Hong Kong Med J 2016 Feb;22(1):30–8 | Epub 23 Oct 2015
DOI: 10.12809/hkmj144470
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Association between pregnancy-associated
plasma protein-A levels in the first trimester and
gestational diabetes mellitus in Chinese women
Queenie KY Cheuk, MB, ChB, FHKAM (Obstetrics and Gynaecology);
TK Lo, MB, BS, FHKAM (Obstetrics and Gynaecology);
SF Wong, FRCOG, FHKAM (Obstetrics and Gynaecology);
CP Lee, FRCOG, FHKAM (Obstetrics and Gynaecology)
Department of Obstetrics and Gynaecology, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong
Corresponding author: Dr Queenie KY Cheuk (dingcky@yahoo.com.hk)
Abstract
Introduction: Several studies have shown that
women with pre-existing diabetes mellitus have
significantly lower pregnancy–associated plasma
protein-A levels than those without. This study aimed
to evaluate whether first-trimester pregnancy–associated plasma protein-A multiple of median
is associated with gestational diabetes mellitus in
Chinese pregnant women.
Methods: This prospectively collected case series
was conducted in a regional hospital in Hong Kong.
All consecutive Chinese women with a singleton
pregnancy who attended the hospital for their
first antenatal visit (before 14 weeks’ gestation)
from April to July 2014 were included. Pregnancy-associated
plasma protein-A multiple of median
was compared between the gestational diabetic
(especially for early-onset gestational diabetes)
and non-diabetic groups. The correlation between
pregnancy-associated plasma protein-A level and
glycosylated haemoglobin level in women with gestational
diabetes was also examined.
Results: Of the 520 women recruited, gestational
diabetes was diagnosed in 169 (32.5%). Among
them, 43 (25.4%) had an early diagnosis, and 167
(98.8%) with the disease were managed by diet
alone. The gestational diabetic group did not
differ significantly to the non-diabetic group in
pregnancy-associated plasma protein-A (0.97 vs
0.99, P=0.40) or free β-human chorionic gonadotrophin multiple of median
(1.05 vs 1.02, P=0.29). Compared
with the non-gestational diabetic group, women
with early diagnosis of gestational diabetes had a
non-significant reduction in pregnancy-associated
plasma protein-A multiple of median (median,
interquartile range: 0.86, 0.57-1.23 vs 0.99, 0.67-1.44;
P=0.11). Pregnancy-associated plasma protein-A
and glycosylated haemoglobin levels were not
correlated in women with gestational diabetes
(r=0.027; P=0.74).
Conclusions: Chinese women with non–insulin-dependent
gestational diabetes did not exhibit
significant changes to pregnancy-associated plasma
protein-A multiple of median nor a correlation
between pregnancy-associated plasma protein-A
with glycosylated haemoglobin levels. Pregnancy-associated
plasma protein-A multiple of median was
not predictive of non–insulin-dependent gestational
diabetes or early onset of gestational diabetes. There
was a high prevalence of gestational diabetes in the
Chinese population.
New knowledge added by this study
- This is the first study to assess the association of first-trimester pregnancy–associated plasma protein-A multiple of median (PAPP-A MoM) with gestational diabetes mellitus (GDM) in a Chinese population. PAPP-A MoM was not predictive of development of non–insulin-dependent GDM in Chinese women.
- There was no correlation between PAPP-A MoM and glycosylated haemoglobin level in Chinese women with GDM. PAPP-A levels were not useful to predict and identify poor glycaemic control in women with GDM.
- There was a high prevalence of GDM (32.5%) in the Chinese population.
- PAPP-A and free β-human chorionic gonadotrophin do not seem to be predictive of non–insulin-dependent GDM. Other predictive model that comprises the maternal and clinical risk factors in Chinese women is warranted to identify women at risk of GDM.
- Further studies that employ the new diagnostic criteria for GDM are warranted to examine the potential of first-trimester biochemical markers to predict GDM as well as their influence on the prevalence of GDM in the Chinese population.
Introduction
Gestational diabetes mellitus (GDM) is defined as
carbohydrate intolerance of any degree that starts or
is first recognised during pregnancy.1 The prevalence
of GDM in pregnant women varies widely in
different populations and is highly dependent on the
screening and diagnosis strategies that are used.2 In
the 1990s, the prevalence of GDM in Hong Kong
was approximately 14.2%.3 Studies in China and the
United States show that the incidence of GDM has
been increasing in recent years,4 5 thus increasing the risk of complications for both mother and child
during pregnancy, childbirth, and beyond.6 Notably,
it is reported that high first-trimester glucose levels
are associated with an increased risk of a diagnosis
of GDM later in pregnancy and adverse pregnancy
outcome.7 This suggests that women who will
develop GDM can exhibit metabolic alterations early
in pregnancy. Thus, it is of interest to determine
whether pregnant women who develop GDM exhibit
changes to first-trimester biochemical markers.
If so, such markers can allow early detection and
treatment of women at risk of GDM, and thus reduce
the associated morbidity.8 9
In Hong Kong, all women undergo first-trimester
screening for Down syndrome using a
combination of maternal age, maternal free β-human
chorionic gonadotrophin (β-HCG), pregnancy-associated
plasma protein-A (PAPP-A), and fetal
nuchal translucency (NT) thickness at 11–13+6
weeks of gestation. Studies have shown that low
free β-HCG and PAPP-A levels in the first trimester
are associated with pregnancy complications.10 11 In particular, low PAPP-A levels are significantly associated with spontaneous fetal loss, low-birth-weight babies, intra-uterine growth restriction, pregnancy-induced
hypertension, pre-eclampsia, preterm
rupture of membranes, and placental abruption.12 13 14
Several studies have shown that women with pre-existing
diabetes mellitus (DM) have significantly
lower PAPP-A levels than those without DM.11 15 16 17 18 Besides, PAPP-A levels in non-pregnant individuals
with type 2 DM correlate inversely with glycosylated
haemoglobin (HbA1c) levels.19 These observations
suggest that PAPP-A levels may reflect the degree
of glycaemic control. Studies of PAPP-A levels in
patients with GDM have yielded conflicting results,
however. In addition, such studies in Chinese women,
who are well known to have a high prevalence of
GDM, have not been performed.
The primary objective of this study was to
investigate whether Chinese women with GDM
exhibit changes in PAPP-A multiple of median
(MoM) in the first trimester. The secondary objectives
were to investigate whether PAPP-A level was an
independent predictor of GDM, especially for early
onset of GDM; whether PAPP-A MoM correlated
with glycaemic control in women with GDM; and the
prevalence of GDM in the Chinese population.
Methods
This prospectively collected case series was
conducted between April and July 2014 at the
obstetric unit of Pamela Youde Nethersole Eastern
Hospital, which is a public tertiary care hospital
in Hong Kong. Ethical approval for the study was
obtained from the local institutional human research
ethics committee.
All consecutive Chinese women with a
singleton pregnancy who attended the hospital
for their first antenatal visit (before 14 weeks of
gestation) during the recruitment period were
invited to participate in this study. Written informed
consent was obtained from all women who agreed
to participate. Women with a multiple pregnancy,
pre-existing DM, chronic disease (eg renal
disease, hypertension, connective tissue disease),
miscarriage, termination of pregnancy, a fetus
with a chromosomal or congenital abnormality, or
preterm delivery before an oral glucose tolerance
test (OGTT) could be performed were excluded.
Universal first-trimester Down syndrome
screening was performed using fetal NT and
maternal biochemistry. The ultrasound machine
used was the Voluson E8 Expert (GE Healthcare, Fairfield [CT], US) or iU22
(Philips Medical System, Bothell [WA], US) equipped
with a 3-5 MHz convex/broadband transducer. To
determine crown rump length and NT thickness, the
protocols outlined by the Fetal Medicine Foundation
were followed.20 The serum levels of free β-HCG
and PAPP-A were measured by the DELFIA Xpress
analytical platform (PerkinElmer Life Sciences,
Turku, Finland). Multiple of median was adjusted
for maternal weight and ethnicity. Down syndrome
risk was calculated using the Alpha software (Logical
Medical Systems, London, UK).
The demographic and clinical data were
routinely collected by an obstetrician during the
first antenatal visit and were entered into the
hospital electronic system (antenatal record system).
Maternal weight, height, and blood pressure were
measured and body mass index (BMI) was calculated.
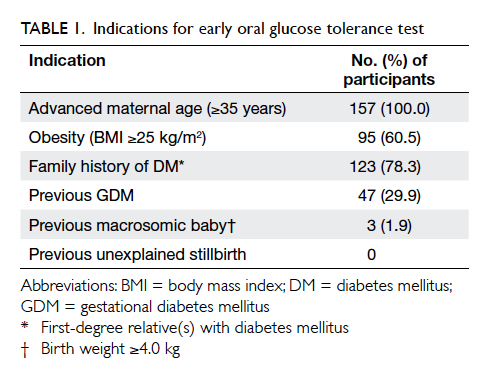
All women who had one or more risk factors
for the development of GDM, such as advanced
maternal age (≥35 years), previous GDM, family
history of DM (first-degree relative with DM), a
previous macrosomic baby (≥4.0 kg), an unexplained
stillbirth, significant glycosuria, or obesity (BMI
≥25 kg/m2) underwent an early 75-g OGTT after
the initial visit. The OGTT results were interpreted
according to the World Health Organization (WHO)
1999 criteria.21 Gestational DM was diagnosed if
the fasting blood glucose level was ≥7.0 mmol/L or if the
2-hour OGTT blood glucose level was ≥7.8 mmol/L. All
low-risk women and those with normal early OGTT
results underwent universal 75-g OGTT screening
at around 28 to 30 weeks. Women with a diagnosis
of GDM underwent a further blood test 2 to 3 weeks
after the initial diagnosis to determine HbA1c level.
They were also given dietary and exercise advice and
encouraged to perform daily capillary blood glucose
monitoring before and 2 hours after a meal. If the pre- and
post-meal glucose levels frequently exceeded
6.0 and 7.8 mmol/L, respectively, the women were
prescribed insulin. All participating women received
routine antenatal care until delivery according to our
department protocol.
All statistical analyses were performed using
PASW Statistics 18, Release Version 18.0.0 (SPSS
Inc, 2009, Chicago [IL], US). Categorical data were
analysed using the Chi squared test or Fisher’s
exact test, depending on the data distribution. For
continuous variables with a normal distribution, the
independent t test was used. For continuous data
with a highly skewed distribution, a non-parametric
test (ie Mann-Whitney U test) was used.
Sample size was calculated based on two
assumptions. First, about 25% of the population
screened will have GDM, according to a previous
local study22 and our departmental annual audit.
Second, there was a 10% difference in PAPP-A MoM
between a GDM and non-GDM group, according
to a previous published series.23 Based on these
assumptions, a total sample size of 380 cases with
95 cases in the GDM group and 285 cases in the
non-GDM group were required for a type 1 error of
0.05, power of 80%, and standard deviation of 0.3 in
both groups.
Results
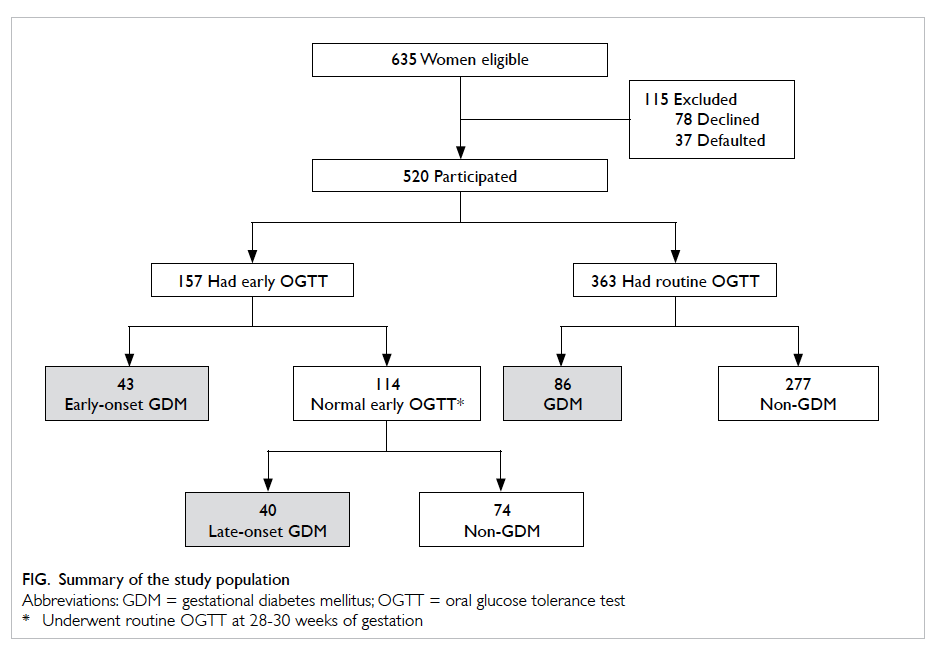
The study sample is summarised in the Figure. In
total, 520 women participated in the study of whom
157 (30.2%) underwent early OGTT. Indications for
early OGTT are summarised in Table 1. Overall,
GDM was diagnosed in 169 women. Among them,
43 (25.4%) had an early diagnosis of GDM. All GDM
cases were diagnosed based on a 2-hour OGTT blood glucose
level of ≥7.8 mmol/L; none had a fasting blood glucose
level of ≥7.0 mmol/L (Table 2). The remaining 351
women did not develop GDM. The GDM prevalence
was 32.5%. No woman underwent preterm delivery
before OGTT. There was no difference in baseline
characteristics between those excluded (eg defaulter
and decliner) and those included in the analysis. The
majority (n=167; 98.8%) of women with GDM were
managed with diet alone. Only two (1.2%) required
insulin.
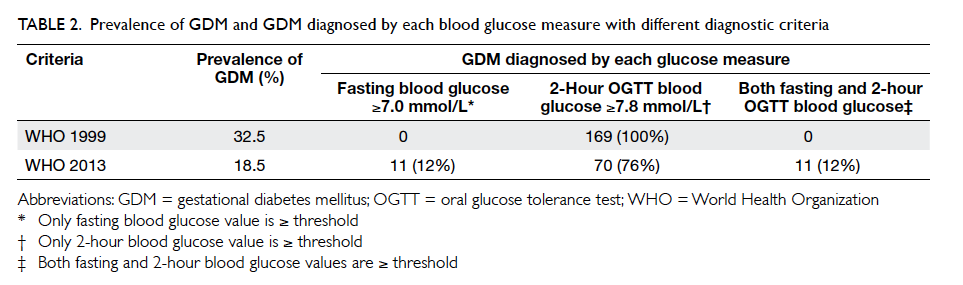
Table 2. Prevalence of GDM and GDM diagnosed by each blood glucose measure with different diagnostic criteria
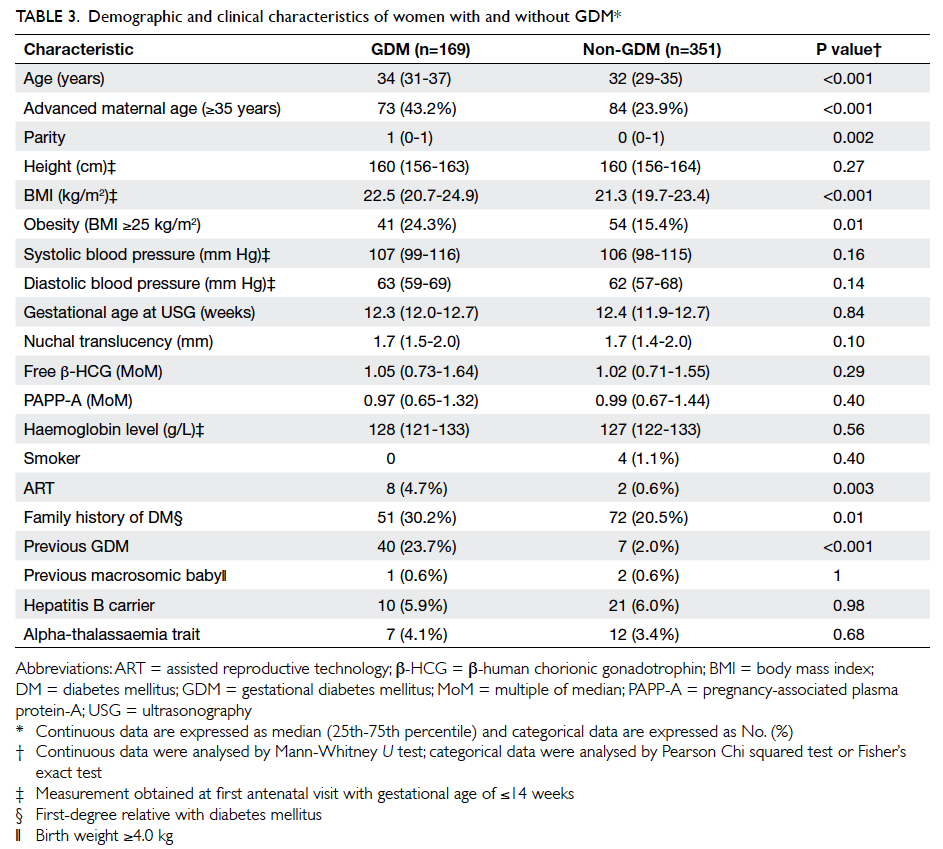
The maternal characteristics of the women
with and without GDM are shown in Table 3. Compared with the non-GDM group, women in the
GDM group were significantly older (34 vs 32 years),
had a higher parity (1 vs 0) and a higher BMI (22.5
vs 21.3 kg/m2). They were also more likely to have
conceived with assisted reproductive technology
and to have a family history of DM and a history of GDM. The two groups did not differ in
terms of PAPP-A MoM (P=0.40) or free β-HCG MoM
(P=0.29), however.
Compared with the non-GDM group,
women with an early diagnosis of GDM had a non-significant
reduction in PAPP-A MoM (median, interquartile range: 0.86, 0.57-1.23 vs 0.99, 0.67-1.44;
P=0.11). There was also a non-significant reduction
in PAPP-A MoM (median, interquartile range: 0.86,
0.57-1.23 vs 1.02, 0.72-1.61; P=0.07) in the women
with early-onset GDM compared with those who
had late-onset GDM (their early OGTT result
was normal but subsequent routine OGTT result at 28
weeks was abnormal). Only two women with GDM
required insulin treatment: their PAPP-A MoM
was 0.54 and 0.78, which was low when compared
with that of women with GDM who did not require
insulin treatment or with the non-GDM group.
In this study, 308 (59.2%) women were
nulliparous. Among them, GDM was diagnosed in
83 women. Compared with the non-GDM group,
nulliparous women with GDM had no significant
change in PAPP-A MoM (median, interquartile
range: 0.92, 0.63-1.27 vs 1.0, 0.72-1.46; P=0.08).
Although univariate analysis showed that
the women with and without GDM did not differ
significantly in PAPP-A MoM, it is possible that an
association between GDM and PAPP-A MoM was
obscured by confounding variables. To identify
potential confounding variables, Spearman’s rho
correlation coefficient analysis was performed
(Table 4). It revealed that PAPP-A MoM did not
correlate with maternal age, height, parity, or NT.
There was a significant but weak correlation between
PAPP-A MoM and free β-HCG MoM (r=0.2). Since
univariate analysis showed that free β-HCG MoM was
not associated with GDM (P=0.29), its confounding
effect would be minimal. Thus, an association
between GDM and PAPP-A MoM was not detected.
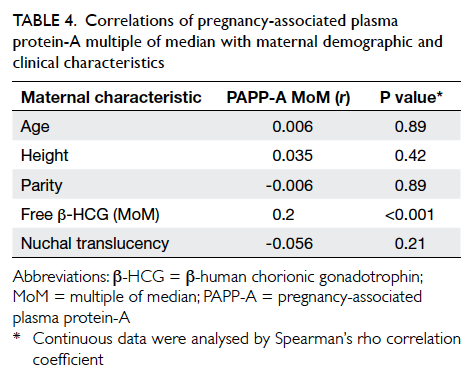
Table 4. Correlations of pregnancy-associated plasma protein-A multiple of median with maternal demographic and clinical characteristics
Our study showed that with maternal risk
factor screening strategies, only 157 (30.2%) women
would undergo OGTT (Fig). If we screened at-risk women by early OGTT alone, only 43 (25.4%) cases
of GDM would be identified. On the other hand, if
we subjected at-risk women to early OGTT followed
by 28-week OGTT for those who had a normal early
OGTT, 83 (49.1%) cases of GDM would be identified.
Although we lacked 1-hour data, we tried
to determine whether PAPP-A was associated
with GDM based on new diagnostic criteria from
the American Diabetes Association (ADA), the
International Association of Diabetes and Pregnancy
Study Groups (IADPSG),24 and WHO 2013.25 A total of 23 women in whom GDM was diagnosed during
early pregnancy based on WHO 1999 criteria (but
normal by WHO 2013 criteria) did not undergo a
second OGTT and were excluded from analysis. The
application of the fasting or 2-hour criteria led to
92 (18.5%) women being identified with GDM. The
majority (76%) of women were diagnosed with GDM
based on a 2-hour glucose level of ≥8.5 mmol/L
(Table 2). Nevertheless, the GDM group again did
not differ to the non-GDM group in terms of PAPP-A
MoM (median, interquartile range: 0.94, 0.67-1.34 vs
0.99, 0.66-1.43; P=0.52) or free β-HCG MoM (1.02, 0.71-1.56 vs 1.03, 0.72-1.61; P=0.80).
To determine whether PAPP-A MoM and
HbA1c levels in women with GDM correlated with
each other, Spearman’s rho correlation coefficient
was used. A correlation was not found (r=0.027;
P=0.74).
Discussion
The present study showed that Chinese women
with GDM did not exhibit a significant change in
PAPP-A MoM during the first trimester. Women
with early-onset GDM had a non-significant
decrease in PAPP-A MoM when compared with
non-GDM women or women with late-onset GDM.
Nevertheless, first-trimester PAPP-A MoM was not
a useful predictor for development of GDM or early-onset
GDM. This is consistent with the results of three
other studies18 26 27 but contradicts others.8 9 11 23 28 29
These published series are summarised in Table
5.8 9 11 18 23 26 27 28 29 There are several possible explanations
for the different results of these studies, including
ours.
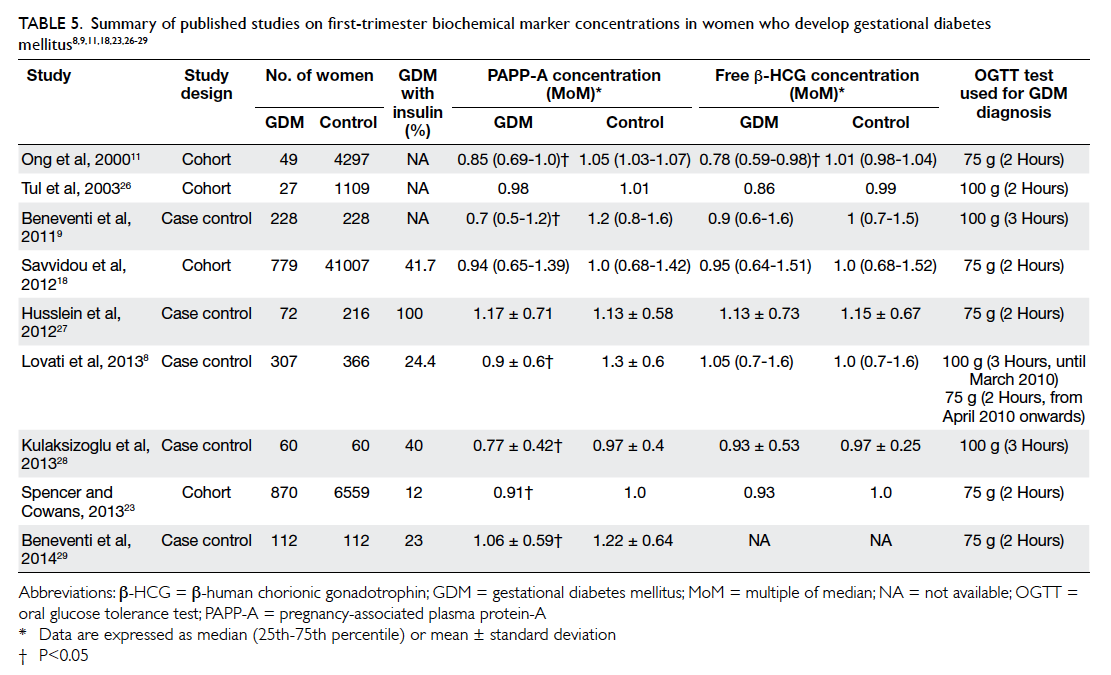
Table 5. Summary of published studies on first-trimester biochemical marker concentrations in women who develop gestational diabetes mellitus8 9 11 18 23 26 27 28 29
First, the studies differed in the selection
criteria used to determine when OGTT should be
performed, which in turn would target a different
study population: some women were tested on the
basis of GDM risk factors,23 some when the women
had an abnormal random blood glucose level11 18 or 50-mg glucose challenge test,9 28 others as a universal screening test as in our study.8 27
Second, the studies differed in terms of the
OGTT method (75-g 2 hours, 100-g 3 hours,
100-g 2 hours) and diagnostic criteria. Tran et al30
showed that GDM varied substantially in the same
population by different diagnostic criteria: 5.9%
ADA, 20.4% IADPSG, and 24.3% WHO 1999.
Third, the studies may differ in GDM severity,
as reflected by the proportion of women with GDM
who required insulin treatment. Women with GDM
who require insulin may have a more severe type of
GDM or undiagnosed pre-existing DM. Lovati et al8
showed that women with GDM had a significantly
lower PAPP-A MoM if they received insulin therapy
than women with GDM who were managed by diet
(0.56 vs 0.76 MoM; P<0.001). Beneventi et al29 showed
a similar result that PAPP-A MoM was significantly
lower in GDM managed with insulin treatment
than GDM without (0.87 vs 1.11 MoM; P=0.031).
These studies showed that insulin-dependent GDM
was more strongly correlated with lower PAPP-A
MoM. There were, however, only two (1.2%) women
with GDM in our study who required insulin, a
much lower frequency compared with other study
populations (12%-100%).8 9 11 18 23 26 27 28 29 Although both
women had a lower PAPP-A MoM than women
with non–insulin-treated GDM or non-GDM group,
such a small proportion is insufficient to determine
whether PAPP-A MoM differs significantly between
women with insulin-treated GDM and non–insulin-treated
women with GDM. The low frequency of
insulin treatment in our study population may
imply that the majority of affected Chinese women
had mild GDM and may also explain why our study
population did not exhibit changes in PAPP-A MoM
during the first trimester.
Fourth, it is known that PAPP-A and free β-HCG are
influenced by other maternal or pregnancy variables
such as gestational age,11 maternal weight,9 28 and
smoking.26 Corrections for these variables were
taken into account when calculating the MoM of
PAPP-A and free β-HCG. While different laboratories
may have corrected the MoM of PAPP-A and free β-HCG
differently using maternal or pregnancy variables,
this may have introduced bias in the assessment of
the association between these biochemical markers
and GDM.
The HAPO study led to considerable debate
about the definition of GDM.6 31 As a result, ADA, IADPSG,24 and WHO 201325 have recently suggested that GDM is diagnosed on the basis of 75-g OGTT
and fasting, 1-hour, or 2-hour glucose levels of
≥5.1, ≥10.0, and ≥8.5 mmol/L as the threshold,
respectively. Since we had the fasting and 2-hour
glucose data (1-hour glucose data were not available),
we reclassified our patients with GDM accordingly.
After reclassification, PAPP-A and free β-HCG MoM in
women with GDM did not differ to that of women
without GDM. Our reclassification had limitations,
however. A total of 23 women who were diagnosed
with GDM during early pregnancy using old WHO
1999 criteria (but normal by WHO 2013 criteria)
did not undergo second OGTT so it is unknown
whether their results of a second OGTT would be
normal or diagnosed as GDM based on the new
WHO criteria. Further studies that employ the new
diagnostic criteria for GDM are warranted to explore
the potential of using first-trimester biochemical
markers to predict GDM.
During pregnancy, PAPP-A is produced by
trophoblasts and is detectable in maternal blood
28 days after conception. An experimental model
found that PAPP-A was a protease of insulin growth
factor binding protein 4, regulating the
activity of insulin-like growth factor (IGF).32 This
may be a plausible explanation for the association
between PAPP-A and glycaemic control because the
IGF axis is involved in glycaemic control. Human
studies, however, have not provided clear evidence
for a metabolic or biochemical mechanism that
can explain a putative association between first-trimester
PAPP-A levels and GDM. In one study,
non-pregnant individuals with type 2 DM were
found to have lower PAPP-A levels than non-diabetic
controls, PAPP-A levels correlated inversely with
HbA1c levels (r= –0.2; P=0.03).19 Another study failed
to detect this correlation in pregnant women with
insulin-dependent DM.17 Similarly, we identified no
correlation between PAPP-A and HbA1c levels in
women with GDM. These observations suggest that
PAPP-A may not be useful for assessing or predicting
glycaemic control in women with GDM due to the
relatively short duration of women’s exposure to
GDM that is confined to the latter part of pregnancy.
This is particularly so in Chinese as the majority of
affected women, as we have demonstrated, have mild
disease only. To our knowledge, our study is the first
to address this issue in women with GDM.
Different tools to assess risk of GDM have
been proposed and most have found that previous
GDM is the best predictor of subsequent GDM.33
In our population, the majority of women (59.2%)
were nulliparous. We tried to investigate whether
PAPP-A would be a possible predictor in this group
of women. Our study showed that in nulliparous
women with GDM, first-trimester PAPP-A MoM did
not differ to that of women without GDM.
There is much debate about the screening
strategies for GDM. Jensen et al34 found that
risk factor–based screening was as effective as
universal screening: those not identified on risk factor
screening were negligible compared with the
high number successfully identified. However, this
may not be the case with Chinese women whose
ethnicity places them at risk of GDM.35 36 37 Our study
showed that with maternal clinical risk factor–based
screening, less than one third of our population
would undergo OGTT and about half of the GDM
cases would be missed. Provided resources are
available, universal screening should be considered
in Chinese women.
Our study, which was based on universal
screening, showed a high prevalence of GDM (32.5%).
This prevalence was much higher than that of a study
conducted by Ko et al3 in the early 1990s (14.2%).
It was also higher than the HAPO study cohort in
2000-200638 that reported a prevalence of GDM in
Hong Kong of 14.4%. It is worth investigating this
change in the trend of GDM prevalence in Hong
Kong that may be due to increasing maternal obesity,
adoption of a westernised diet and lifestyle, genetic
shift, or other unknown factors. Some authors have
proposed that with the new GDM diagnostic criteria,
the prevalence of GDM may increase further.23 This
may not be the case in Chinese, however. The HAPO study38 found that in Hong Kong, a higher proportion (29%) of GDM was diagnosed based on
a raised 2-hour glucose level than in other countries
(6%-19%). Our study concurred with the HAPO
study: all women diagnosed with GDM (WHO
1999) had a raised 2-hour glucose level only (Table
2). With the raised 2-hour glucose cut-off value in
the new WHO diagnostic criteria (from ≥7.8 to ≥8.5
mmol/L), the prevalence of GDM in Chinese may
not be raised. Our figure of reclassifying GDM using
new diagnostic criteria without 1 hour did show a
reduction in GDM prevalence. Further study using
the full WHO 2013 diagnostic criteria to assess the
prevalence of GDM in Chinese is warranted.
Departmental resources and limited
manpower did not allow us to use the new WHO
2013 diagnostic criteria. Moreover, possible selection
bias (eg defaulters and decliners) and possible
confounding factors were not taken into account
in the present study. Nevertheless all women in our
study underwent routine OGTT to identify GDM,
covering both low- and high-risk groups, whereas
other studies were more likely to focus only on a
high-risk group. Some previous studies11 15 18 have also
included women with pre-existing DM.
This was the first study to assess the association
between first-trimester PAPP-A levels and GDM in a
Chinese population. It showed that the vast majority
of Chinese women with GDM did not require insulin
nor exhibit significant change in PAPP-A MoM
during the first trimester. First-trimester PAPP-A
MoM was not a useful predictor for development of
GDM. A correlation between PAPP-A and HbA1c
levels was not observed. Our study showed a high
prevalence of GDM at 32.5%, which is higher than that in
previous studies.
Declaration
No conflicts of interest were declared by authors.
References
1. American Diabetes Association. Diagnosis and
classification of diabetes mellitus. Diabetes Care 2009;32
Suppl 1:S62-7. Crossref
2. Galtier F. Definition, epidemiology, risk factors. Diabetes
Metab 2010;36:628-51. Crossref
3. Ko GT, Tam WH, Chan JC, Rogers M. Prevalence of
gestational diabetes mellitus in Hong Kong based on the
1998 WHO criteria. Diabet Med 2002;19:80. Crossref
4. Dabelea D, Snell-Bergeon JK, Hartsfield CL, Bischoff KJ,
Hamman RF, McDuffie RS; Kaiser Permanente of Colorado
GDM Screening Program. Increasing prevalence of
gestational diabetes mellitus (GDM) over time and by birth
cohort: Kaiser Permanente of Colorado GDM Screening
Program. Diabetes Care 2005;28:579-84. Crossref
5. Zhang F, Dong L, Zhang CP, et al. Increasing prevalence of
gestational diabetes mellitus in Chinese women from 1999
to 2008. Diabet Med 2011;28:652-7. Crossref
6. HAPO Study Cooperative Research Group, Metzger
BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse
pregnancy outcomes. N Engl J Med 2008;358:1991-2002. Crossref
7. Riskin-Mashiah S, Younes G, Damti A, Auslender R. First-trimester fasting hyperglycemia and adverse pregnancy
outcomes. Diabetes Care 2009;9:1639-43. Crossref
8. Lovati E, Beneventi F, Simonetta M, et al. Gestational
diabetes mellitus: including serum pregnancy-associated
plasma protein-A testing in the clinical management of
primiparous women? A case-control study. Diabetes Res
Clin Pract 2013;100:340-7. Crossref
9. Beneventi F, Simonetta M, Lovati E, et al. First trimester
pregnancy–associated plasma protein-A in pregnancies
complicated by subsequent gestational diabetes. Prenat
Diagn 2011;31:523-8. Crossref
10. Pedersen JF, Sørensen S, Ruge S. Human placental
lactogen and pregnancy-associated plasma protein A in
first trimester and subsequent fetal growth. Acta Obstet
Gynecol Scand 1995;74:505-8. Crossref
11. Ong CY, Liao AW, Spencer K, Munim S, Nicolaides KH.
First trimester maternal serum free beta human chorionic
gonadotrophin and pregnancy associated plasma protein
A as predictors of pregnancy complications. BJOG
2000;107:1265-70. Crossref
12. Farina A, Rapacchia G, Freni Sterrantino A, Pula G, Morano
D, Rizzo N. Prospective evaluation of ultrasound and
biochemical-based multivariable models for the prediction
of late pre-eclampsia. Prenat Diagn 2011;31:1147-52. Crossref
13. Montanari L, Alfei A, Albonico G, et al. The impact
of first-trimester serum free beta-human chorionic
gonadotropin and pregnancy-associated plasma protein A
on the diagnosis of fetal growth restriction and small for
gestational age infant. Fetal Diagn Ther 2009;25:130-5. Crossref
14. Dugoff L, Hobbins JC, Malone FD, et al. First-trimester
maternal serum PAPP-A and free-beta subunit human
chorionic gonadotropin concentrations and nuchal
translucency are associated with obstetric complications: a
population-based screening study (the FASTER Trial). Am
J Obstet Gynecol 2004;4:1446-51. Crossref
15. Spencer K, Cowans NJ, Spencer CE, Achillea N. A re-evaluation
of the influence of maternal insulin-dependent
diabetes on fetal nuchal translucency thickness and
first-trimester maternal serum biochemical markers of
aneuploidy. Prenat Diagn 2010;30:937-40. Crossref
16. Kuc S, Wortelboer EJ, Koster MP, de Valk HW, Schielen
PC, Visser GH. Prediction of macrosomia at birth in
type-1 and 2 diabetic pregnancies with biomarkers of early
placentation. BJOG 2011;118:748-54. Crossref
17. Pedersen JF, Sørensen S, Mølsted-Pedersen L. Serum levels
of human placental lactogen, pregnancy-associated plasma
protein A and endometrial secretory protein PP14 in first
trimester of diabetic pregnancy. Acta Obstet Gynecol
Scand 1998;77:155-8. Crossref
18. Savvidou MD, Syngelaki A, Muhaisen M, Emelyanenko
E, Nicolaides KH. First trimester maternal serum free
β-human chorionic gonadotropin and pregnancy-associated
plasma protein A in pregnancies complicated
by diabetes mellitus. BJOG 2012;119:410-6. Crossref
19. Pellitero S, Reverter JL, Pizarro E, et al. Pregnancy-associated
plasma protein-a levels are related to glycemic
control but not to lipid profile or hemostatic parameters in
type 2 diabetes. Diabetes Care 2007;30:3083-5. Crossref
20. Spencer K, Souter V, Tul N, Snijders R, Nicolaides KH. A
screening program for trisomy 21 at 10-14 weeks using
fetal nuchal translucency, maternal serum free beta-human
chorionic gonadotropin and pregnancy-associated plasma
protein-A. Ultrasound Obstet Gynecol 1999;4:231-7. Crossref
21. Definition, diagnosis and classification of diabetes mellitus
and its complications. Part 1. Diagnosis and classification
of diabetes mellitus. WHO/NCD/NCS/99.2. Geneva:
World Health Organization; 1999.
22. Li KE, Cheung YS, Lau BY. Use of fasting plasma glucose
and haemoglobin A1c in screening for gestational diabetes
mellitus in high-risk antenatal patients in Hong Kong.
Hong Kong J Gynaecol Obstet Midwifery 2014;14:31-7.
23. Spencer K, Cowans NJ. The association between gestational
diabetes mellitus and first trimester aneuploidy screening
markers. Ann Clin Biochem 2013;50:603-10. Crossref
24. International Association of Diabetes and Pregnancy
Study Groups Consensus Panel, Metzger BE, Gabbe SG,
Persson B, et al. International association of diabetes
and pregnancy study groups recommendations on the
diagnosis and classification of hyperglycemia in pregnancy.
Diabetes Care 2010;33:676-82. Crossref
25. Diagnostic criteria and classification of hyperglycaemia
first detected in pregnancy. WHO_NMH_MND_13.2_eng.
Geneva: World Health Organization; 2013. Available from:
http://www.who.int/iris/handle/10665/85975. Accessed 5
Dec 2013.
26. Tul N, Pusenjak S, Osredkar J, Spencer K, Novak-Antolic Z.
Predicting complications of pregnancy with first-trimester
maternal serum free-betahCG, PAPP-A and inhibin-A.
Prenat Diagn 2003;23:990-6. Crossref
27. Husslein H, Lausegger F, Leipold H, Worda C. Association
between pregnancy-associated plasma protein-A and
gestational diabetes requiring insulin treatment at
11-14 weeks of gestation. J Matern Fetal Neonatal Med
2012;25:2230-3. Crossref
28. Kulaksizoglu S, Kulaksizoglu M, Kebapcilar AG, Torun
AN, Ozcimen E, Turkoglu S. Can first-trimester screening
program detect women at high risk for gestational diabetes
mellitus? Gynecol Endocrinol 2013;29:137-40. Crossref
29. Beneventi F, Simonetta M, Locatelli E, et al. Temporal
variation in soluble human leukocyte antigen-G (sHLA-G)
and pregnancy-associated plasma protein A (PAPP-A) in
pregnancies complicated by gestational diabetes mellitus
and in controls. Am J Reprod Immunol 2014;72:413-21. Crossref
30. Tran TS, Hirst JE, Do MA, Morris JM, Jeffery HE. Early
prediction of gestational diabetes mellitus in Vietnam:
clinical impact of currently recommended diagnostic
criteria. Diabetes Care 2013;36:618-24. Crossref
31. Moses RG. New consensus criteria for GDM: problem
solved or a pandora’s box? Diabetes Care 2010;33:690-1. Crossref
32. Lawrence JB, Oxvig C, Overgaard MT, et al. The insulin-like
growth factor (IGF)–dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated
plasma protein-A. Proc Natl Acad Sci USA
1999;96:3149-53. Crossref
33. Teede HJ, Harrison CL, Teh WT, Paul E, Allan CA.
Gestational diabetes: development of an early risk
prediction tool to facilitate opportunities for prevention.
Aust N Z J Obstet Gynaecol 2011;51:499-504. Crossref
34. Jensen DM, Mølsted-Pedersen L, Beck-Nielsen H,
Westergaard JG, Ovesen P, Damm P. Screening for
gestational diabetes mellitus by a model based on risk
indicators: a prospective study. Am J Obstet Gynecol
2003;189:1383-8. Crossref
35. American Diabetic Association. Gestational diabetes
mellitus. Diabetes Care 1998;21 Suppl 1:S60-1. Crossref
36. Alberti KG, Zinnet PZ. Definition, diagnosis and
classification of diabetes mellitus and its complications;
Part 1 : Diagnosis and classification of diabetes mellitus.
Provisional report of a WHO consultation. Diabet Med
1998;15:539-53. Crossref
37. Naylor CD, Sermer M, Chen E, Farine D. Selective
screening for gestational diabetes mellitus. Toronto
Trihospital Gestational Diabetes Project Investigators. N
Engl J Med 1997;337:1591-6. Crossref
38. Sacks DA, Hadden DR, Maresh M, et al. Frequency of
gestational diabetes mellitus at collaborating centers based
on IADPSG consensus panel–recommended criteria: the
Hyperglycemia and Adverse Pregnancy Outcome (HAPO)
Study. Diabetes Care 2012;35:526-8. Crossref