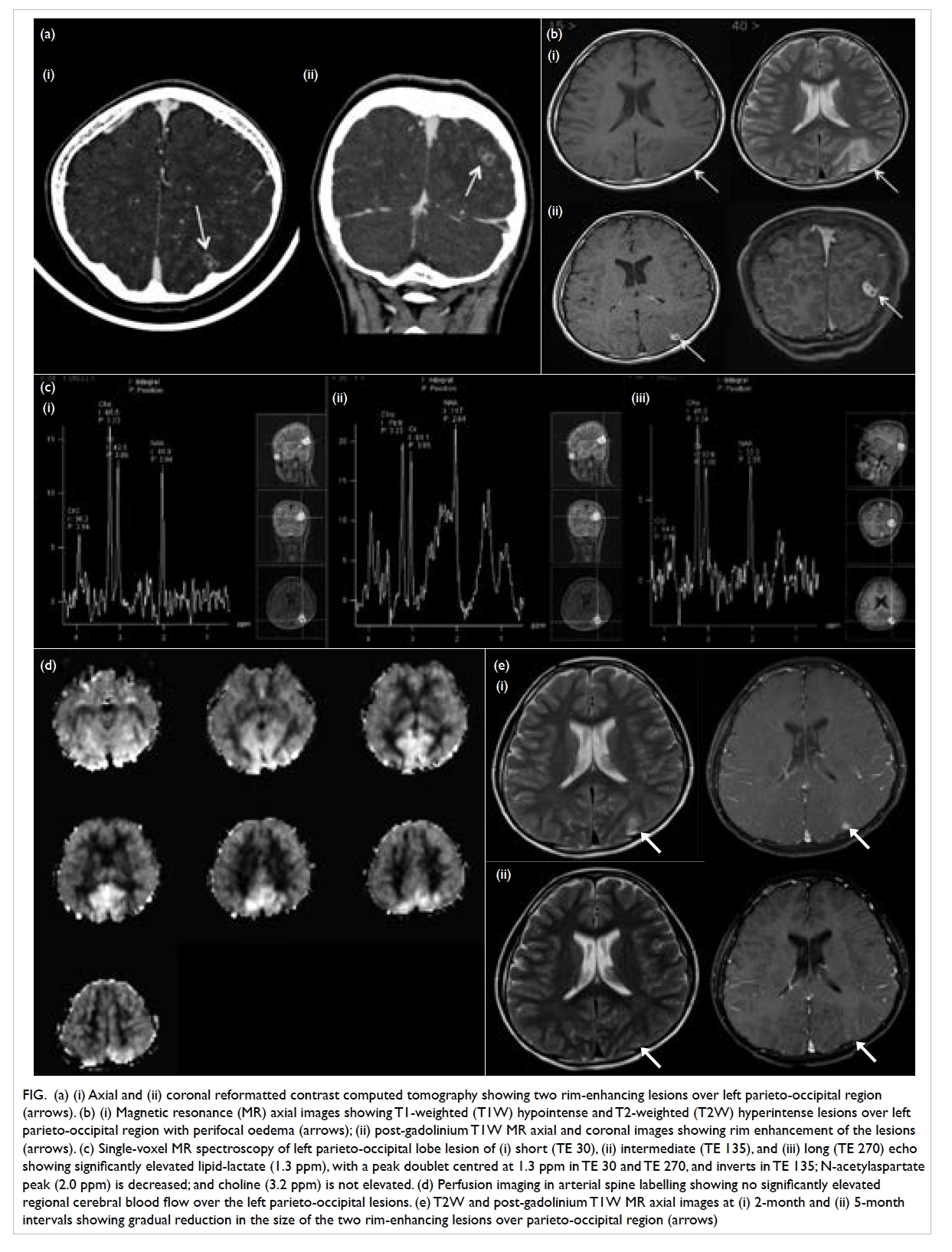
Figure. (a) (i) Axial and (ii) coronal reformatted contrast computed tomography showing two rim-enhancing lesions over left parieto-occipital region (arrows). (b) (i) Magnetic resonance (MR) axial images showing T1-weighted (T1W) hypointense and T2-weighted (T2W) hyperintense lesions over left parieto-occipital region with perifocal oedema (arrows); (ii) post-gadolinium T1W MR axial and coronal images showing rim enhancement of the lesions (arrows). (c) Single-voxel MR spectroscopy of left parieto-occipital lobe lesion of (i) short (TE 30), (ii) intermediate (TE 135), and (iii) long (TE 270) echo showing significantly elevated lipid-lactate (1.3 ppm), with a peak doublet centred at 1.3 ppm in TE 30 and TE 270, and inverts in TE 135; N-acetylaspartate peak (2.0 ppm) is decreased; and choline (3.2 ppm) is not elevated. (d) Perfusion imaging in arterial spine labelling showing no significantly elevated regional cerebral blood flow over the left parieto-occipital lesions. (e) T2W and post-gadolinium T1W MR axial images at (i) 2-month and (ii) 5-month intervals showing gradual reduction in the size of the two rim-enhancing lesions over parieto-occipital region (arrows)
Subsequent extensive investigations of blood
(including anti-Taenia solium immunoglobulin G),
CSF, urine and stool for tuberculosis and T solium
were performed, and all results were negative.
Overall review of the clinical presentation,
travel history, laboratory and imaging findings,
and the two cerebral rim-enhancing lesions were
suggestive of an infective process, in particular,
neurocysticercosis or tuberculosis.
After thorough investigations and
interdepartmental discussions, it was decided to treat
the patient as a probable case of neurocysticercosis
and a course of empirical albendazole and
prednisolone was prescribed.
The patient remained asymptomatic after
treatment. Follow-up MRI performed after 2 and 5
months (Fig e) showed reduction in size of the two
cerebral rim-enhancing lesions (3 mm and 1 mm), as
well as the extent of perifocal oedema.
Discussion
Neurocysticercosis is a parasitic infection of
the central nervous system by T solium (ie pork
tapeworm) usually through accidental ingestion of
contaminated food containing its eggs. It has been a
diagnostic challenge in developed areas owing to its
infrequent occurrence, low sensitivity of serological
tests, and highly variable and non-specific
manifestations, both clinically and radiologically. It
is further complicated by clinical and radiological
features that overlap considerably with tuberculosis
infection, particularly in tuberculosis-endemic areas.
A few international diagnostic criteria for
neurocysticercosis have been proposed, and aim
to stratify the diagnostic strength based on the
interpretation of clinical, radiological, serological,
and epidemiological information. One of the most
widely accepted revised criteria was proposed by
Del Brutto et al in 2001,1 and classifies patients into
two degrees of diagnostic certainty—definitive or
probable. Definitive diagnosis requires the presence
of one absolute criterion or two major plus one minor
epidemiological criteria. Probable diagnosis can be
made if there are one major plus two minor criteria;
one major plus one minor and one epidemiological
criteria; or presence of three minor plus one
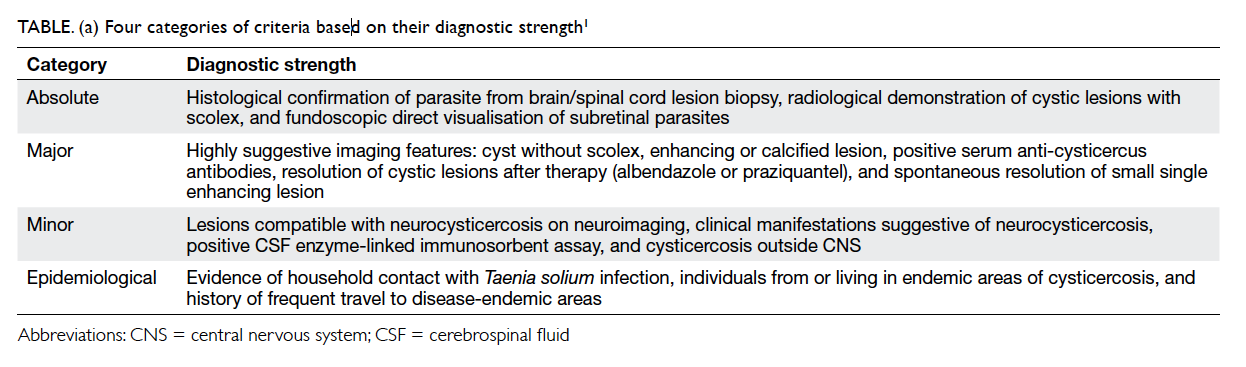
epidemiological criteria. Four categories of criteria
based on their diagnostic strength are shown in
Table a.1
The diagnostic dilemma in our index case
was the lack of characteristic imaging features and
positive serological proof, hence difficult differentiation
from tuberculosis infection, i.e. tuberculomas.
Other common differential diagnoses of cystic
lesions include pyogenic abscesses and metastases.
Both were unlikely in our patient given the aseptic
clinical presentation, normal blood and CSF profiles,
absence of a known primary and lack of associated
radiological findings (eg cerebritis, ventriculitis or
meningeal enhancement). Biopsy or excision of the
cerebral cystic lesions might offer a straightforward
histopathological diagnosis, however, it is an invasive
investigation and imposes potential risks.
Attempts have been made in the literature
to distinguish cysticercosis and tuberculosis
neurological infection by combined interpretation of
the clinical, radiological, and serological features.2 3 4
Clinically, tuberculomas are more often
associated with increased intracranial pressure and
focal neurological deficits, whereas patients with
neurocysticercosis are usually less symptomatic or
present with seizures.2
Radiologically, the imaging findings of
neurocysticercosis are variable, depending on the
stage and location of infestation. A diagnostic dilemma
lies in those who present with intracranial cystic
lesions without presence of scolex, and that may be
present in both neurocysticercosis and tuberculosis.
Table b shows some MRI imaging features that may
help distinguish the two diagnoses.2 3 4 5
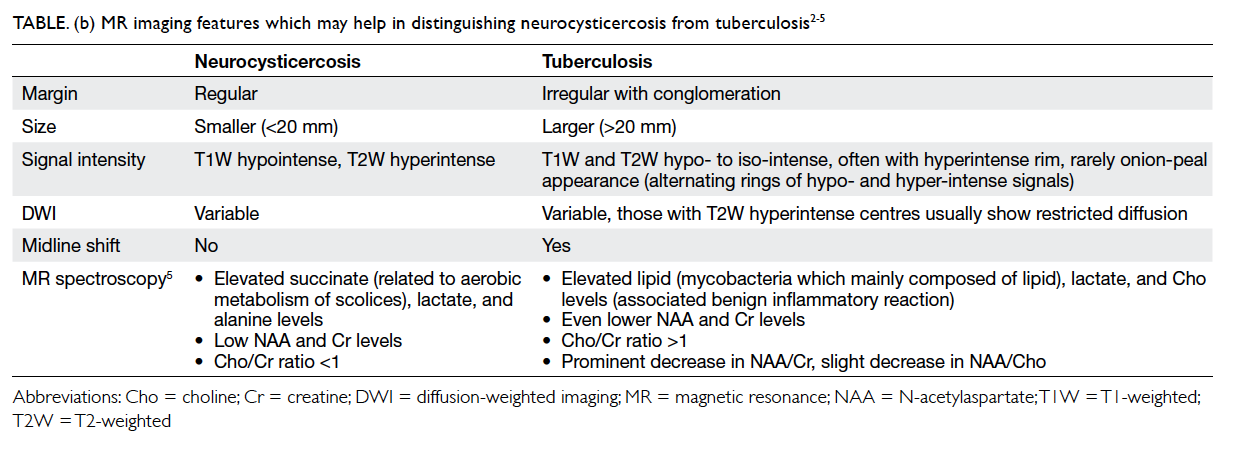
Table (b). MR imaging features which may help in distinguishing neurocysticercosis from tuberculosis2 3 4 5
Based on the neuroimaging features and the
revised diagnostic criteria, a probable diagnosis of
neurocysticercosis was made: presence of lesions
compatible with neurocysticercosis that resolved
after treatment with albendazole, and a history of
travelling to an endemic area.
Neurocysticercosis is an uncommon parasitic
infection of the central nervous system in Hong
Kong and requires a high degree of clinical suspicion
for diagnosis. Despite the advances in neuroimaging,
accurate diagnosis is still sometimes difficult, which
is related to the pleomorphic disease nature and
significant overlapping features with tuberculosis. A
combination of proper interpretation of diagnostic
criteria and imaging findings is helpful in making the
diagnosis without invasive and potentially harmful
investigations.
References
1. Del Brutto OH, Rajshekhar V, White AC Jr, et al. Proposed
diagnostic criteria for neurocysticercosis. Neurology
2001;57:177-83. Crossref
2. Rajshekhar V, Haran RP, Prakash GS, Chandy MJ.
Differentiating solitary small cysticercus granulomas
and tuberculomas in patients with epilepsy. Clinical
and computerized tomographic criteria. J Neurosurg
1993;78:402-7. Crossref
3. Garg RK, Kar AM, Kumar T. Neurocysticercosis like
presentation in a case of CNS tuberculosis. Neurol India
2000;48:260-2.
4. Garg RK. Diagnosis of intracranial tuberculoma. Indian J
Tuberc 1996;43:35-9.
5. Pretell EJ, Martinot C Jr, Garcia HH, Alvarado M, Bustos
JA, Martinot C; Cysticercosis Working Group in Peru.
Differential diagnosis between cerebral tuberculosis and
neurocysticercosis by magnetic resonance spectroscopy. J
Comput Assist Tomogr 2005;29:112-4. Crossref