Hong Kong Med J 2015 Apr;21(2):107–13 | Epub 27 Feb 2015
DOI: 10.12809/hkmj144389
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Duplex sonography for detection of deep vein thrombosis of upper extremities: a 13-year
experience
Amy SY Chung, MSc, MHKCRRT1;
WH Luk, FRCR, FHKAM (Radiology)2;
Adrian XN Lo, FRCR, FHKAM (Radiology)3;
CF Lo, PDDR1
1Department of Radiology, United Christian Hospital, Kwun Tong, Hong Kong
2Department of Radiology, Princess Margaret Hospital, Laichikok, Hong Kong
3Department of Radiology, Hong Kong Adventist Hospital, 40 Stubbs Road, Hong Kong
Corresponding author: Dr Amy SY Chung (chungsya@gmail.com)
Abstract
Objectives: To determine the prevalence and
characteristics of sonographically evident upper-extremity
deep vein thrombosis in symptomatic
Chinese patients and identify its associated risk
factors.
Design: Case series.
Setting: Regional hospital, Hong Kong.
Patients: Data on patients undergoing upper-extremity
venous sonography examinations during
a 13-year period from November 1999 to October
2012 were retrieved. Variables including age, sex,
history of smoking, history of lower-extremity deep
vein thrombosis, major surgery within 30 days,
immobilisation within 30 days, cancer (history of
malignancy), associated central venous or indwelling
catheter, hypertension, diabetes mellitus, sepsis
within 30 days, and stroke within 30 days were tested
using binary logistic regression to understand the risk
factors for upper-extremity deep vein thrombosis.
Main outcome measures: The presence of upper-extremity
deep vein thrombosis identified.
Results: Overall, 213 patients with upper-extremity
sonography were identified. Of these patients, 29
(13.6%) had upper-extremity deep vein thrombosis.
The proportion of upper-extremity deep vein
thrombosis using initial ultrasound was 0.26% of all
deep vein thrombosis ultrasound requests. Upper
limb swelling was the most common presentation
seen in a total of 206 (96.7%) patients. Smoking
(37.9%), history of cancer (65.5%), and hypertension
(27.6%) were the more prevalent conditions among
patients in the upper-extremity deep vein thrombosis–positive group. No
statistically significant predictor of upper-extremity
deep vein thrombosis was noted if all variables
were included. After backward stepwise logistic
regression, the final model was left with only age
(P=0.119), female gender (P=0.114), and history of malignancy
(P=0.024) as independent variables. History of
malignancy remained predictive of upper-extremity
deep vein thrombosis.
Conclusions: Upper-extremity deep vein thrombosis
is uncommon among symptomatic Chinese
population. The most common sign is swelling and
the major risk factor for upper-extremity deep vein
thrombosis identified in this study is malignancy.
New knowledge added by this
study
- Data suggest that upper-extremity deep vein thrombosis among ethnic Chinese is different from western population.
- Patients with a history of malignancy should be given priority for ultrasound screening of upper-extremity deep vein thrombosis.
Introduction
It has been a long-held notion that in United
Christian Hospital in Hong Kong, requests for
upper-extremity vein sonography to screen for deep
vein thrombosis (DVT) were rare. This may have
been because upper-extremity deep vein thrombosis
(UEDVT) was considered a benign phenomenon
and not an urgent condition. However, UEDVT
potentially carries certain risks like pulmonary
embolism (PE), and leads to morbidity and mortality.
Therefore, understanding the associated risk factors
would help in improving the ability to predict and
prevent the risk of PE.
In the past decade, most of the research
focused on identification and management of lower-extremity
deep vein thrombosis (LEDVT), because
UEDVT was believed to be clinically insignificant
and quite rare, representing less than 2% of DVT.1 A study by Baarslag et al2 in 2004, however, reported
that around half of their patients with UEDVT died
during the follow-up period. More recent studies
have challenged this belief.3 4 5 In 2004, Chan et al6 reported a study comparing Chinese and Caucasian
patients, and showed prevalence of LEDVT was
different between the two populations (9.1%
proximal LEDVT without prophylaxis for Chinese
and 16% proximal LEDVT with prophylaxis for
Caucasians). This suggested that a study to assess the
prevalence of UEDVT in Chinese population needs
to be undertaken.
There are many imaging strategies to aid
diagnosis of UEDVT. When comparing the different
strategies, contrast venograms and computed
tomography (CT) venograms require the injection of
contrast agents and involve radiation. With magnetic
resonance venogram, however, no radiation is
involved and can be performed without contrast
injection. Unfortunately, the use of magnetic
resonance venogram is limited by its high cost
and inconvenience associated with the procedure.
On the other hand, colour duplex sonography is
relatively cheap and more easily available. Colour
duplex sonography provides excellent sensitivity and
specificity as shown in a study by Köksoy et al7 in
which the sensitivity and specificity were 94% and
96%, respectively. According to these authors, the
downside is that this technique cannot completely
exclude the presence of thrombus in axillary,
subclavian, superior vena cava, or brachiocephalic
vessels.7 The presence of UEDVT may only be inferred
from secondary signs such as absence of respiratory
variation and cardiac plasticity.8 In view of its safety
and cost-effectiveness, duplex sonography is usually
preferred as the first-line imaging technique in the
evaluation of UEDVT.
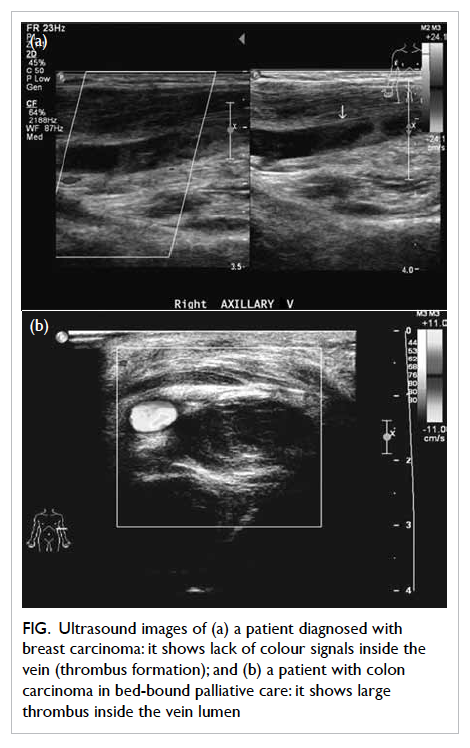
Figure. Ultrasound images of (a) a patient diagnosed with breast carcinoma: it shows lack of colour signals inside the vein (thrombus formation); and (b) a patient with colon carcinoma in bed-bound palliative care: it shows large thrombus inside the vein lumen
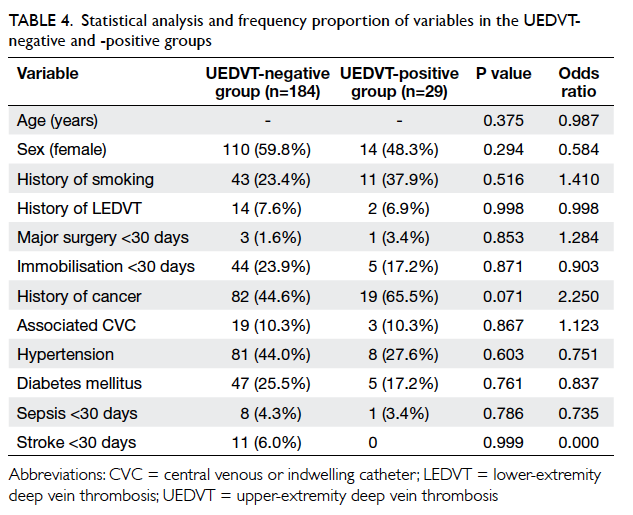
Table 4. Statistical analysis and frequency proportion of variables in the UEDVT-negative and -positive groups
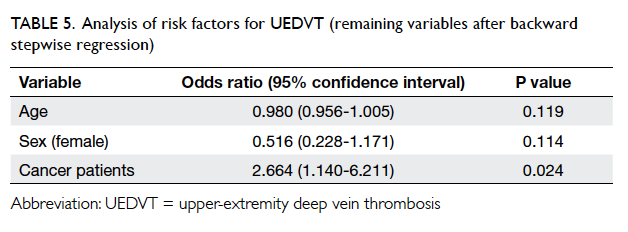
Table 5. Analysis of risk factors for UEDVT (remaining variables after backward stepwise regression)
The aims of this study were to determine the
prevalence and characteristics of sonographically
evident UEDVT in symptomatic Chinese patients
and identify the associated risk factors.
Methods
Methodology
A retrospective study was conducted in a regional
hospital in a district where the socio-economic status
was similar to the rest of the population in Hong
Kong.9 The study sample was comprised of patients
undergoing an initial duplex sonography of the
upper extremity for suspicion of UEDVT during the
period November 1999 to October 2012. The study
began with an initial search on the computerised
Radiology Information System of the Hong Kong
Hospital Authority and patients undergoing duplex
sonography of upper- or lower-extremity veins were
identified. From the radiology reports, positive cases
of DVT (both UEDVT and LEDVT) were sourced
using key words “incomplete compressibility”, “non-compressible”,
“incompressible”, “not compressible”,
or “compressibility: (no)”. The search was further
narrowed down to retrieve patients with radiology
reports and images of all upper-extremity vein
sonography using key words in reports like “upper
extremity vein” or “upper limb vein”.
Since the demographic profile of Hong Kong
is mainly ethnic Chinese, our study included only
Chinese patients who underwent initial upper-extremity
sonography for the detection of UEDVT
within the defined period. Studies that were
incomplete for any reason and patients who had a
positive finding of UEDVT from a previous scan were
excluded. Medical record search was performed for
the selected patients through the electronic Patient
Record System.
Data collection and analysis
The medical records were reviewed and data on
patient demographic characteristics, possible risk
factors, and co-morbidities were collected. All
confidential patient data were de-identified and each
patient was assigned a study number before analysis.
Standardised data collection charts were used to
gather information, and details of information
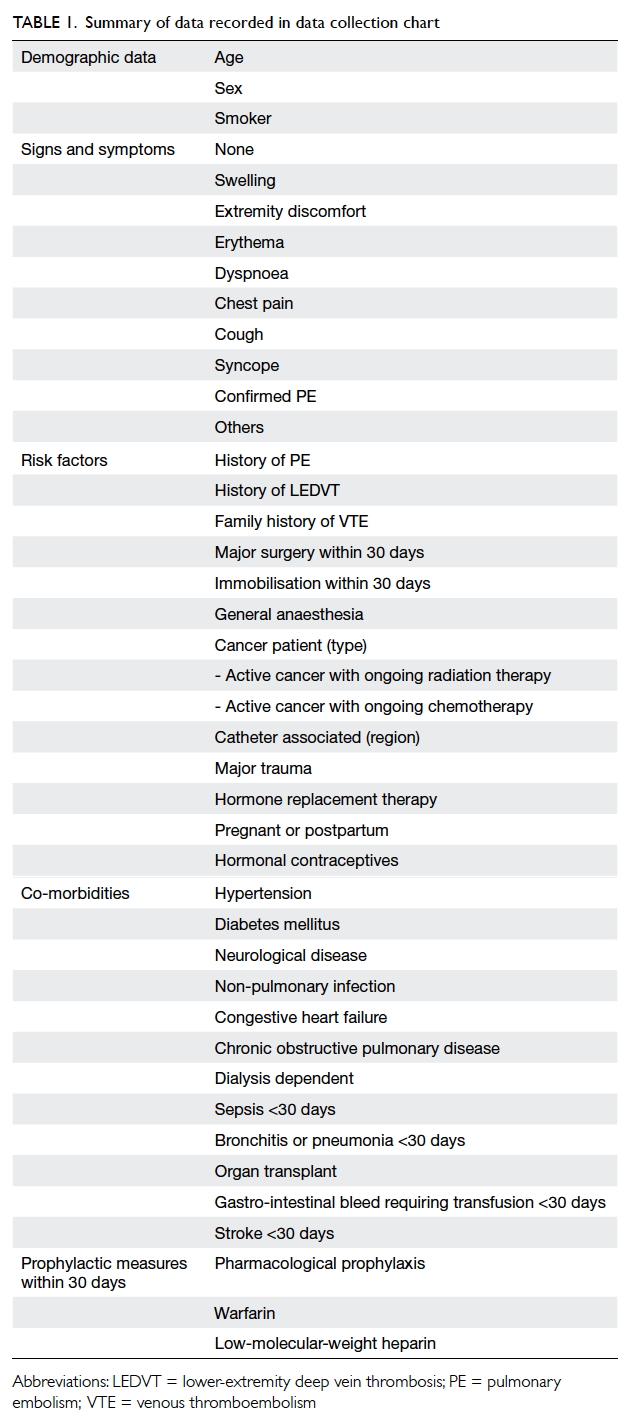
recorded are shown in Table 1.
The radiology reports and images were
reviewed by two experienced, qualified radiologists,
with each radiologist having more than 10 years of
experience. The diagnosis of UEDVT was primarily
based on the incomplete compressibility of the veins
on sonography.3 When Doppler evaluation was
used, absence of flow, lack of respiratory variation,
or cardiac plasticity were used as secondary criteria
for diagnosis.3 Central lines were considered to be
present if mentioned in the sonography report,
in the medical record, or documented on chest
radiography, venography, CT or other imaging
modality within 4 weeks prior to sonography.
The catheter size and catheter material were not
considered or correlated, as such information was
not readily available retrospectively. Patients who
presented with a history of vigorous exercise within
4 weeks of UEDVT were classified as effort-related.10
In contrary, when no forceful activity of limb or
predisposing factor was observed before onset of
symptoms, UEDVT was classified as idiopathic or
spontaneous.9 Any discrepancies in the report or
findings were addressed according to a consensus by
the two reviewing radiologists.
Preliminary data analysis was performed
using descriptive statistics. The mean values of
patient’s age and frequency distribution among both
genders were calculated in the UEDVT-negative and
UEDVT-positive groups. t Test was used to examine
the differences in age between the two groups and
P<0.05 was regarded as significant. The frequency
distributions of signs and symptoms including
swelling, extremity discomfort, erythema, dyspnoea,
chest pain, and cough were compared in the two
groups. The frequency proportions of the variables
in the two groups were calculated. Variables
including age, sex, history of smoking and LEDVT,
major surgery within 30 days, immobilisation within
30 days, cancer (history of malignancy), associated
CVC (central venous or indwelling catheter),
hypertension, diabetes mellitus, sepsis within 30
days, and stroke within 30 days were tested using
binary logistic regression. Using backward stepwise
logistic regression, the variables with the highest
P values were eliminated one by one until all the
remaining variables had P≤0.2, and P<0.05 was
considered significant. The most prevalent risk
factor in the UEDVT-positive group was identified
and compared with data from Caucasian population.
All statistical comparisons were done using the
Statistical Package for the Social Sciences (Windows
version 19.0; SPSS Inc, Chicago [IL], US).
Results
Between November 1999 and October 2012, 11 019
patients had undergone upper- or lower-extremity
vein ultrasound examinations in the hospital. Major
proportion of requests (10 783 patients, 97.9%) was
for lower-extremity vein ultrasound. Ultrasound
diagnosis of DVT (UEDVT and LEDVT) was seen in
822 (7.6%) patients, of which UEDVT was seen in 34
(4.1%) patients and LEDVT in 788 (95.9%) patients
during that period.
Overall there were 236 upper-extremity vein
ultrasound requests, of which 23 patients (5 out of
23 patients had UEDVT) were excluded as they did
not meet the inclusion criteria (an initial upper-extremity
vein sonography). A total of 213 patients
were included in the study sample; UEDVT was
diagnosed in 29 (13.6%) of the study sample (Fig).
Therefore, the proportion of UEDVT diagnosed
by initial ultrasound was only 0.26% (29/11 019) of
all DVT (upper and lower extremity) ultrasound
requests. The demographic characteristics of
patients in the UEDVT-negative and UEDVT-positive
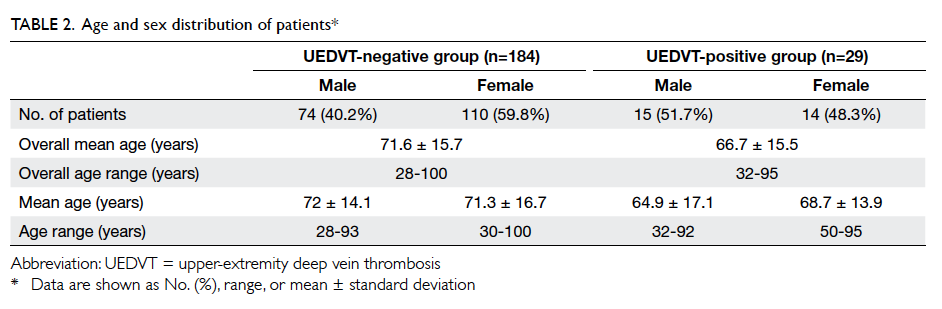
groups are shown in Table 2.

Figure. Ultrasound images of (a) a patient diagnosed with breast carcinoma: it shows lack of colour signals inside the vein (thrombus formation); and (b) a patient with colon carcinoma in bed-bound palliative care: it shows large thrombus inside the vein lumen
When comparing the age distribution between
the two groups with t test, the results were not
significant (P=0.06). In the UEDVT-negative group,
74 (40.2%) patients were males and 110 (59.8%)
patients were females. There was no significant
difference in age distribution among the two genders
(P=0.394). Among the UEDVT-positive group, 15
(51.7%) patients were males and 14 (48.3%) were
females. t Test to compare the age distribution
between the two genders in this group was also not
significantly different (P=0.257).
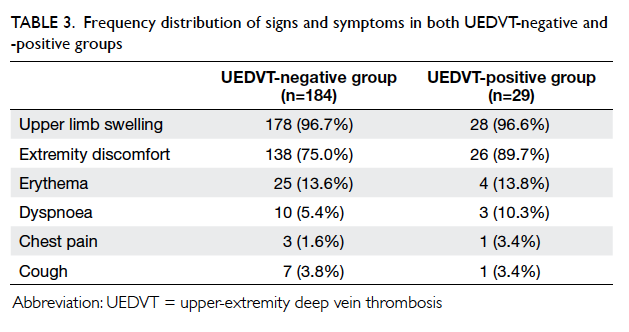
The frequency distributions of the signs and
symptoms in the two groups are summarised in
Table 3. Most patients in the UEDVT-negative group
presented with upper limb swelling, and was seen
in 178 (96.7%) patients. Even among the UEDVT-positive
group patients, upper limb swelling was the
most common sign, and was present in 28 (96.6%)
patients.
Statistical analysis and frequency proportion
of variables in the two groups are summarised in
Table 4. In the UEDVT-negative group, history of
cancer, hypertension, and diabetes mellitus appeared
to be the more prevalent variables and was seen in 82 (44.6%), 81 (44.0%) and 47 (25.5%), respectively.
On the other hand, among the 29 patients in the
UEDVT-positive group, history of smoking, history of cancer,
and hypertension were the prevalent risk factors,
and was seen in 11 (37.9%), 19 (65.5%) and 8 (27.6%)
patients, respectively.

Table 4. Statistical analysis and frequency proportion of variables in the UEDVT-negative and -positive groups
Binary logistic regression was used to test
the variables (Table 4). There were no statistically
significant predictors of UEDVT if all variables
were included. There was a trend towards higher
risk of UEDVT in patients with a history of
malignancy (odds ratio [OR]=2.250, P=0.071)
but this was not statistically significant. Stepwise
backward regression was performed to eliminate the
independent variables with the highest P value until
P≤0.2. The final regression model was left with only
age, sex, and history of malignancy as independent
variables, as the other variables persistently showed
high P values (Table 5).

Table 5. Analysis of risk factors for UEDVT (remaining variables after backward stepwise regression)
In this study, the remaining variables in the
model were age (P=0.119), female gender (P=0.114), and history
of malignancy (P=0.024). History of malignancy
remained predictive of UEDVT, and positive history
of malignancy had an OR of 2.664 (95% confidence
interval, 1.140-6.211) for the presence of UEDVT.
In the UEDVT-positive group, there was
no obvious predisposing cause observed in three
patients. Therefore, these three (10.3%) patients
were classified as having primary UEDVT, while
the remaining 26 (89.7%) patients were classified as
secondary UEDVT.
Discussion
In our study, the number of UEDVT cases diagnosed
during the 13-year period using initial sonography
was about 2.2 patients per year. As stated earlier,
it has been a long-held perspective that UEDVT
screening was a rare request in our hospital, and
this is clearly evident from this study. Requests
for UEDVT sonography constituted only 2.1% (236/11 019) of
all extremity (upper and lower) vein ultrasound
requests. The proportion of UEDVT diagnosed by
initial ultrasound was only 0.26% of all DVT (upper
and lower extremity) ultrasound requests, and
therefore very rare.
Among 29 patients with UEDVT in our
study, three patients presented with no obvious
predisposing cause. One young healthy 32-year-old
male claimed to have developed symptoms after
exercise, and so this particular case was classified
as primary effort-related thrombosis. Effort-related
UEDVT often affected individuals who were
young and healthy, with a male-to-female ratio of
approximately 2:1.11 The incidence is higher in males
and similar findings were also found in this study, and
males were younger than females. Pain and swelling
are commonly present in patients with UEDVT as
shown in a study by Mustafa et al.4 Similarly, swelling
was the most prevalent sign in our study, which was
seen in 96.6% of patients, and represented the most
common sign of UEDVT.
In our study, the prevalence of UEDVT among
those undergoing ultrasound examinations for
suspected UEDVT was 13.6%, and is the lowest
when compared with other studies conducted among
Caucasian population (18%,12 40%,13 25%,14 and
40%5).
We also observed that there were fewer patients with
indwelling catheters in our study sample compared
with other studies (10.3% vs 11.6%,13 12%,12 23%,14
and 57%5). Earlier reports by Joffe et al3 suggested that indwelling catheter was the strongest predictor
of UEDVT, and this may be the reason for the lower
incidence in our study compared with other studies.
Overall, in our study it was found that
history of smoking (37.9%), malignancy (65.5%),
and hypertension (27.6%) were the common risk
factors and particularly in UEDVT group (Table 4). Statistical analysis showed that a history of
malignancy remained predictive of UEDVT. In
our study, malignancy was a major risk factor for
UEDVT, similar to studies conducted in Caucasian
population.1 3 4 In our study, the frequency of cancer
(65.5%) was even higher than those in Caucasian
population in other studies, which had 43%,15 30%,16 38%,17 and 45%.4
Similar studies on Chinese population have
already been published. Chen et al18 have investigated
the differences in limb, age, and sex of Chinese
patients with LEDVT. Abdullah et al19 studied the
incidence of UEDVT associated with peripherally
inserted central catheters. Liu et al20 estimated the
incidence of venous thromboembolism instead
of UEDVT in a study from a Hong Kong regional
hospital. However, no study relating to prevalence
of UEDVT comparing Chinese and western
population have been performed. This study, while
important, highlighted malignancy as the major risk
factor for the prevalence of UEDVT. In a resource-limited
health care system, patients with a history
of malignancy should be prioritised in the triage
of symptomatic patients referred for UEDVT
screening, because malignancy is a major predictor
of UEDVT and carries risk of PE. Such prioritisation
will be beneficial to UEDVT patients as they can be
identified and treated early.
Limitations
We employed retrospective observation in this study,
and data were collected only from those available
in the medical records. Therefore, the frequency
of UEDVT reported might grossly underestimate
the true number. The reason for this could be that
signs and symptoms of UEDVT are usually non-specific,
and as reported in other prospective studies
many patients with UEDVT may remain completely
asymptomatic.21
In our study, diagnosis of UEDVT was made
solely by ultrasound. Studies have shown that
ultrasound imaging has excellent sensitivity and
specificity for LEDVT.22 23 In a study, the sensitivity had reached 97% to 100% and specificity of 98% to
99%.18 However, previous studies have reported
lower sensitivity and specificity for upper-extremity
ultrasound at 78% to 100% and 82% to 100%,
respectively.18 19 There are several possible reasons
why the sensitivity and specificity for detecting
UEDVT are lower compared with LEDVT. One main
reason is because of the anatomic drawback. The
sternum and clavicle create acoustic shadowing or
artefact on ultrasound imaging which limits the
visualisation of proximal upper-extremity veins and
thereby explains the relatively low sensitivity and
specificity.3 Additionally, it would be difficult to
visualise the centrally situated veins like the medial
segment of the subclavian vein, the brachiocephalic
vein, and their confluence with the superior vena
cava.24 Moreover, the presence of a catheter might
not only alter the venous tone, but also affect the
venous flow making it more difficult to interpret
the Doppler findings visualised on ultrasound.
Further, differentiation between a normal vein and
a large collateral in a patient with chronic venous
thrombosis might sometimes be difficult.20 Another
limitation of our study was the relatively small sample
size, especially for catheter-related patients. Such
small numbers might preclude subgroup analysis
and lower the statistical power for identifying risk
factors.
Conclusions
The major risk factor for UEDVT identified from
this study is malignancy. Therefore, patients with a
history of malignancy should be prioritised in the
triage of symptomatic patients referred for UEDVT
screening because malignancy is a major predictor
of UEDVT and carries risk for PE.
References
1. Tilney ML, Griffiths HJ, Edwards EA. Natural history of
major venous thrombosis of the upper extremity. Arch
Surg 1970;101:792-6. Crossref
2. Baarslag HJ, Koopman MM, Hutten BA, et al. Long-term
follow-up of patients with suspected deep vein thrombosis
of the upper extremity: survival, risk factors and post-thrombotic
syndrome. Eur J Intern Med 2004;15:503-7. Crossref
3. Joffe HV, Kucher N, Tapson VF, Goldhaber SZ; Deep Vein
Thrombosis (DVT) FREE Steering Committee. Upper-extremity
deep vein thrombosis: a prospective registry of
592 patients. Circulation 2004;110:1605-11. Crossref
4. Mustafa S, Stein PD, Patel KC, Otten TR, Holmes R,
Silbergleit A. Upper extremity deep venous thrombosis.
Chest 2003;123:1953-6. Crossref
5. Giess CS, Thaler H, Bach AM, Hann LE. Clinical experience
with upper extremity sonography in a high-risk cancer
population. J Ultrasound Med 2002;21:1365-70.
6. Chan YK, Chiu KY, Cheng SW, Ho P. The incidence of
deep vein thrombosis in elderly Chinese suffering hip
fracture is low without prophylaxis: a prospective study
using serial duplex ultrasound. J Orthop Surg (Hong Kong)
2004;12:178-83.
7. Köksoy C, Kuzu A, Kutlay J, Erden I, Ozcan H, Ergîn K.
The diagnostic value of colour Doppler ultrasound in
central venous catheter related thrombosis. Clin Radiol
1995;50:687-9. Crossref
8. Marshall PS, Cain H. Upper extremity deep vein
thrombosis. Clin Chest Med 2010;31:783-97. Crossref
9. Statistical tables of the 2006 population by-census.
Available from: http://www.bycensus2006.gov.hk/en/data/data3/statistical_tables/index.htm#A2. Accessed 9 Dec
2014.
10. Joffe HV, Goldhaber SZ. Upper-extremity deep vein
thrombosis. Circulation 2002;106:1874-80. Crossref
11. Illig KA, Doyle AJ. A comprehensive review of Paget-Schroetter syndrome. J Vasc Surg 2010;51:1538-47. Crossref
12. Kerr TM, Lutter KS, Moeller DM, et al. Upper extremity
venous thrombosis diagnosed by duplex scanning. Am J
Surg 1990;160:202-6. Crossref
13. Kröger K, Schelo C, Gocke C, Rudofsky G. Colour Doppler
sonographic diagnosis of upper limb venous thromboses.
Clin Sci (Lond) 1998;94:657-61.
14. Lee JA, Zierler BK, Zierler RE. The risk factors and clinical
outcomes of upper extremity deep vein thrombosis. Vasc
Endovascular Surg 2012;46:139-44. Crossref
15. Marinella MA, Kathula SL, Markert RJ. Spectrum of
upper-extremity deep venous thrombosis in a community
teaching hospital. Heart Lung 2000;29:113-7. Crossref
16. Isma N, Svensson PJ, Gottsäter A, Lindblad B. Upper
extremity deep venous thrombosis in the population-based
Malmö thrombophilia study (MATS). Epidemiology,
risk factors, recurrence risk, and mortality. Thromb Res
2010;125:335-8. Crossref
17. Muñoz FJ, Mismetti P, Poggio R, et al. Clinical outcome
of patients with upper-extremity deep vein thrombosis:
results from the RIETE Registry. Chest 2008;133:143-8. Crossref
18. Chen F, Xiong JX, Zhou WM. Differences in limb, age and
sex of Chinese deep vein thrombosis patients. Phlebology
2014 Feb 14. Epub ahead of print. Crossref
19. Abdullah BJ, Mohammad N, Sangkar JV, et al. Incidence of
upper limb venous thrombosis associated with peripherally
inserted central catheters (PICC). Br J Radiol 2005;78:596-600. Crossref
20. Liu HS, Kho BC, Chan JC, et al. Venous thromboembolism
in the Chinese population—experience in a regional
hospital in Hong Kong. Hong Kong Med J 2002;8:400-5.
21. Luciani A, Clement O, Halimi P, et al. Catheter-related
upper extremity deep venous thrombosis in cancer
patients: a prospective study based on Doppler US.
Radiology 2001;220:655-60. Crossref
22. Prandoni P, Polistena P, Bernardi E, et al. Upper-extremity
deep vein thrombosis. Risk factors, diagnosis, and
complications. Arch Intern Med 1997;157:57-62. Crossref
23. Baarslag HJ, van Beek EJ, Koopman MM, Reekers JA.
Prospective study of color duplex ultrasonography
compared with contrast venography in patients suspected
of having deep venous thrombosis of the upper extremities.
Ann Intern Med 2002;136:865-72. Crossref
24. Chin EE, Zimmerman PT, Grant EG. Sonographic
evaluation of upper extremity deep venous thrombosis. J
Ultrasound Med 2005;24:829-38; quiz 839-40.