DOI: 10.12809/hkmj144230
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Isolated spinal artery aneurysm: a rare culprit of subarachnoid haemorrhage
Tony HT Sung, MB, ChB, FRCR;
Warren KW Leung, FHKCR, FHKAM (Radiology);
Bill MH Lai, MB, BS, FRCR;
Jennifer LS Khoo, FHKCR, FHKAM (Radiology)
Department of Radiology, Pamela Youde Nethersole Eastern Hospital, Chai Wan, Hong Kong
Corresponding author: Dr Tony HT Sung (sht557@ha.org.hk), (tttony100@gmail.com)
Abstract
Isolated spinal artery aneurysm is a rare lesion
which could be accountable for spontaneous spinal
subarachnoid haemorrhage. We describe the case
of a 74-year-old man presenting with sudden
onset of chest pain radiating to the neck and
back, with subsequent headache and confusion.
Initial computed tomography aortogram revealed
incidental finding of subtle acute spinal subarachnoid
haemorrhage. A set of computed tomography scans
of the brain showed further acute intracranial
subarachnoid haemorrhage with posterior
predominance, small amount of intraventricular
haemorrhage, and absence of intracranial vascular
lesions. Subsequent magnetic resonance imaging
demonstrated a thrombosed intradural spinal
aneurysm with surrounding sentinel clot, which was
trapped and excised during surgical exploration.
High level of clinical alertness is required in order
not to miss this rare but detrimental entity. Its
relevant aetiopathological features and implications
for clinical management are discussed.
Introduction
Aneurysmal subarachnoid haemorrhage (SAH) is
an uncommon but fatal clinical event. The reported
incidence in a recent international population-based
epidemiological study ranged between 2 and 16 per
100 000 population,1 and the mortality rate ranged
from 8% to 67%.2 Among all SAH cases, less than
1% are considered to originate from the spine.3 We
present a case of isolated spinal artery aneurysm
as a rare culprit of spinal and intracranial SAH to
illustrate the diagnostic challenge, its relevant
aetiopathological features, and implications for
clinical management.
Case report
A 74-year-old Chinese man with a history of
hypertension and ischaemic heart disease presented
to the Accident and Emergency Department in
February 2013 for sudden onset of chest pain
radiating to the neck and back. Clinical examination
and electrocardiogram on admission showed no
evidence of myocardial infarction. Initial working
diagnosis of acute aortic dissection was also
excluded with urgent computed tomography (CT)
aortogram. On retrospective analysis, subtle but
definite hyperdensities within the thecal sac at upper
thoracic levels were noted, suggestive of acute spinal
SAH (Fig 1).
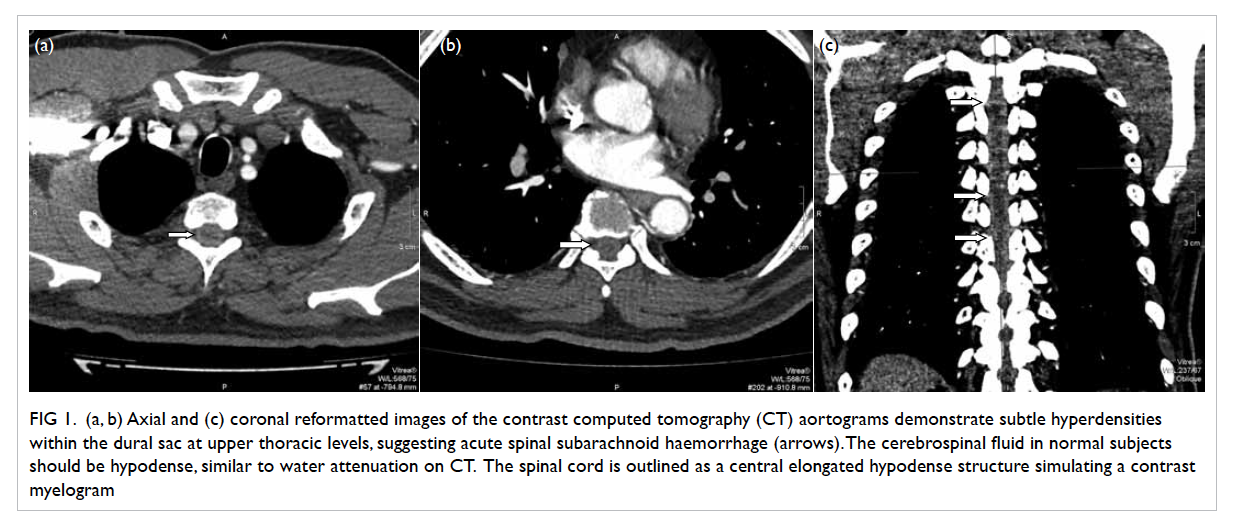
Figure 1. (a, b) Axial and (c) coronal reformatted images of the contrast computed tomography (CT) aortograms demonstrate subtle hyperdensities within the dural sac at upper thoracic levels, suggesting acute spinal subarachnoid haemorrhage (arrows). The cerebrospinal fluid in normal subjects should be hypodense, similar to water attenuation on CT. The spinal cord is outlined as a central elongated hypodense structure simulating a contrast myelogram
The patient developed gradual progressive
headache and confusion requiring intubation on
the fourth day of admission. Plain CT of the brain
at that time revealed further diffuse acute SAH
with predominance over the posterior aspect, as
well as a small amount of acute intraventricular
haemorrhage. No skull vault fracture was observed.
Concurrent CT cerebral arteriogram and venogram
did not demonstrate any aneurysms or venous
sinus thrombosis. Subsequent digital subtraction
angiogram (DSA) of cerebral arteries performed on
the next day was unremarkable.
In view of posterior predominance of the
intracranial SAH with negative cerebral angiograms,
an urgent magnetic resonance imaging (MRI) of the
brain and cervical spine (Fig 2) was performed for
suspected subtle intracranial pathology and a spinal
origin of SAH (observed rarely). A lobulated 8-mm
intradural, extramedullary lesion with internal
hypointense signal was seen at the T1/2 level abutting
the lateral surface of the spinal cord. Trace contrast
enhancement was noted within the lesion. Adjacent
hyperintense signal and susceptibility artefacts
within the dural sac were also observed, suggestive of
sentinel clot formation. The spinal cord was displaced
slightly by the lesion with associated mild cord
oedema. Radiological differential diagnosis at this
juncture included cavernoma, largely thrombosed
spinal aneurysm, other exophytic haemorrhagic
intramedullary tumour or slow-flow arteriovenous
malformation (AVM).
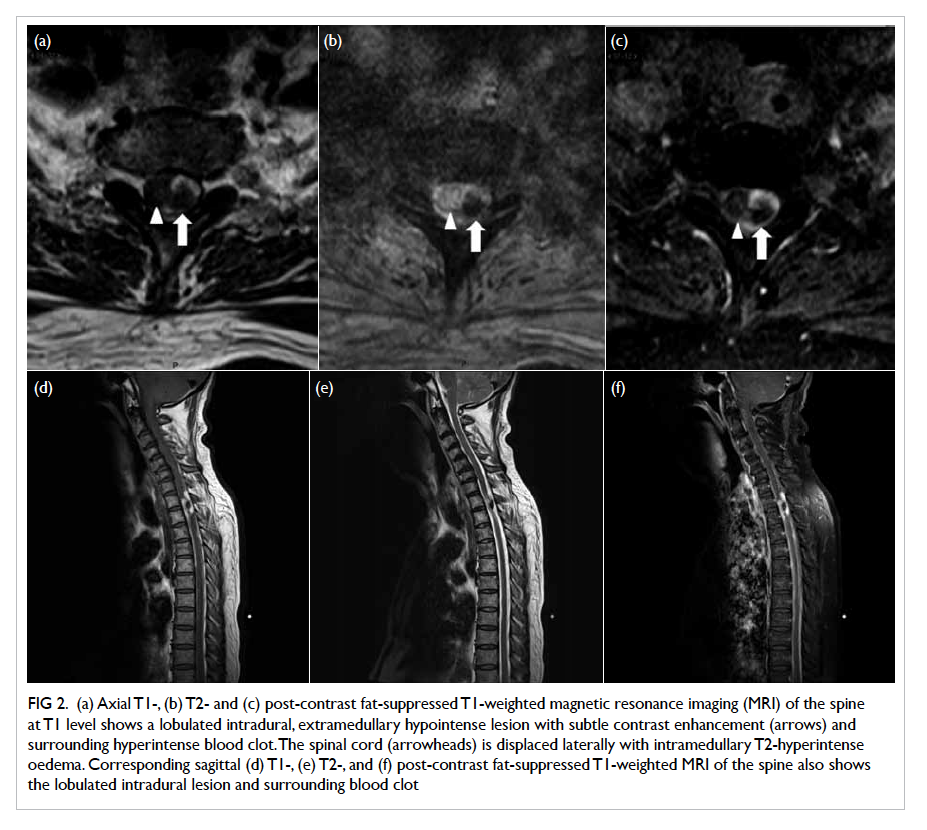
Figure 2. (a) Axial T1-, (b) T2- and (c) post-contrast fat-suppressed T1-weighted magnetic resonance imaging (MRI) of the spine at T1 level shows a lobulated intradural, extramedullary hypointense lesion with subtle contrast enhancement (arrows) and surrounding hyperintense blood clot. The spinal cord (arrowheads) is displaced laterally with intramedullary T2-hyperintense oedema. Corresponding sagittal (d) T1-, (e) T2-, and (f) post-contrast fat-suppressed T1-weighted MRI of the spine also shows the lobulated intradural lesion and surrounding blood clot
Subsequent laminectomy and surgical
exploration were performed for definitive diagnosis,
which revealed a 7-mm saccular aneurysm
surrounded by fibrin and an old haematoma from
C7 to T2 level. The aneurysm arose from and
was incorporated with the radicular artery at T1
level, showing internal partial thrombosis. The
aneurysmal sac was trapped and carefully excised
without jeopardising the rest of the arterial supply
to the cord. The patient had good clinical recovery
in postoperative rehabilitation with no further
neurological complaints.
Discussion
Compared with more common causes of spinal SAH
like AVM, dural arteriovenous fistula (dAVF) and
haemorrhagic spinal cord tumours, spinal artery
aneurysm remains a rare entity with a reported
incidence of less than 1 in 3000 spinal angiograms,
according to a large-scale review by Pia and
Djindjian.4 Ever since the first case report on spinal
aneurysm back in 1930,5 experience from various
studies remains limited mainly to isolated case
reports and case series with small sample sizes.
Spinal aneurysms are distinct from intracranial
counterparts in several ways. First, they occur
mostly along the course of their parent arteries
which have a small calibre and are less affected
by atherosclerosis.6 On the contrary, intracranial
aneurysms are well known for their predilection
for branching points of large-sized arteries which
are more haemodynamically challenged. Second,
spinal aneurysms tend to be small in size, whereas
giant aneurysms are exclusively found intracranially,
especially in patients with connective tissue disease.
Third, many spinal aneurysms are found to be
dissecting aneurysms in histology, which explains
their fusiform shape and lack of a surgical neck.5 This
hinders direct surgical clipping during treatment
planning.
For aetiology, isolated spinal aneurysms, as
in our case, are rare lesions. More commonly, they
are associated with concomitant vascular lesions
or occlusions which recruit the spinal artery as a
collateral route for supplying and increasing local
blood flow. This, in turn, imposes haemodynamic
stress and induces aneurysm formation along the
parent arteries. Reported associations include spinal
cord AVM,7 dAVF,8 aortic coarctation,5 Moyamoya
disease,9 and bilateral vertebral artery occlusion.10
Another group of spinal aneurysms is related to
underlying vasculopathies. Common examples
include collagen vascular disease such as rheumatoid
arthritis,11 mycosis,12 and syphilis.5
The diagnosis of spinal artery aneurysm
could be challenging and delayed due to its rarity.
Most patients present with headache and backache
related to aneurysmal rupture and SAH. Neurological deficits including
paraparesis, quadriplegia, and cord compression
have also been reported.13 As for investigations,
MRI and DSA of the spine are sufficient to
demonstrate the location and vascular anatomy of
a spinal artery aneurysm on most occasions which,
in turn, are valuable for treatment planning by
neurosurgeons. The position of the aneurysm with
respect to the spinal cord determines whether the
anterior approach for transthoracic vertebrectomy
or posterior laminectomy is most appropriate. In a
large-scale literature review by Geibprasert et al,14
32 spinal artery aneurysms were studied, in which
the majority (62.5%) arose from the anterior spinal
artery. Origin from the radicular artery, as in our
case, was scarcely seen (n=2). The authors also
observed that posterior spinal artery aneurysms
were predominantly isolated dissecting aneurysms.
On the contrary, those from the anterior spinal
artery are more diverse in aetiology, eg, related to
candidiasis and connective tissue disease, which
implies a non-surgical management approach with
medical treatment for the underlying disorder. Even
for isolated dissecting aneurysms from the anterior
axis, surgical ligation or endovascular embolisation
is still limited by increased risk of postoperative
complications including spinal cord infarction due to
possible compromise of the dominant arterial supply
to the cord. Options of surgical approach include
resection and wrapping of the aneurysmal sac, with
possible microvascular reconstruction in individual
cases. Rare cases of spontaneous complete healing
of the dissecting aneurysm have been reported,12 but
prompt treatment should not be delayed due to the
associated severe complications.
Conclusion
Spinal artery aneurysms are rare culprits of SAH, with different
morphological features of their intracranial
counterparts. Associations with concurrent
vascular lesions and vasculopathy are frequent,
with a minority of cases being isolated in aetiology.
Magnetic resonance imaging and DSA of the spine
show their merit in delineating the location and
vascular anatomy of a spinal artery aneurysm,
which are key determinants in management
planning. Surgical treatment should be prompt and
performed cautiously in view of possible substantial
neurological deficit when the arterial supply of the
cord is jeopardised.
References
1. Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag
V. Worldwide stroke incidence and early case fatality
reported in 56 population-based studies: a systematic
review. Lancet Neurol 2009;8:355-69. Crossref
2. Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij
NK, Rinkel GJ. Changes in case fatality of aneurysmal
subarachnoid haemorrhage over time, according to age,
sex, and region: a meta-analysis. Lancet Neurol 2009;8:635-42. Crossref
3. van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage.
Lancet 2007;369:306-18. Crossref
4. Pia HW, Djindjian R. Spinal angiomas: advances in
diagnosis and therapy. Berlin: Springer Verlag; 1987.
5. Rengachary SS, Duke DA, Tsai FY, Kragel PJ. Spinal arterial
aneurysm: case report. Neurosurgery 1993;33:125-9;
discussion 129-30. Crossref
6. Leech PJ, Stokes BA, ApSimon T, Harper C. Unruptured
aneurysm of the anterior spinal artery presenting as
paraparesis. Case report. J Neurosurg 1976;45:331-3. Crossref
7. Konan AV, Raymond J, Roy D. Transarterial embolization
of aneurysms associated with spinal cord arteriovenous
malformations. Report of four cases. J Neurosurg 1999;90(1 Suppl):148-54.
8. Malek AM, Halbach VV, Phatouros CC, et al. Spinal dural
arteriovenous fistula with an associated feeding artery
aneurysm: case report. Neurosurgery 1999;44:877-80. Crossref
9. Walz DM, Woldenberg RF, Setton A. Pseudoaneurysm of
the anterior spinal artery in a patient with Moyamoya: an
unusual cause of subarachnoid hemorrhage. AJNR Am J
Neuroradiol 2006;27:1576-8.
10. Kawamura S, Yoshida T, Nonoyama Y, Yamada M, Suzuki
A, Yasui N. Ruptured anterior spinal artery aneurysm: a
case report. Surg Neurol 1999;51:608-12. Crossref
11. Toyota S, Wakayama A, Fujimoto Y, Sugiura S, Yoshimine
T. Dissecting aneurysm of the radiculomedullary artery
originating from extracranial vertebral artery dissection
in a patient with rheumatoid cervical spine disease: an
unusual cause of subarachnoid hemorrhage. Case report.
J Neurosurg Spine 2007;7:660-3. Crossref
12. Berlis A, Scheufler KM, Schmahl C, Rauer S, Götz F,
Schumacher M. Solitary spinal artery aneurysms as a
rare source of spinal subarachnoid hemorrhage: potential
etiology and treatment strategy. ANJR Am J Neuroradiol
2005;26:405-10.
13. Yahiro T, Hirakawa K, Iwaasa M, Tsugu H, Fukushima
T, Utsunomiya H. Pseudoaneurysm of the thoracic
radiculomedullary artery with subarachnoid hemorrhage.
Case report. J Neurosurg 2004;100(3 Suppl Spine):312-5.
14. Geibprasert S, Krings T, Apitzsch J, Reinges MH, Nolte
KW, Hans FJ. Subarachnoid hemorrhage following
posterior spinal artery aneurysm. A case report and review
of the literature. Interv Neuroradiol 2010;16:183-90.

