Hong Kong Med J 2016 Aug;22(4):399.e1–3
DOI: 10.12809/hkmj164815
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
PICTORIAL MEDICINE
Neurocysticercosis in a young Indian male
Eugene PL Ng, MB, ChB;
Peter YM Woo, MB, BS, FHKAM (Surgery);
Alain KS Wong, MB, ChB, FHKAM (Surgery);
KY Chan, MB, ChB, FHKAM (Surgery)
Department of Neurosurgery, Kwong Wah Hospital, Yaumatei, Hong Kong
Corresponding author: Dr Eugene PL Ng (npl566@ha.org.hk)
A 33-year-old Indian male was hospitalised for
a 3-day history of headache and left lower limb
weakness in December 2014. He had experienced no
fever or seizures. He had visited New Delhi, India,
the year before. Physical examination revealed the
patient to be fully conscious with left lower limb
monoparesis. There was no sensory deficit. Computed
tomography (CT) revealed a superior parietal intra-axial
lesion with a calcified focus (Fig 1a). Magnetic resonance imaging (MRI) delineated a 2 x 1.5 x 1.5
cm circumscribed hypointense cystic lesion with a
contrast-enhancing wall and an eccentric intracystic
signal with perilesional oedema (Figs 1b to 1d). Differential diagnoses included neurocysticercosis,
brain abscess, brain metastasis, and malignant
glioma. Craniotomy for excision was performed
in view of the possibility of malignancy and the
surgically accessible superficial location of the lesion.
Intra-operatively, a firm capsular mass containing
thick opaque material was seen (Fig 2a). Histology revealed a cysticercus within a fibrous capsule,
compatible with the diagnosis of neurocysticercosis
(Figs 2b and 2c). A 2-week course of oral albendazole was commenced and the patient was discharged with
full recovery.
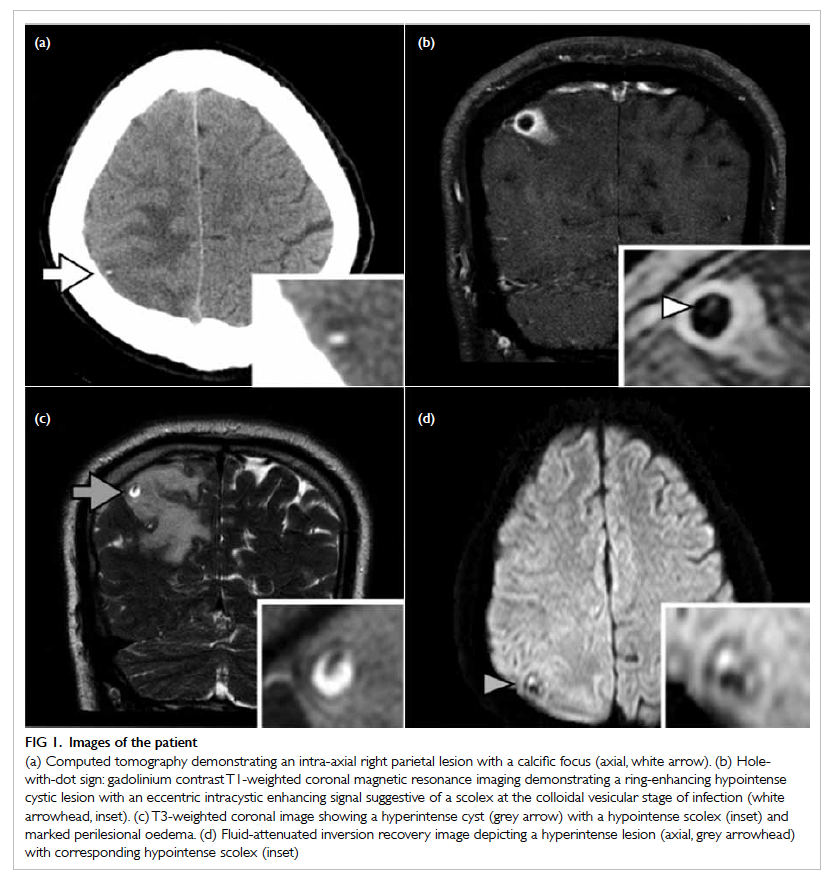
Figure 1. Images of the patient
(a) Computed tomography demonstrating an intra-axial right parietal lesion with a calcific focus (axial, white arrow). (b) Hole-with-dot sign: gadolinium contrast T1-weighted coronal magnetic resonance imaging demonstrating a ring-enhancing hypointense cystic lesion with an eccentric intracystic enhancing signal suggestive of a scolex at the colloidal vesicular stage of infection (white arrowhead, inset). (c) T3-weighted coronal image showing a hyperintense cyst (grey arrow) with a hypointense scolex (inset) and marked perilesional oedema. (d) Fluid-attenuated inversion recovery image depicting a hyperintense lesion (axial, grey arrowhead) with corresponding hypointense scolex (inset)
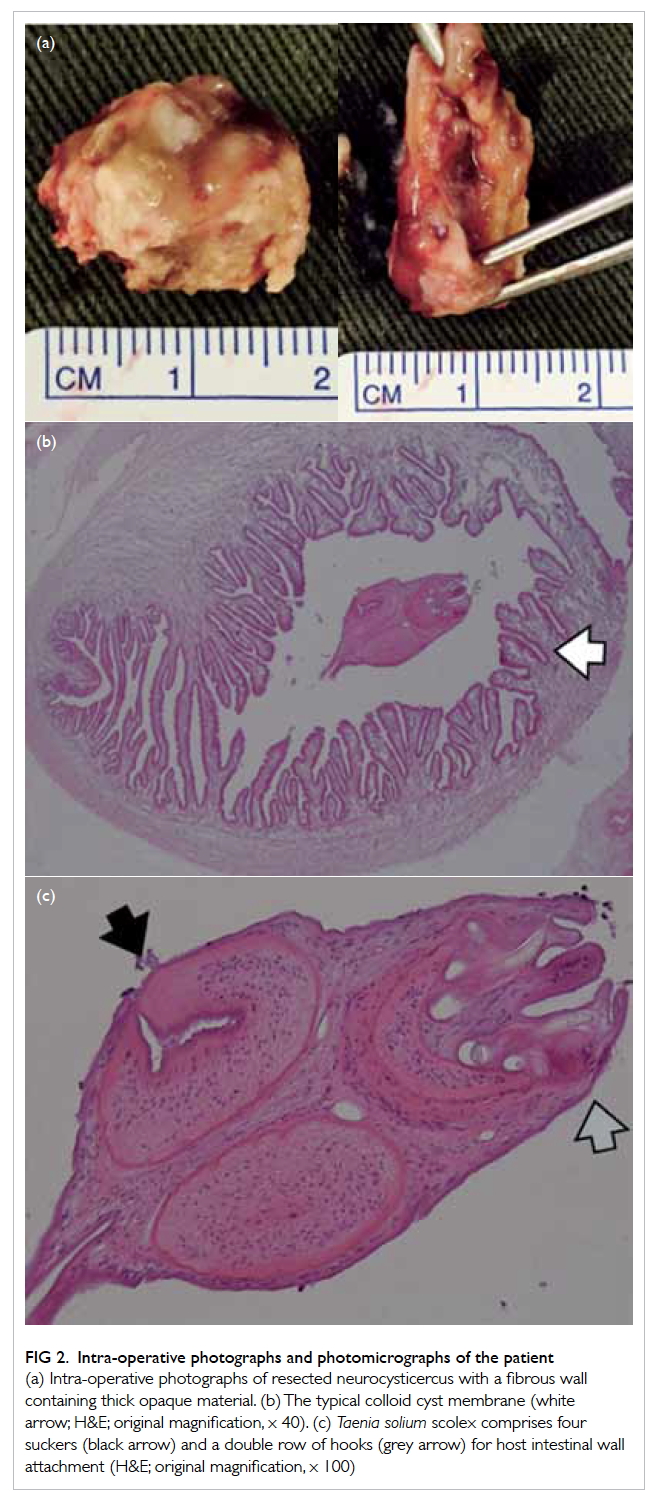
Figure 2. Intra-operative photographs and photomicrographs of the patient
(a) Intra-operative photographs of resected neurocysticercus with a fibrous wall containing thick opaque material. (b) The typical colloid cyst membrane (white arrow; H&E; original magnification, x 40). (c) Taenia solium scolex comprises four suckers (black arrow) and a double row of hooks (grey arrow) for host intestinal wall attachment (H&E; original magnification, x 100)
Neurocysticercosis is the most common
parasitic infection of the central nervous system
(CNS) caused by the larval form of Taenia solium.
The peak age of incidence is between 25 and 35
years1 and the condition is endemic to the Indian
subcontinent, coastal North Africa, sub-Saharan
Africa, Latin America, and China. The main mode of
transmission is by faecal-oral ingestion of tapeworm
embryos.1 2 Consumption of contaminated poorly
cooked pork is a less-frequent alternative source of
infection since pigs are intermediate hosts.2 3 Within
72 hours of ingestion, larvae known as oncospheres
are released and pass through the intestinal wall into
the circulation, subsequently depositing in the CNS,
retina, and skeletal muscle as cysticerci.1 2 The parasite can remain viable in the brain for several years after
which it undergoes calcific degeneration.3 In endemic
areas, the most common presentation is epilepsy,
responsible for 30% of cases.1 Focal neurological
deficits may occur including cranial nerve palsy
due to basal meningitis. Obstructive hydrocephalus
develops when lesions occupy the fourth ventricle.1 2
Characteristic radiological features include
dystrophic calcification on CT imaging, cyst wall
contrast enhancement on T1-weighted MRI and
identification of the pathognomonic scolex, an
eccentric focus of enhancement representing
the tapeworm’s head, best delineated with fluid-attenuated
inversion recovery sequences (hole-with-dot sign).3 4 Brain abscess or metastases are important differential diagnoses as they are
also similarly located at the grey-white matter
junction of the middle cerebral artery distribution,
associated with disproportionately significant
perilesional cerebral oedema and classically exhibit
heterogeneous contrast enhancement. Malignant
glioma was less likely in our patient since they are
morphologically more infiltrative, and the present
lesion was well-circumscribed. For this patient,
the major distinguishing feature that supported a
diagnosis of neurocysticercosis was the presence of
dystrophic calcification on CT and, in retrospect,
the presence of a scolex on MRI. Absolute criteria
for a definitive diagnosis are either histological
parasitic proof, imaging demonstration of a scolex,
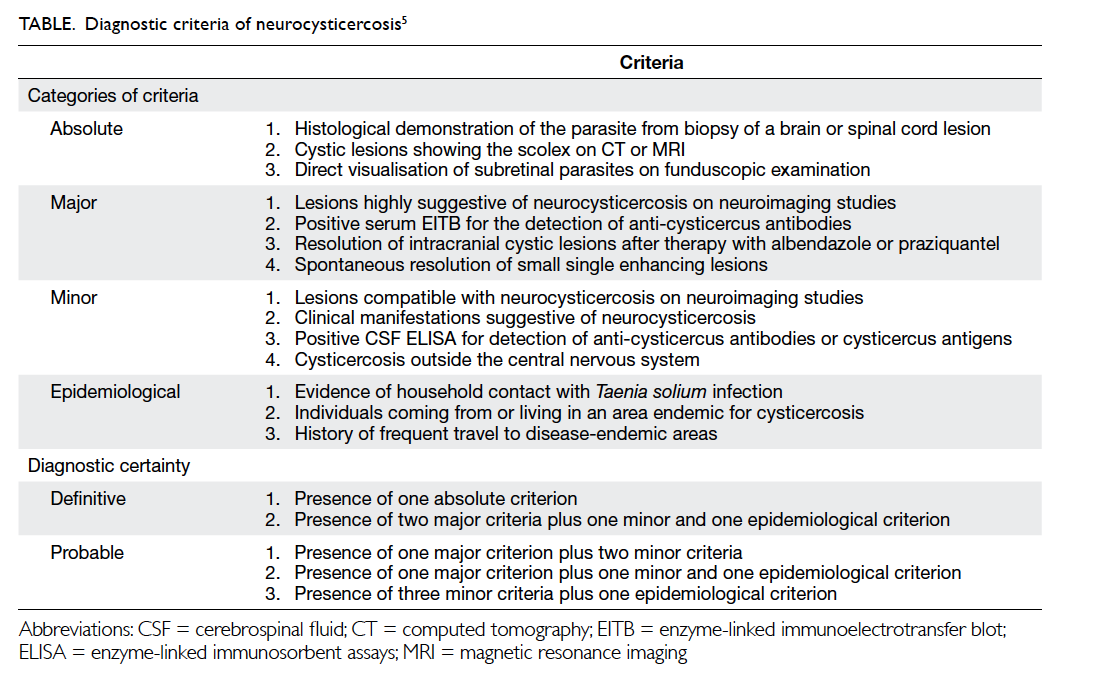
or subretinal parasites on fundoscopy (Table5).
Serological enzyme-linked immunoelectrotransfer
blot detection of anti-cysticercus antibodies or
cerebrospinal fluid enzyme-linked immunosorbent
assays are adjunctive investigations.1 6 Management of
active neurocysticercosis includes antiepileptic drug
administration, anti-inflammatory glucocorticoid
therapy, and definitive antiparasitic therapy with
albendazole (15 mg/kg per day) or praziquantel
(50 mg/kg per day) for 2 to 4 weeks.1 Surgical
excision is reserved for cysts that cause mass effect,
hydrocephalus, or if the diagnosis is unclear.2
In an era of increasing migration and
international travel, patients from developing
countries who present with seizures, raised
intracranial pressure symptoms, or focal
neurological deficit should be suspected of having
neurocysticercosis when characteristic imaging
findings are identified. In probable cases, a trial of
antiparasitic therapy is recommended with serial
scans arranged to monitor treatment response.
References
1. Garcia HH, Nash TE, Del Brutto OH. Clinical symptoms,
diagnosis, and treatment of neurocysticercosis. Lancet
Neurol 2014;13:1202-15. Crossref
2. Zymberg ST. Neurocysticercosis. World Neurosurg
2013;79(2 Suppl):S24.e5-8.
3. Dhesi B, Karia SJ, Adab N, Nair S. Imaging in
neurocysticercosis. Pract Neurol 2015;15:135-7. Crossref
4. Lerner A, Shiroishi MS, Zee CS, Law M, Go JL. Imaging
of neurocysticercosis. Neuroimaging Clin N Am
2012;22:659-76. Crossref
5. Del Brutto OH, Rajshekhar V, White AC Jr, et al. Proposed
diagnostic criteria for neurocysticercosis. Neurology
2001;57:177-83. Crossref
6. Gekeler F, Eichenlaub S, Mendoza EG, Sotelo J, Hoelscher
M, Löscher T. Sensitivity and specificity of ELISA and
immunoblot for diagnosing neurocysticercosis. Eur J Clin
Microbiol Infect Dis 2002;21:227-9. Crossref