Hong Kong Med J 2015 Oct;21(5):417–25 | Epub 28 Aug 2015
DOI: 10.12809/hkmj144472
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Patient acceptability, efficacy, and skin biophysiology of a cream and cleanser containing lipid complex with shea butter extract versus a ceramide product for eczema
KL Hon, MD, FCCM1; YC Tsang, BSc1; NH Pong, MPhil1; Vivian WY Lee, PharmD2;
NM Luk, FRCP3; CM Chow, FRCPCH1; TF Leung, FRCPCH, FAAAAI1
1 Department of Paediatrics, Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, Hong Kong
2 School of Pharmacy, The Chinese University of Hong Kong, Shatin, Hong Kong
3 Hong Kong Dermatology Foundation, Hong Kong
 Full
paper in PDF
Full
paper in PDF
Corresponding author: Prof KL Hon (ehon@hotmail.com)
Abstract
Objectives: To investigate patient acceptability,
efficacy, and skin biophysiological effects of a
cream/cleanser combination for childhood atopic
dermatitis.
Design: Case series.
Setting: Paediatric dermatology clinic at a university
teaching hospital in Hong Kong.
Patients: Consecutive paediatric patients with
atopic dermatitis who were interested in trying a new
moisturiser were recruited between 1 April 2013 and
31 March 2014. Swabs and cultures from the right
antecubital fossa and the worst eczematous area,
disease severity (SCORing Atopic Dermatitis index),
skin hydration, and transepidermal water loss were
obtained prior to and following 4-week usage of a
cream/cleanser containing lipid complex with shea
butter extract (Ezerra cream; Hoe Pharma, Petaling
Jaya, Malaysia). Global or general acceptability of
treatment was documented as ‘very good’, ‘good’,
‘fair’, or ‘poor’.
Results: A total of 34 patients with atopic dermatitis
were recruited; 74% reported ‘very good’ or ‘good’,
whereas 26% reported ‘fair’ or ‘poor’ general
acceptability of treatment of the Ezerra cream;
and 76% reported ‘very good’ or ‘good’, whereas
24% reported ‘fair’ or ‘poor’ general acceptability
of treatment of the Ezerra cleanser. There were
no intergroup differences in pre-usage clinical
parameters of age, objective SCORing Atopic
Dermatitis index, pruritus, sleep loss, skin hydration,
transepidermal water loss, topical corticosteroid
usage, oral antihistamine usage, or general
acceptability of treatment of the prior emollient.
Following use of the Ezerra cream, mean pruritus
score decreased from 6.7 to 6.0 (P=0.036) and mean
Children’s Dermatology Life Quality Index improved
from 10.0 to 8.0 (P=0.021) in the ‘very good’/‘good’
group. There were no statistically significant
differences in the acceptability of wash (P=0.526)
and emollients (P=0.537) with pre-trial products.
When compared with the data of another ceramide-precursor
moisturiser in a previous study, there was
no statistical difference in efficacy and acceptability
between the two products.
Conclusions: The trial cream was acceptable in
three quarters of patients with atopic dermatitis.
Patients who accepted the cream had less pruritus
and improved quality of life than the non-accepting
patients following its usage. The cream containing
shea butter extract did not differ in acceptability or
efficacy from a ceramide-precursor product. Patient
acceptability is an important factor for treatment
efficacy. There is a general lack of published
clinical trials to document the efficacy and skin
biophysiological effects of many of the proprietary
moisturisers.
New knowledge added by this
study
- Patient acceptability is an important factor for treatment efficacy.
- There is a general lack of published clinical trials to document the efficacy and skin biophysiological effects of many of the proprietary moisturisers.
Introduction
Eczema or atopic dermatitis (AD) is a chronically
relapsing dermatosis associated with atopy, and
characterised by reduced skin hydration (SH),
impaired skin integrity (transepidermal water
loss [TEWL]), and poor quality of life as a result
of deficient ceramides in the epidermis.1 Regular
application of a moisturiser is the key step in
its management. Moisturiser therapy for AD is
significantly complicated by the diversity of disease
manifestations and by a variety of complex immune
abnormalities.1 Filaggrin (filament-aggregating
protein) and related moisturising factors have an
important function in epidermal differentiation
and barrier function, and null mutations within the
filaggrin gene are major risk factors for developing
AD.2 3 4 5 6 Ceramides and related lipid products are also important components in skin barrier
function.7 Recent advances in the understanding
of the pathophysiological process of AD have led
to the production of new moisturisers targeted at
correcting the reduced amount of ceramides and
natural moisturising factors in the stratum corneum
with ceramides, pseudoceramides, or natural
moisturising factors.7 Many proprietary products
claim to have these ingredients, but have no or
limited studies to document their clinical efficacy.
Our group previously tested a number of these
commercial products and found patient preference
and acceptability may influence outcomes of
topical treatment independent of the ingredients
in these products.8 The purposes of this study were
to investigate patient acceptability of a cream/cleanser combination containing lipid complex and
shea butter extract with claimed antihistaminergic
properties, and evaluate its efficacy in improving
the clinical and biophysiological properties of the
skin in AD patients. A MEDLINE search was also
performed to evaluate whether evidence of efficacy
of many of the proprietary moisturisers exists.
Methods
Consecutive patients with AD who were interested
in trying a new moisturiser were recruited from
the paediatric dermatology clinic at a university
teaching hospital in Hong Kong. Diagnosis of AD
was based on the UK working group criteria.9 In
this study, SH and TEWL in the right forearm (2 cm
below the antecubital flexure), and disease severity
(SCORing Atopic Dermatitis [SCORAD] index)
were measured. We have previously described our
method of standardising measurements of SH and
TEWL.10 After acclimatisation in the consultation
room with the patient sitting comfortably in a chair
for 20 to 30 minutes, SH (in arbitrary units) and
TEWL (in g/m2/h) were then measured according
to the manufacturer’s instructions with the Mobile
Skin Center MSC 100 equipped with a Corneometer
CM 825 (Courage + Khazaka electronic GmbH,
Cologne, Germany), and a Tewameter TM 210
probe (Courage + Khazaka electronic GmbH).
We documented that a site 2 cm distal to the right
antecubital flexure was optimal for standardisation.
Oozing and infected areas were avoided by moving
the probe slightly sideways.10 The clinical severity of
AD was assessed with the SCORAD index.11 12 The SCORAD index also scores pruritus and sleep loss/disturbance on a scale of 0 to 10 (0 being not affected
and 10 being most severely affected).
Patients were given a liberal supply of a trial
cream containing lipid complex with shea butter
extract for eczema (Ezerra [E]; Hoe Pharma, Petaling
Jaya, Malaysia) and body wash (E, Hoe Pharma). The
moisturiser contained STIMU-TEX AS (Centerchem
Inc, Norwalk [CT], US) and saccharide isomerate. The
wash contained STIMU-TEX AS and Amisoft (Amisoft
Technologies Ltd, Brentwood, UK). The patients
were instructed not to use any other moisturiser
or topical treatment. Use of any medications such
as topical corticosteroid or oral antihistamine was
documented. Patients were encouraged to use the
test moisturiser at least twice daily on the flexures
and areas with eczema. In case the emollient effect
was not satisfactory, they could use their usual
emollient and medications, but the frequency of
such use was to be reported and they must continue
with the E moisturiser. The patients were reviewed
at the end of 4 weeks. Measurements of SCORAD
index, Children’s Dermatology Life Quality Index
(CDLQI), SH, and TEWL were repeated. Patients’
global or general acceptability of treatment (GAT)
was recorded as ‘very good’, ‘good’, ‘fair’, or ‘poor’.8 13 Approval was obtained from the Clinical Research Ethics Committee of the Chinese University of Hong
Kong and written informed consents were obtained
from the guardian and patient.
Continuous data were expressed as mean
and standard deviation. Mann-Whitney U test was
used for intergroup comparison and Wilcoxon
signed rank test for within-group comparison as a
small number of patients was included. Categorical
data were presented in counts. Chi squared test
or Fisher’s exact test where appropriate was used
to compare intergroup categorical data, while
McNemar’s test was used to compare within-group
categorical data. Fisher’s exact test was
used to determine the GAT of previously used
proprietary products and E moisturiser and wash.
All comparisons were two-tailed, and P values of
<0.05 were considered to be statistically significant.
The results were also compared with the data for an
emollient (C) containing ceramide-precursor lipids
and moisturising factors (n=24).14
Results
Between 1 April 2013 and 31 March 2014, 34 patients
(56% boys; mean [± standard deviation] age, 12.1 ± 4.4 years) with AD were recruited and treated with applications of a moisturising cream (E). Compliance
was good and patients generally managed to use
the moisturiser daily. Among the patients, 74%
reported ‘very good’ or ‘good’ acceptability, whereas
26% reported ‘fair’ or ‘poor’ acceptability of the
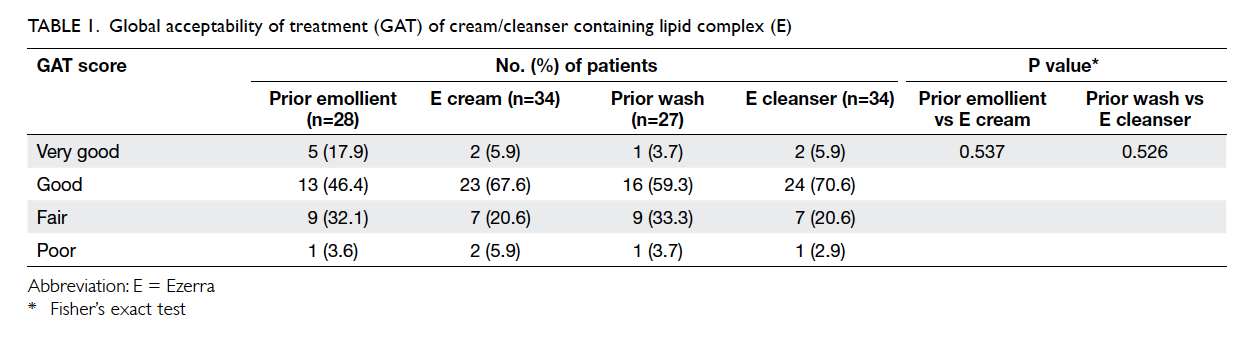
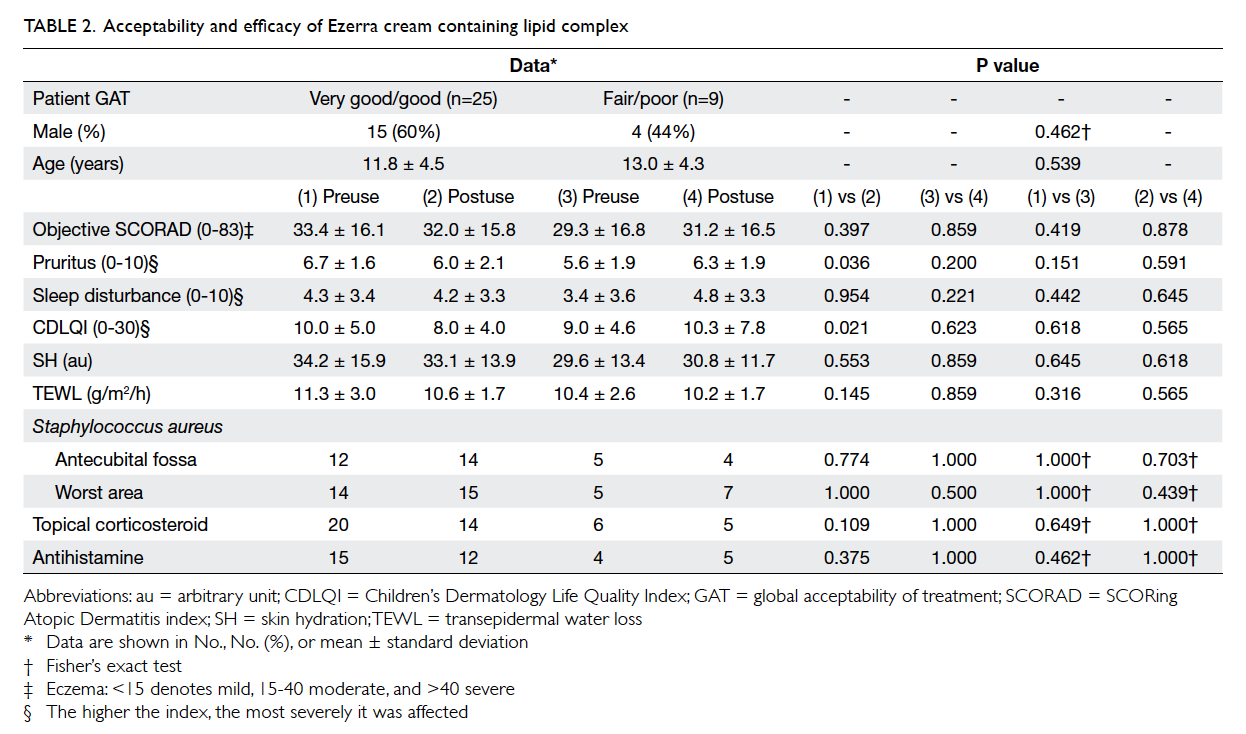
moisturiser (Tables 1 and 2).
There were no intergroup differences in pre-usage
clinical parameters of age, objective SCORAD
index, pruritus, sleep loss, SH, TEWL, topical
corticosteroid usage, oral antihistamine usage, or
GAT of prior emollient (Table 2). Following
use of the E cream, pruritus score and CDLQI were lower
in the ‘very good’/‘good’ group than in the ‘fair’/‘poor’
group. Mean pruritus score decreased from 6.7 to 6.0
(P=0.036) and mean CDLQI improved from 10.0 to
8.0 (P=0.021) in the ‘very good’/‘good’ group (Table 2).
When analysed for the association of the
rating of acceptability, the acceptability of E
cleanser (P=0.526) and E cream (P=0.537) was not
significantly associated with their respective pre-trial
products (Table 1). Patients who preferred the trial moisturiser or wash might or might not have
come from the group of poor/fair acceptability of
their prior emollient or wash, and vice versa. Prior
products included emulsifying ointment and various
other proprietary products.
When compared historically with another
product containing ceramide-precursor lipids (C)
that we tested in a previous report,14 the present shea
butter extract–containing cream showed similar
efficacy and acceptability (Table 3). It appears that
ceramide does not confer superiority in terms of
acceptability and clinical efficacy.
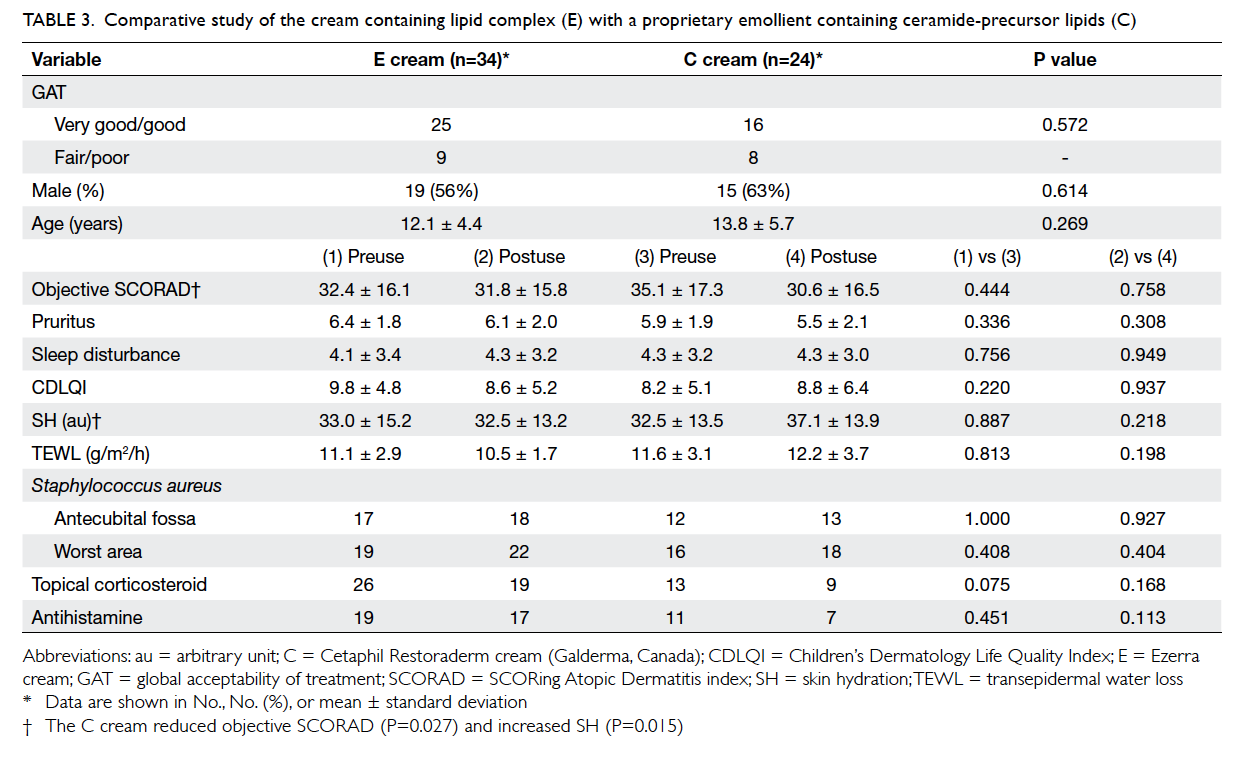
Table 3. Comparative study of the cream containing lipid complex (E) with a proprietary emollient containing ceramide-precursor lipids (C)
A MEDLINE search was performed on selected
common proprietary moisturisers/emollients
for eczema using the following search terms in
combinations: “eczema” OR “atopic dermatitis”,
AND “emollient” OR “moisturizer” OR “barrier” OR
“barrier repair” OR “natural moisturizing factor”
OR “ceramide” OR “pseudoceramide”. We selected
literature mainly from the past 10 years, but did not
exclude commonly referenced and highly cited older
articles. We included and described all randomised
trials, case series, and bench studies in barrier repair
therapy for eczema, with limits activated (Humans,
Clinical Trial, Meta-Analysis, Randomized
Controlled Trial, English, published in the past 10
years). Editorials, letters, practice guidelines, reviews,
and animal studies were excluded. In addition, the
bibliographies of the retrieved articles and our own
research database were also hand searched. As of
23 April 2014, 18 articles were obtained (Table
413 27 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48). The common proprietary moisturisers
were included. The publications generally provided
limited evidence of efficacy and biophysical effects
(such as SH and TEWL), but virtually no data on
patient acceptability and effects on Staphylococcus
aureus colonisation.
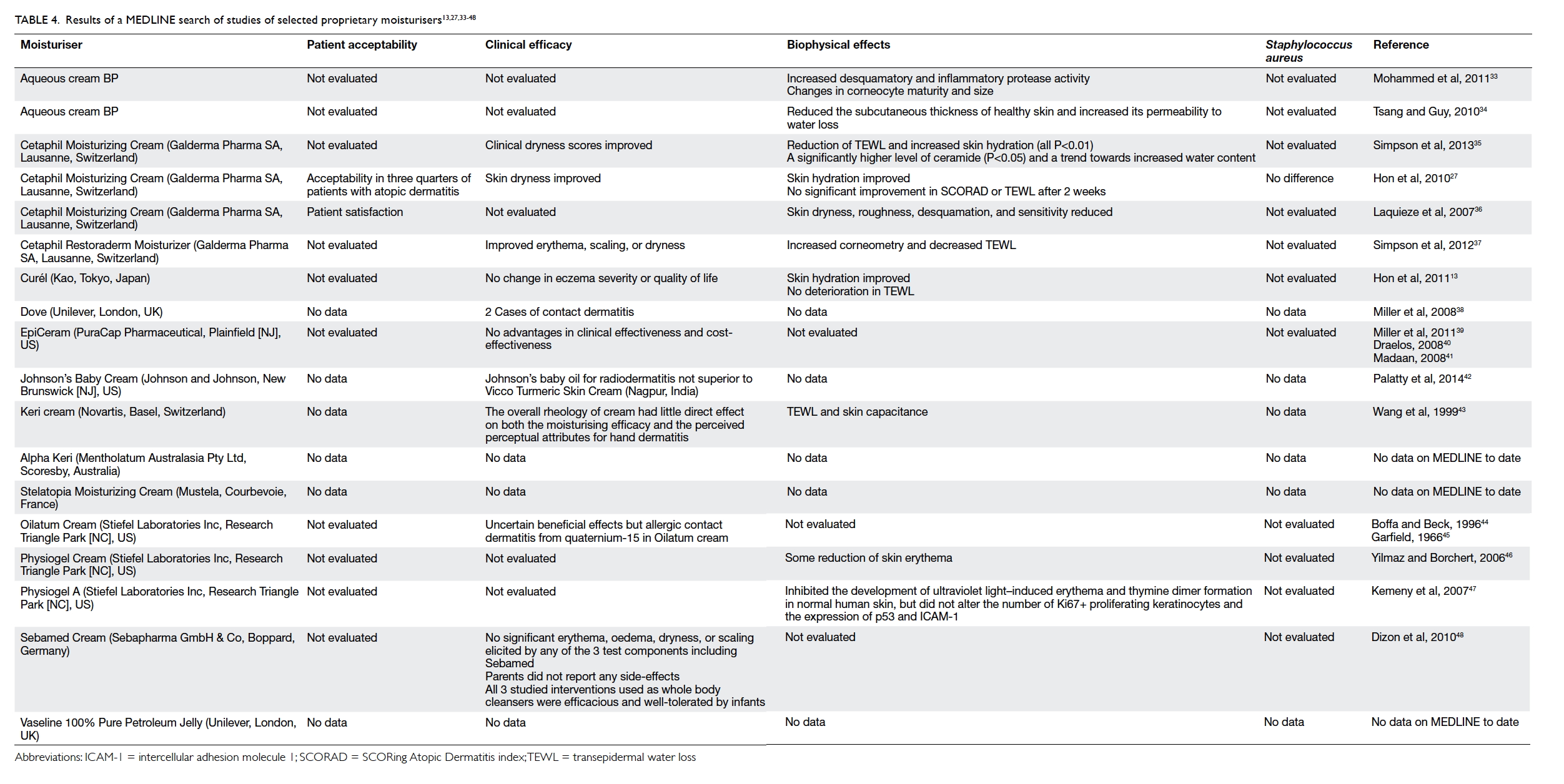
Table 4. Results of a MEDLINE search of studies of selected proprietary moisturisers13 27 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48
Discussion
Atopic dermatitis is a chronically relapsing
dermatosis characterised by pruritus, skin dryness,
inflammation, secondary bacterial infection by S
aureus, and poor quality of life.1 15 16 17 The stratum
corneum normally consists of fully differentiated
corneocytes surrounded by natural moisturising
factor and a lipid-rich matrix containing cholesterol,
free fatty acids, and ceramide. In AD, metabolism
of lipid and filaggrin protein is abnormal, causing
a deficiency of ceramide and natural moisturising
factors and impairment of epidermal barrier
function that leads to increased TEWL and
abnormal skin integrity.1 4 7 18 19 20 21 Moisturisers form
the first-line therapy as maintenance and therapeutic
management in childhood-onset AD.1 22 23 Hydration
of the skin helps to improve dryness, reduce pruritus,
and restore disturbed barrier function. Bathing
without the use of moisturiser may compromise
SH.24 25 26
In this study, we explored clinical efficacy
and acceptability of a proprietary moisturiser (E)
containing shea butter extract. The cream was
acceptable as ‘very good’ or ‘good’ in about three quarters
of patients with AD who tried the moisturiser,
and ameliorated their pruritus and improved their
quality of life.
Compliance or adherence to usage of the
moisturising cream was reflected by the GAT and
reported frequency of usage (times per day).27 We
did not calculate the amount of moisturising cream
used because many parents/patients have discarded
the tubes or failed to bring them back for weighing
in previous trials. Topical steroid usage is also an
important confounding factor in this study. We
standardise treatment for all our patients by not
changing their existing topical steroid (mometasone
furoate) and other medications (ie oral antihistamine,
topical immunomodulant, and Chinese medicine).
In previous studies, we found that documentation
of the exact amount of steroid usage (weight or
frequency of usage) was difficult for similar reasons
as those for moisturisers.28 Most parents are still
concerned about topical steroid usage and tend to
use the minimal amount of steroid as far as possible.29
Alternative explanations for the modest
within-group changes in pruritus and CDLQI
(Table 2) include regression to the mean, detection
bias, or confounding by co-treatment with topical
corticosteroid or usual emollients. Our study did not
demonstrate any reduction in clinical severity or S
aureus colonisation. When compared historically
with another product (C) containing ceramide-precursor lipids that we tested in a previous report,14
although different patients were involved and the E
or C products were not received by patients in the
same period, the present E cream showed similar
efficacy and acceptability with the use of a similar
study protocol as the previous study. It appears that
specific ceramide-precursor lipids do not confer
superiority in terms of acceptability and clinical
efficacy.
Regarding intra-group comparisons, the C
cream reduced objective SCORAD index (P=0.027)
and increased SH (P=0.015), whereas the E cream
reduced pruritus and improved CDLQI only in the
‘very good’/‘good’ group (Table 3). Regarding intergroup
comparisons, overall there were no significant
differences between the pretreatment and post-treatment
parameters for the two moisturisers. We
note that in the subgroup analysis, pruritus and
CDLQI could be the possible contributing factors
for the acceptability in the ‘very good’/‘good’
group for the E cream.
Many proprietary emollients/moisturisers are
available in the market.7 22 30 31 Despite claims about
their efficacy, little evidence has demonstrated the
short- or long-term usefulness of many of these
proprietary products. Ceramides, pseudoceramides,
or filaggrin protein products have been studied
and added to commercial moisturisers to mimic
natural skin lipids and moisturising factors.32
Anxious parents often consult their physicians for
recommendation as to the choice of an ideal or perfect
moisturiser for their child with AD. Physicians need
to have some evidence-based understanding about
these moisturisers in order to address issues raised
by the parents. We performed a MEDLINE search
and found that only a few of these products have
published clinical data (Table 413 27 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48). The majority either do not have patient acceptability or clinical
efficacy data in the scientific literature. The efficacy
of ceramides and natural moisturiser factors is
generally not scientifically documented. Larger-scale,
properly conducted randomised controlled trials
with recruitment of more study participants may
validate subtle differences in clinical efficacy between
different emollients. It is likely that there will be
similar outcomes in efficacy if the tested emollient is
compared with any other traditional emollient such
as aqueous cream or Vaseline (Unilever, London,
England). Commercial pharmaceutical companies
are often unwilling to supply free samples of their
product to compare with an inexpensive product,
even if more validated and conclusive results
may be obtained by increasing the sample size in
clinical trials. That is perhaps why there are so few
comparative clinical studies in the medical literature.
In a wider context, AD is a complex multifactorial
atopic disease. Monotherapy targeting merely at
replacement of ceramides, pseudoceramides, or
filaggrin degradation products at the epidermis is
often suboptimal. In particular, colonisation with S
aureus must be adequately treated before emollient
treatment can be optimised.17
The major hindrance to the efficacy of a
moisturiser is the patient’s perception as to what
an ideal moisturiser should be.8 Indeed, it is often
not the product, but the patient’s acceptability that
determines whether it may be used consistently.
Therefore, the physician caring for a patient with AD
must educate and guide the parents and the patient
to choose the most acceptable formulation to ensure
optimal compliance.
This open-label series confirms our previous
experience in emollient research. First, patient
acceptance of the strengths, types, and formulations
of any novel products need to be studied, preferably
in randomised controlled trials. Second, holistic
efficacy studies of all clinical parameters (namely
severity scores, quality-of-life indices, SH, TEWL,
S aureus colonisation, and patient acceptance) must
be included. Third, as AD is not a simple epidermal
skin disease but rather a complex atopic disease,
emollient alone is bound to be suboptimal in efficacy.
Conclusions
Well-designed, large-scale, randomised, placebo-controlled
trials to document therapeutic effects on
disease severity, skin biophysiological parameters,
quality of life, and patient acceptability are needed.
Patient’s acceptability of a certain product is pivotal
for compliance and clinical outcome. Only few of
the many proprietary moisturisers for AD have
undergone clinical trials to evaluate clinical efficacy
and patient acceptability.
Declaration
Drs KL Hon and TF Leung have performed research
on eczema therapeutics, and written about the
subject matter of filaggrin, ceramides, and emollients.
Acknowledgements
We thank Hoe Pharma in Hong Kong for freely
supplying the studied materials. The company,
however, was not involved in the design or analysis of
the research data. The products used in the present
study could be examples of other similar products
containing shea butter.
References
1. Leung AK, Hon KL, Robson WL. Atopic dermatitis. Adv
Pediatr 2007;54:241-73. Crossref
2. Sandilands A, Terron-Kwiatkowski A, Hull PR, et al.
Comprehensive analysis of the gene encoding filaggrin
uncovers prevalent and rare mutations in ichthyosis
vulgaris and atopic eczema. Nat Genet 2007;39:650-4. Crossref
3. Sandilands A, Smith FJ, Irvine AD, McLean WH. Filaggrin’s
fuller figure: a glimpse into the genetic architecture of
atopic dermatitis. J Invest Dermatol 2007;127:1282-4. Crossref
4. Enomoto H, Hirata K, Otsuka K, et al. Filaggrin null
mutations are associated with atopic dermatitis and
elevated levels of IgE in the Japanese population: a family
and case-control study. J Hum Genet 2008;53:615-21. Crossref
5. Chamlin SL, Kao J, Frieden IJ, et al. Ceramide-dominant
barrier repair lipids alleviate childhood atopic dermatitis:
changes in barrier function provide a sensitive indicator of
disease activity. J Am Acad Dermatol 2002;47:198-208. Crossref
6. Maintz L, Novak N. Getting more and more complex:
the pathophysiology of atopic eczema. Eur J Dermatol
2007;17:267-83.
7. Hon KL, Leung AK. Use of ceramides and related products
for childhood-onset eczema. Recent Pat Inflamm Allergy
Drug Discov 2013;7:12-9. Crossref
8. Hon KL, Wang SS, Pong NH, Leung TF. The ideal
moisturizer: a survey of parental expectations and practice
in childhood-onset eczema. J Dermatolog Treat 2013;24:7-12. Crossref
9. Williams HC, Burney PG, Pembroke AC, Hay RJ. The U.K.
Working Party’s Diagnostic Criteria for Atopic Dermatitis.
III. Independent hospital validation. Br J Dermatol
1994;131:406-16. Crossref
10. Hon KL, Wong KY, Leung TF, Chow CM, Ng PC.
Comparison of skin hydration evaluation sites and
correlations among skin hydration, transepidermal water
loss, SCORAD index, Nottingham Eczema Severity Score,
and quality of life in patients with atopic dermatitis. Am J
Clin Dermatol 2008;9:45-50. Crossref
11. Severity scoring of atopic dermatitis: the SCORAD index.
Consensus Report of the European Task Force on Atopic
Dermatitis. Dermatology 1993;186:23-31. Crossref
12. Kunz B, Oranje AP, Labreze L, Stalder JF, Ring J, Taieb A.
Clinical validation and guidelines for the SCORAD index:
consensus report of the European Task Force on Atopic
Dermatitis. Dermatology 1997;195:10-9. Crossref
13. Hon KL, Wang SS, Lau Z, et al. Pseudoceramide for
childhood eczema: does it work? Hong Kong Med J 2011;17:132-6.
14. Hon KL, Pong NH, Wang SS, Lee VW, Luk NM, Leung
TF. Acceptability and efficacy of an emollient containing
ceramide-precursor lipids and moisturizing factors
for atopic dermatitis in pediatric patients. Drugs R D
2013;13:37-42. Crossref
15. Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid
QA. New insights into atopic dermatitis. J Clin Invest
2004;113:651-7. Crossref
16. Hon KL, Lam MC, Leung TF, et al. Clinical features
associated with nasal Staphylococcus aureus colonisation
in Chinese children with moderate-to-severe atopic
dermatitis. Ann Acad Med Singapore 2005;34:602-5.
17. Hon KL, Wang SS, Lee KK, Lee VW, Fan LT, Ip M.
Combined antibiotic/corticosteroid cream in the empirical
treatment of moderate to severe eczema: friend or foe? J
Drugs Dermatol 2012;11:861-4.
18. Hanifin JM, Rajka RG. Diagnostic features of atopic
dermatitis. Acta Derm Venereol (Stockh) 1980;2:44-7.
19. Hanifin JM. Atopic dermatitis. J Am Acad Dermatol
1982;6:1-13. Crossref
20. Candi E, Schmidt R, Melino G. The cornified envelope:
a model of cell death in the skin. Nat Rev Mol Cell Biol
2005;6:328-40. Crossref
21. Leung DY, Nicklas RA, Li JT, et al. Disease management
of atopic dermatitis: an updated practice parameter. Joint
Task Force on Practice Parameters. Ann Allergy Asthma
Immunol 2004;93(3 Suppl 2):S1-21. Crossref
22. Hon KL, Leung AK, Barankin B. Barrier repair therapy
in atopic dermatitis: an overview. Am J Clin Dermatol
2013;14:389-99. Crossref
23. Krakowski AC, Eichenfield LF, Dohil MA. Management
of atopic dermatitis in the pediatric population. Pediatrics
2008;122:812-24. Crossref
24. Lancaster W. Atopic eczema in infants and children.
Community Pract 2009;82:36-7.
25. Tarr A, Iheanacho I. Should we use bath emollients for
atopic eczema? BMJ 2009;339:b4273. Crossref
26. Hon KL, Leung TF, Wong Y, So HK, Li AM, Fok TF. A
survey of bathing and showering practices in children with
atopic eczema. Clin Exp Dermatol 2005;30:351-4. Crossref
27. Hon KL, Ching GK, Leung TF, Choi CY, Lee KK, Ng PC.
Estimating emollient usage in patients with eczema. Clin
Exp Dermatol 2010;35:22-6. Crossref
28. Hon KL, Leung TF, Ng PC, et al. Efficacy and tolerability
of a Chinese herbal medicine concoction for treatment of atopic dermatitis: a randomized, double-blind, placebo-controlled study. Br J Dermatol 2007;157:357-63. Crossref
29. Hon KL, Kam WY, Leung TF, et al. Steroid fears in children
with eczema. Acta Paediatr 2006;95:1451-5. Crossref
30. Roos TC, Geuer S, Roos S, Brost H. Recent advances
in treatment strategies for atopic dermatitis. Drugs 2004;64:2639-66. Crossref
31. Baumer JH. Atopic eczema in children, NICE. Arch Dis
Child Educ Pract Ed 2008;93:93-7.
32. Park KY, Kim DH, Jeong MS, Li K, Seo SJ. Changes of
antimicrobial peptides and transepidermal water loss after
topical application of tacrolimus and ceramide-dominant
emollient in patients with atopic dermatitis. J Korean Med
Sci 2010;25:766-71. Crossref
33. Mohammed D, Matts PJ, Hadgraft J, Lane ME. Influence of Aqueous Cream BP on corneocyte size, maturity, skin protease activity, protein content and transepidermal water loss. Br J Dermatol 2011;164:1304-10. Crossref
34. Tsang M, Guy RH. Effect of Aqueous Cream BP on human
stratum corneum in vivo. Br J Dermatol 2010;163:954-8. Crossref
35. Simpson E, Böhling A, Bielfeldt S, Bosc C, Kerrouche N.
Improvement of skin barrier function in atopic dermatitis patients with a new moisturizer containing a ceramide
precursor. J Dermatolog Treat 2013;24:122-5. Crossref
36. Laquieze S, Czernielewski J, Baltas E. Beneficial use of Cetaphil moisturizing cream as part of a daily skin care regimen for individuals with rosacea. J Dermatolog Treat
2007;18:158-62. Crossref
37. Simpson E, Trookman NS, Rizer RL, et al. Safety and tolerability of a body wash and moisturizer when applied to infants and toddlers with a history of atopic dermatitis:
results from an open-label study. Pediatr Dermatol 2012;29:590-7. Crossref
38. Miller MA, Borys D, Riggins M, Masneri DC, Levsky ME. Two cases of contact dermatitis resulting from use of body wash as a skin moisturizer. Am J Emerg Med 2008;26:246.e1-2.
39. Miller DW, Koch SB, Yentzer BA, et al. An over-the-counter
moisturizer is as clinically effective as, and
more cost-effective than, prescription barrier creams
in the treatment of children with mild-to-moderate
atopic dermatitis: a randomized, controlled trial. J Drugs
Dermatol 2011;10:531-7.
40. Draelos ZD. The effect of ceramide-containing skin care
products on eczema resolution duration. Cutis 2008;81:87-91.
41. Madaan A. Epiceram for the treatment of atopic dermatitis.
Drugs Today (Barc) 2008;44:751-5. Crossref
42. Palatty PL, Azmidah A, Rao S, et al. Topical application
of sandal wood oil and turmeric based cream prevents radiodermatitis in head and neck cancer patients
undergoing external beam radiotherapy: a pilot study. Br J Radiol 2014;87:20130490. Crossref
43. Wang S, Kislalioglu MS, Breuer M. The effect of rheological
properties of experimental moisturizing creams/lotions on
their efficacy and perceptual attributes. Int J Cosmet Sci
1999;21:167-88. Crossref
44. Boffa MJ, Beck MH. Allergic contact dermatitis from
quaternium 15 in Oilatum cream. Contact Dermatitis
1996;35:45-6. Crossref
45. Garfield SS. The beneficial effects of oilatum cream on
hyperhydrosis. J Am Podiatry Assoc 1966;56:22-4. Crossref
46. Yilmaz E, Borchert HH. Effect of lipid-containing, positively
charged nanoemulsions on skin hydration, elasticity and
erythema—an in vivo study. Int J Pharm 2006;307:232-8. Crossref
47. Kemeny L, Koreck A, Kis K, et al. Endogenous phospholipid
metabolite containing topical product inhibits ultraviolet light–induced inflammation and DNA damage in human
skin. Skin Pharmacol Physiol 2007;20:155-61. Crossref
48. Dizon MV, Galzote C, Estanislao R, Mathew N, Sarkar
R. Tolerance of baby cleansers in infants: a randomized controlled trial. Indian Pediatr 2010;47:959-63. Crossref