Hong Kong Med J 2016 Aug;22(4):365–71 | Epub 17 Jun 2016
DOI: 10.12809/hkmj154781
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Chronic peritoneal dialysis in Chinese infants and children younger than two years
YH Chan, FHKCPaed, FHKAM (Paediatrics);
Alison LT Ma, FHKCPaed, FHKAM (Paediatrics);
PC Tong, FHKCPaed, FHKAM (Paediatrics);
WM Lai, FHKCPaed, FHKAM (Paediatrics);
Niko KC Tse, FHKCPaed, FHKAM (Paediatrics)
Paediatric Nephrology Centre, Department of Paediatric and Adolescent
Medicine, Princess Margaret Hospital, Laichikok, Hong Kong
Corresponding author: Dr YH Chan (genegene.chan@gmail.com)
Abstract
Objective: To review the outcome for Chinese
infants and young children on chronic peritoneal
dialysis.
Methods: The Paediatric Nephrology Centre of
Princess Margaret Hospital is the designated site
offering chronic dialysis to children in Hong Kong.
Medical records of children who started chronic
peritoneal dialysis before the age of 2 years, from 1
July 1995 to 31 December 2013, were retrieved and
retrospectively reviewed.
Results: Nine Chinese patients (male-to-female ratio, 3:6)
were identified. They were commenced on automated
peritoneal dialysis at a median age of 4.7 (interquartile
range, 1.1-13.3) months. The median duration of
chronic peritoneal dialysis was 40.9 (interquartile
range, 22.9-76.2) months. The underlying aetiologies
were renal dysplasia (n=3), pneumococcal-associated
haemolytic uraemic syndrome (n=3), ischaemic
nephropathy (n=2), and primary hyperoxaluria I
(n=1). Peritonitis and exit-site infection rate was
1 episode per 46.5 patient-months and 1 episode
per 28.6 patient-months, respectively. Dialysis
adequacy (Kt/Vurea >1.8) was achieved in 87.5% of
patients. Weight gain was achieved in our patients
although three required gastrostomy. Four patients
were delayed in development. All patients survived
except one patient with primary hyperoxaluria I who
died of acute portal vein thrombosis following liver
transplantation. One patient with pneumococcal-associated
haemolytic uraemic syndrome had
sufficient renal function to be weaned off dialysis.
Four patients received deceased donor renal transplantation
after a mean waiting time of 76.7 months. Three
patients remained on chronic peritoneal dialysis at
the end of the study.
Conclusions: Chronic peritoneal dialysis is technically
difficult in infants. Nonetheless, low peritonitis rate,
low exit-site infection rate, and no chronic peritoneal
dialysis–related mortality can be achieved. Chronic
peritoneal dialysis offers a promising strategy to
bridge the way to renal transplantation.
New knowledge added by this study
- Literature on infant chronic peritoneal dialysis (CPD) is scarce. This is the first report about long-term outcome of Chinese infants on CPD.
- The local catheter-related infection rate is low compared with western countries.
- CPD in infancy is a feasible modality as a bridge to transplantation with low infection and mortality rate. A shared decision-making process between parents and paediatric nephrologists is necessary to provide an optimal care plan for this group of patients, considering the predicted outcome, associated co-morbidities, and family burden.
Introduction
End-stage renal disease (ESRD) is a rare disease with
high mortality in infants and young children under 2
years of age. In the past, the decision to initiate infant
dialysis was not easy due to technical difficulties
and poor clinical outcome, as evidenced by a 1990
survey showing that only 50% of paediatric
nephrologists would offer dialysis to ESRD children
younger than 1 year, and only 40% would offer
dialysis to neonates.1 With technological advances
and improving outcome for children on dialysis in
terms of physical growth, development and quality
of life,2 3 most paediatric nephrologists will now consider peritoneal dialysis (PD) as a bridge to renal
transplantation. Data in North American Pediatric
Renal Trials and Collaborative Studies (NAPRTCS)
2011 indicated that 92% of ESRD children younger
than 2 years were on chronic PD (CPD).4
Literature in this area is scarce2 3 5 6 especially
on the long-term outcome of these infants in the
Chinese population. As the only tertiary referral
paediatric nephrology centre in Hong Kong, we
retrospectively reviewed our experience in the
epidemiology, dialysis prescription, complications,
and outcome in this group of patients.
Methods
The Paediatric Nephrology Centre of Princess
Margaret Hospital is the designated site offering
renal replacement therapy to children in Hong Kong.
Medical records of children who started CPD before
2 years old, from 1 July 1995 to 31 December 2013,
were retrieved and reviewed. Information regarding
their primary renal diagnosis, co-morbidities, growth
profile, infectious and non-infectious complications,
dialysis prescription, dialysis adequacy, peritoneal
membrane transport status, relevant laboratory
investigations, and final outcome were reviewed.
Data collected were recorded on data entry forms.
Patients who underwent CPD for less than 6 months
were excluded. The study was approved by the ethics
committee of Princess Margaret Hospital.
In our centre, CPD was the preferred dialysis
modality in young children; PD was performed
by automated cycler in the modes of nocturnal
intermittent peritoneal dialysis (NIPD), continuous
cyclic peritoneal dialysis (CCPD), continuous optimal
peritoneal dialysis, and tidal peritoneal dialysis.
Peritoneal equilibration test was performed annually
with membrane transport status classified as high,
high-average, low-average, or low transporters.7 8 Dialysis adequacy was monitored by both clinical
parameters and biochemical parameters. Due to
limited information on residual renal function,
solute clearance referred to contribution by CPD
only, and was expressed in terms of Kt/Vurea.
Peritonitis was defined as cloudy peritoneal
effluent, with white cell count of >100/mm3 in the
dialysate with at least 50% polymorphonuclear
leukocytes.9 Additionally, clinical symptoms of fever
with or without abdominal pain were included. Exit-site
infection (ESI) was diagnosed in the presence
of peri-catheter swelling, redness, tenderness, and
discharge at the exit site.9 Developmental delay was
defined as children who received special education
or failed to reach a normal developmental milestone
in two or more developmental domains (eg gross
motor, cognition, etc).
Chronic kidney disease–mineral bone disease
(CKD-MBD) was defined as a systemic disorder
of mineral bone metabolism due to renal failure,
manifesting as biochemical abnormalities (calcium,
phosphate, parathyroid hormone [PTH], or vitamin
D metabolism), abnormal bone turnover, or vascular
calcification.10 Renal osteodystrophy, the skeletal component
of CKD-MBD, was defined as alteration of
bone morphology in patients with ESRD.10 Target of
PTH ranged from 11 to 33 pmol/L (100-300 pg/mL)
in children on CPD, supported by recent data from
the International Pediatric Peritoneal Dialysis
Network (IPDN).11
Statistical analysis
Data collection and analysis were performed with
Microsoft Excel 2010. The demographic data and
biochemical parameters were expressed as mean ±
standard deviation, range, median, interquartile
range (IQR), number, or percentage as appropriate.
Height and weight were expressed as standard
deviation scores (SDSs), calculated according to a
local study on growth of children.12
Results
Patient characteristics
From 1995 to 2013, nine Chinese children under 2
years of age (3 boys and 6 girls) receiving CPD
were identified. The mean estimated glomerular
filtration rate immediately prior to dialysis was 6.9
± 3.8 (range, 3.9-15) mL/min/1.73 m2, calculated by
Schwartz Formula. The median age at initiating CPD
was 4.7 (IQR, 1.1-13.3) months. The median duration
of CPD was 40.9 (IQR, 22.9-76.2) months. The most
common causes of ESRD were renal dysplasia (n=3,
33%) and pneumococcal-associated haemolytic
uraemic syndrome (pHUS) [n=3, 33%], followed
by ischaemic nephropathy due to severe perinatal
asphyxia (n=2, 22%) and primary hyperoxaluria
I (PH1) [n=1, 11%] (Tables 1 and 2). All three patients with pHUS presented with pneumococcal
pneumonia, microangiopathic haemolytic anaemia,
and acute kidney injury. Either direct Coombs test or
T-antigen test was positive to support the diagnosis
of pHUS.
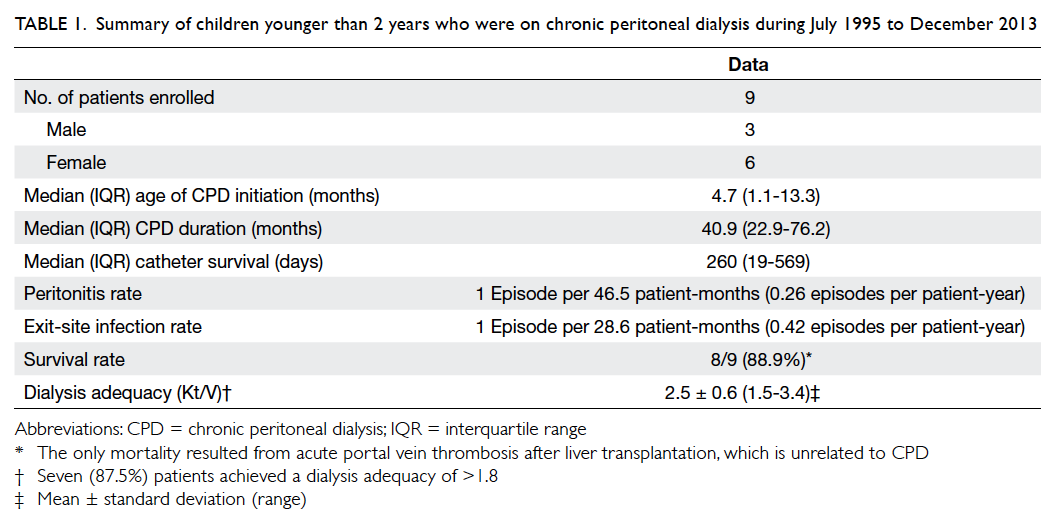
Table 1. Summary of children younger than 2 years who were on chronic peritoneal dialysis during July 1995 to December 2013
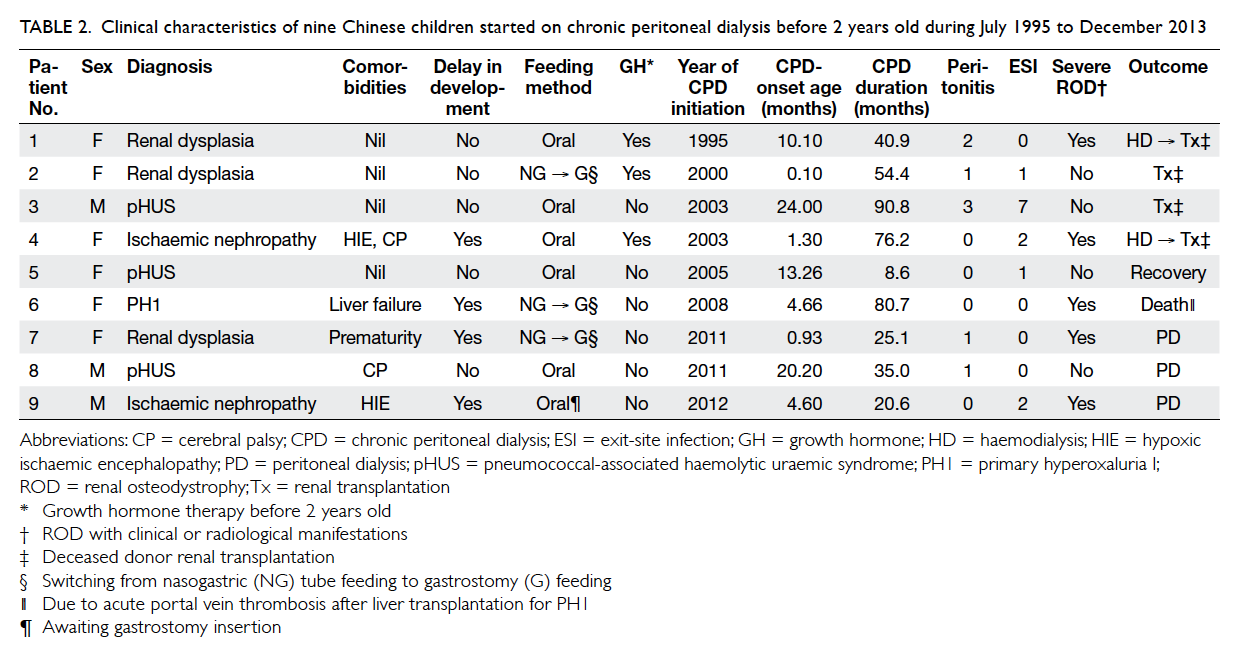
Table 2. Clinical characteristics of nine Chinese children started on chronic peritoneal dialysis before 2 years old during July 1995 to December 2013
Peritoneal dialysis prescription, transporter status, and peritoneal dialysis adequacy
All patients were put on automated peritoneal
dialysis (APD). Initially, eight children were on NIPD
and only one was on CCPD. Over the course of CPD,
five (56%) patients changed to CCPD, and one (11%)
patient changed to tidal PD because of drainage pain.
Three (33%) patients remained on NIPD. Decreasing
residual renal function and inadequate dialysis were
the most common reasons for changing modes of
CPD.
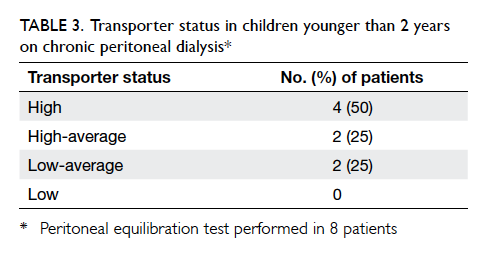
Peritoneal equilibration test and dialysis
adequacy assessment were performed in eight
patients. Four patients were high transporters, while
two patients were high-average transporters and two
patients were low-average transporters (Table 3). Seven (87.5%) patients achieved a dialysis adequacy
(Kt/Vurea) of >1.8. The mean Kt/Vurea was 2.5 ± 0.6
(range, 1.5-3.4). The mean weekly creatinine clearance
was 38.3 ± 6.2 (range, 25.6-47.1) L/week/1.73 m2.
Catheter survival
During the study period, 23 episodes of Tenckhoff
catheter insertion were carried out in these nine
patients. The median catheter survival was 260 (IQR,
19-569) days. Only one patient did not require any
catheter change. Fourteen catheter changes were
performed in eight patients. Catheters were replaced
once in four patients, twice in three patients, and
4 times in one patient. The most common reason
for catheter change was catheter blockage due to
omental wrap (n=7, 50%), followed by chronic ESI
or refractory peritonitis (n=4, 29%), migration
or malposition (n=2, 14%), and cuff extrusion
(n=1, 7%). While omentectomy was not routinely
performed, 44% patients eventually required partial
omentectomy due to omental wrap.
Peritonitis, exit-site infection, and surgical complications
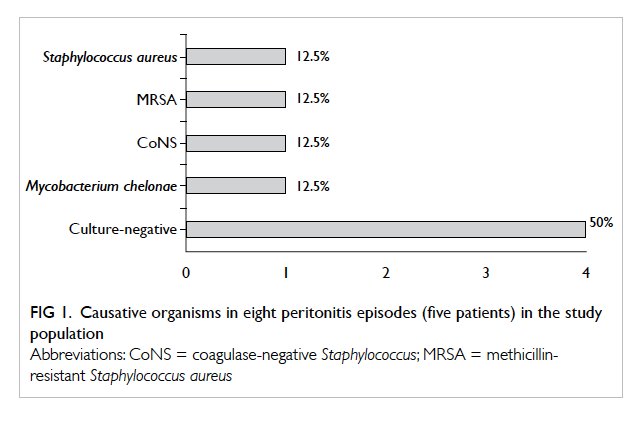
Five patients experienced a total of eight episodes
of peritonitis. Four patients did not have peritonitis.
Peritonitis rate was 0.26 episode per patient-year
or 1 episode per 46.5 patient-months. Two
(25%) episodes were caused by Staphylococcus
aureus, one of which was methicillin-resistant.
One (12.5%) episode was caused by coagulase-negative
Staphylococcus (CoNS) and the other by
Mycobacterium chelonae (12.5%). The remaining
four (50%) episodes were culture-negative peritonitis
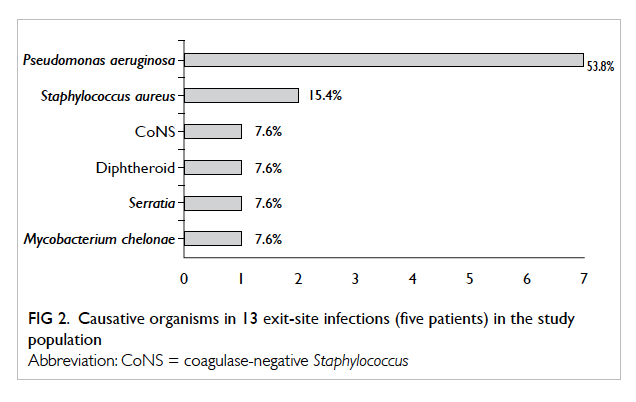
(Fig 1). Altogether 13 episodes of ESI occurred in five patients, and patient 3 contributed seven episodes.
The rate of ESI was 0.42 episode per patient-year
or 1 episode per 28.6 patient-months. The most
common organisms were Pseudomonas aeruginosa
(n=7, 54%) and methicillin-sensitive S aureus (n=2,
15%). Other causative pathogens included CoNS,
diphtheroid, Serratia, and M chelonae, each of which
resulted in one ESI (Fig 2).
One patient required surgical correction of
patent processus vaginalis that led to hydrocoele.
One patient required repair of bilateral inguinal
hernia. One patient had cuff extrusion and required
replacement of PD catheter. No catheters developed
leakage.
Growth and nutrition
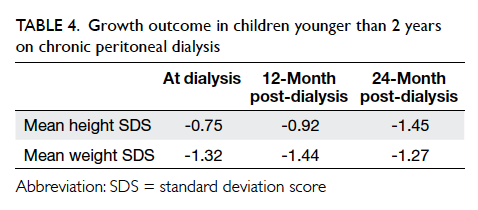
Weight gain was observed after initiation of CPD.
At the start of dialysis, 12 months and 24 months
post-dialysis, the mean weight SDS (wtSDS) was
-1.32, -1.44, and -1.27, while height SDS (htSDS) was
-0.75, -0.92, and -1.45, respectively (Table 4). Three (33%) patients were commenced on nasogastric
(NG) enteral feeding and eventually were switched
to gastrostomy feeding. Six (67%) patients were fed
on demand, one of whom was awaiting gastrostomy
insertion at the end of the study. Three (33%) patients
were prescribed growth hormone therapy before the
age of 2 years.
Development
Four (44%) children were delayed in development or
received special education. Two of them had severe
perinatal asphyxia associated with hypoxic ischaemic
encephalopathy, one was born prematurely at 32
weeks of gestation and the other had PH1, all of
which could account for the developmental delay.
Anaemia, chronic kidney disease–mineral bone disease, and hypertension
During the first 2 years of CPD, all patients received
erythropoiesis-stimulating agent, except patient 5
who later became dialysis-free. The mean maximum
dose of recombinant human erythropoietin (rHuEPO)
was 169 ± 91 (range, 65-300) units/kg/week. Three
patients received rHuEPO at a dose exceeding 200
units/kg/week. Seven patients were put on oral
iron supplements; one of whom was switched to
intravenous iron replacement subsequently due to
functional iron deficiency. The mean haemoglobin
level was 109 ± 8 g/L; only two patients (patients 1
and 7) failed to achieve a mean haemoglobin level of
≥100 g/L.
All patients showed some degree of CKD-MBD,
as evidenced by raised PTH level and the
need for activated vitamin D and phosphate binder.
Five patients had severe renal osteodystrophy
with clinical or radiological manifestations (Table 2). All of them had markedly elevated mean
PTH (90-111 pmol/L) outside the recommended
target. Of note, two patients (patients 6 and 7)
had pathological fractures. Two patients (patients
7 and 9) received a calcimimetic (cinacalcet) for
tertiary hyperparathyroidism. Five (56%) patients
had hypertension and were on antihypertensive
medications with satisfactory control.
Outcome
All patients survived except patient 6 with PH1
who died of acute portal vein thrombosis following
liver transplantation at the age of 5 years. Patient 5
with pHUS became dialysis-free after 8.6 months
of CPD. Four patients underwent deceased donor
renal transplantation (DDRT) with a mean waiting
time of 76.7 (range, 54-90) months, of whom two
were switched to chronic haemodialysis before
transplantation because of inadequate dialysis. Three
patients remained on PD at the end of the study.
Discussion
End-stage renal disease is rare in infants and young
children. The reported incidence is variable but
remains low around the globe. Up to 16 cases per
age-related population per year have been reported
in the UK.13 In NAPRTCS 2011, 13.2% of children
on dialysis were under 2 years old.4 In Hong Kong,
recent data from the Hong Kong Renal Registry
showed that the incidence and prevalence of ESRD
in those <20 years old was around 5 and 28 per
million children, respectively.14
The most common aetiology of ESRD in
this age-group is congenital anomalies of the
kidney and urinary tract, including renal dysplasia
and obstructive uropathy.15 Nonetheless, pHUS
constituted an important cause of ESRD in Hong
Kong. A potential explanation is the late introduction
of a universal pneumococcal vaccination programme
in 2009, compared with 2000 in the US population.
In our study, all patients started with CPD.
Difficult vascular access for haemodialysis and a
high volume of daily milk intake make CPD the
more favourable choice of renal replacement therapy
in young infants. While local mean DDRT waiting
time in children younger than 18 years was 4.4 ±
2.4 years,16 the waiting time in our young patients
was much longer (mean, 6.4 years). This is because
patients have to weigh more than 15 kg before DDRT
can be carried out due to technical difficulties.
Therefore, CPD acts as a bridge to transplantation
and reserves vascular access for future use.15
Ethical considerations and infection, together
with growth and nutrition, are the most challenging
aspects of infant CPD.
Ethical considerations
Decisions to initiate or withhold dialysis remain
one of the most challenging aspects in infant
ESRD. Recent data, which showed improvement
in mortality and developmental outcome, support
initiation of dialysis. Shroff et al6 reported a survival
rate of 77% at 5 years in children commenced
on chronic dialysis before the age of 1 year. Our
unpublished data revealed 91 patients were put on
APD from 1996 to 2013. The overall survival rate was
90%. In this series, survival rate in young infants was
similar and there was no CPD-related mortality. The
only mortality resulted from surgical complications
after liver transplantation.
Warady et al17 reported the 79% infants who
started CPD had normal developmental scores at
1 and 4 years old and 94% of school-aged children
attended school. In our series, 44% of patients
were delayed in development, all of which could
be accounted for by co-morbidities or underlying
aetiology of ESRD.
Nonetheless, unpredictable outcome,
psychosocial burdens, and cost continually fuel
the ethical dilemma.13 15 18 The family burden is
tremendous. Since CPD is a home-based treatment,
caregivers must perform dialysis daily. Up to 55%
of paediatric nephrologists felt a parental decision
to refuse dialysis should be respected for neonates
and 26% for children of 1 to 12 months old.13 In
two surveys, serious co-existing co-morbidities and
predicted morbidity were the most important factors
when a physician considered withholding dialysis.1 19 While serious non-renal co-morbidities such as
pulmonary hypoplasia are strongly associated with a
poor prognosis,20 patients with isolated renal disease
should be considered separately as their prognosis
is generally better.18 It should be a shared decision-making
process between parents and paediatric
nephrologists, after detailed counselling on potential
burdens and after considering co-morbidities,
expected quality of life, and available resources
and expertise.18 21 Designated nurses, clinical
psychologists, and medical social workers are crucial
in supporting patients and parents.
Peritoneal dialysis–related infection
Infants and young children are at risk of PD-related
infectious complications. In the US, the annualised
rate of peritonitis in children younger than 2 years
was 0.79 episode per patient-year, compared with
0.57 episode per patient-year in adolescents aged over 12 years.4 In our
current series, the annual peritonitis rate was 0.26,
which is less frequent than the US data. As previously
reported, the overall annual peritonitis rate among
all our paediatric patients on APD was low at 0.22.22 A low infection rate has similarly been reported in several Asian countries.22
There are a few possible explanations. First, all
our patients were on APD that is associated with a
reduced risk of infection as shown in a systematic
review by Rabindranath et al23 and previous data in
NAPRTCS 2005.24 Second, we strictly complied with
the guidelines and recommendations on prevention
of PD-related infection.4 9 21 25 26 Measures included the use of double-cuffed Tenckhoff catheters,
downward or laterally pointing exit sites away
from diaper and ostomies, antibiotic prophylaxis
at catheter insertion, post-insertion immobilisation
of the catheter, nasal methicillin-resistant S aureus
screening and decolonisation with mupirocin, and
selective use of prophylactic topical antibiotics for
patients with a history of ESI. Third, all patients
and their carers completed an intensive PD training
programme before commencing home APD.
Training was conducted by a senior renal nurse
with regular reviews and phone follow-ups. The
high culture-negative peritonitis rate in our series
highlights the need for proper specimen collection
and handling.9
Growth and nutrition
Growth in infancy is important because one third of
postnatal height is achieved during the first 2 years
of life.27 Growth during this period largely relies on
nutritional intake, rather than growth hormone.
Growth in ESRD is often impaired because of poor
appetite, increased circulatory leptin, nutritional loss
through peritoneal dialysate and repeated vomiting
due to dysmotility, gastroesophageal reflux, and
raised intraperitoneal pressure.15 27 Infants can lose more than 2 htSDS that can be irreversible.15
Importantly, it is also a period of catch-up growth;
NAPRTCS reported improvement in both htSDS
and wtSDS in children who started dialysis before
the age of 2 years—htSDS improved from -2.59 at
baseline to -2.15 at 24 months post-dialysis, while
wtSDS improved from -2.26 to -1.05.4
In our cohort, there was weight gain, but a
decline in htSDS was observed. The IPDN recently
analysed growth in 153 very young children on CPD.27
Interestingly, htSDS decreased further in the first 6
to 12 months of CPD and then stabilised. Although
catch-up in height was noted in the NAPRTCS
report, such improvement was only observed in
children with worse baseline height deficit, defined as
htSDS ≤ –1.88. Children with htSDS > –1.88 instead
had a decline in htSDS by 0.11 and 0.2 at 12 and 24
months, respectively.4 Only two of our patients had
worse baseline height deficit (≤ –1.88), with htSDS
being -2 at CPD initiation. Similar to the findings in
IPDN and NAPRTCS, catch-up growth in height was
observed in these two patients. At 12 months post-dialysis,
their htSDS improved to -0.94 and -1.6, respectively.
Oral intake is often unsatisfactory and enteral
feeding is required, either by NG or gastrostomy
tube. This allows overnight feeding and reduces
vomiting. In the recent IPDN study on growth, 37%
young children were fed on demand, 39% by NG
tube, 7% by gastrostomy tube, and 17% switched
from NG to gastrostomy feeding.27 Both NG and
gastrostomy feeding led to significant increase in
body mass index SDS, although regional variation
was observed. Gastrostomy but not NG feeding was
associated with improved linear growth, an effect
that was no longer significant after adjusting the
baseline length. Feeding by gastrostomy appeared
to be superior to NG tube in growth promotion and
may be related to less vomiting.27
Over the years, the use of a gastrostomy
to enhance nutritional supplementation has
been promoted in our centre, with intensified
collaboration with a paediatric renal dietitian. In our
series, only one (20%) patient who commenced CPD
before 2008 received enteral feeding, owing to low
parental acceptance. Of the remaining four patients
who started CPD after 2008, two had gastrostomies,
one was awaiting gastrostomy insertion, and one
thrived satisfactorily without the need for enteral
feeding. This suggests an improved nutritional
management and parental acceptance. Extra efforts
should also be made to optimise factors such as
acidosis, anaemia, and metabolic bone disease.27
In addition, KDOQI (Kidney Disease Outcomes
Quality Initiative) suggests consideration of growth
hormone when children have htSDS and height
velocity SDS of ≤ –1.88 after optimising nutrition
and metabolic abnormalities.28
There are a few limitations to this study. First,
because of the retrospective study design, there was
recall bias. Some information could not be retrieved
from medical records, especially for children who
presented in the late 1990s and early 2000s. Second,
the total case number was small since patients were
recruited from a single nephrology centre. Last,
infant dialysis has changed considerably over the
past two decades and might in turn affect patient
outcome.
Conclusions
End-stage renal disease in very young children is
uncommon. Chronic PD is feasible and the outcome
is improving. Vigilant adoption of guidelines,
universal use of APD, and a well-structured PD
training programme are crucial to achieve low
peritonitis and ESI rates with no CPD-related
mortality in our centre. Optimisation of dialysis,
nutritional support, and developmental training are
important while successful renal transplantation is
the ultimate goal for these infants.
Declaration
All authors have disclosed no conflicts of interest.
References
1. Geary DF. Attitudes of pediatric nephrologists to
management of end-stage renal disease in infants. J Pediatr
1998;133:154-6. Crossref
2. Kari JA, Gonzalez C, Ledermann SE, Shaw V, Rees L.
Outcome and growth of infants with severe chronic renal
failure. Kidney Int 2000;57:1681-7. Crossref
3. Ledermann SE, Scanes ME, Fernando ON, Duffy PG,
Madden SJ, Trompeter RS. Long-term outcome of
peritoneal dialysis in infants. J Pediatr 2000;136:24-9. Crossref
4. North American Pediatric Renal Trials and Collaborative
Studies (NAPRTCS). 2011 Annual dialysis report. Available from: https://web.emmes.com/study/ped/annlrept/annualrept2011.pdf. Accessed Nov 2015.
5. Vidal E, Edefonti A, Murer L, et al. Peritoneal dialysis in
infants: the experience of the Italian Registry of Paediatric
Chronic Dialysis. Nephrol Dial Transplant 2012;27:388-95. Crossref
6. Shroff R, Rees L, Trompeter R, Hutchinson C, Ledermann
S. Long-term outcome of chronic dialysis in children.
Pediatr Nephrol 2006;21:257-64. Crossref
7. Warady BA, Alexander SR, Hossli S, et al. Peritoneal
membrane transport function in children receiving long-term
dialysis. J Am Soc Nephrol 1996;7:2385-91.
8. Warady BA, Alexander S, Hossli S, Vonesh E, Geary
D, Kohaut E. The relationship between intraperitoneal
volume and solute transport in pediatric patients. Pediatric
Peritoneal Dialysis Study Consortium. J Am Soc Nephrol
1995;5:1935-9.
9. Warady BA, Bakkaloglu S, Newland J, et al. Consensus
guidelines for the prevention and treatment of catheter-related
infections and peritonitis in pediatric patients
receiving peritoneal dialysis: 2012 update. Perit Dial Int
2012;32 Suppl 2:S32-86. Crossref
10. Kidney Disease: Improving Global Outcomes (KDIGO)
CKD-MBD Work Group. KDIGO clinical practice
guideline for the diagnosis, evaluation, prevention, and
treatment of Chronic Kidney Disease-Mineral and Bone
Disorder (CKD-MBD). Kidney Int Suppl 2009;113:S1-130.
11. Borzych D, Rees L, Ha IS, et al. The bone and mineral
disorder of children undergoing chronic peritoneal dialysis.
Kidney Int 2010;78:1295-304. Crossref
12. Leung SS, Tse LY, Wong GW, et al. Standards for
anthropometric assessment of nutritional status of Hong
Kong children. Hong Kong J Paediatr 1995;12:5-15.
13. Rees L. Paediatrics: Infant dialysis—what makes it special?
Nat Rev Nephrol 2013;9:15-7. Crossref
14. Yap HK, Bagga A, Chiu MC. Pediatric nephrology in Asia.
In: Avner ED, Harmon WE, Niaudet P, Yoshikawa N,
Emma F, Goldstein SL, editors. Pediatric nephrology. 6th
ed. Springer; 2010: 1981-90.
15. Zaritsky J, Warady BA. Peritoneal dialysis in infants and
young children. Semin Nephrol 2011;31:213-24. Crossref
16. Chiu MC. An update overview on paediatric renal
transplantation. Hong Kong J Paediatr 2004;9:74-7.
17. Warady BA, Belden B, Kohaut E. Neurodevelopmental
outcome of children initiating peritoneal dialysis in early
infancy. Pediatr Nephrol 1999;13:759-65. Crossref
18. Lantos JD, Warady BA. The evolving ethics of infant
dialysis. Pediatr Nephrol 2013;28:1943-7. Crossref
19. Teh JC, Frieling ML, Sienna JL, Geary DF. Attitudes of
caregivers to management of end-stage renal disease in
infants. Perit Dial Int 2011;31:459-65. Crossref
20. Wood EG, Hand M, Briscoe DM, et al. Risk factors for
mortality in infants and young children on dialysis. Am J
Kidney Dis 2001;37:573-9. Crossref
21. Zurowska AM, Fischbach M, Watson AR, Edefonti A,
Stefanidis CJ, European Paediatric Dialysis Working
Group. Clinical practice recommendations for the care
of infants with stage 5 chronic kidney disease (CKD5).
Pediatr Nephrol 2013;28:1739-48. Crossref
22. Chiu MC, Tong PC, Lai WM, Lau SC. Peritonitis and exit-site
infection in pediatric automated peritoneal dialysis.
Perit Dial Int 2008;28 Suppl 3:S179-82.
23. Rabindranath KS, Adams J, Ali TZ, Daly C, Vale L,
MacLeod AM. Automated vs continuous ambulatory
peritoneal dialysis: a systematic review of randomized
controlled trials. Nephrol Dial Transplant 2007;22:2991-8. Crossref
24. North American Pediatric Renal Transplant Cooperative
Study (NAPRTCS). 2005 Annual report. Available
from: https://web.emmes.com/study/ped/annlrept/annlrept2005.pdf. Accessed Nov 2015.
25. Piraino B, Bailie GR, Bernardini J, et al. Peritoneal dialysis-related
infections recommendations: 2005 update. Perit
Dial Int 2005;25:107-31.
26. Auron A, Simon S, Andrews W, et al. Prevention of
peritonitis in children receiving peritoneal dialysis. Pediatr
Nephrol 2007;22:578-85. Crossref
27. Rees L, Azocar M, Borzych D, et al. Growth in very young
children undergoing chronic peritoneal dialysis. J Am Soc
Nephrol 2011;22:2303-12. Crossref
28. KDOQI Work Group. KDOQI Clinical Practice Guideline
for Nutrition in Children with CKD: 2008 update.
Executive summary. Am J Kidney Dis 2009;53:S11-104. Crossref