DOI: 10.12809/hkmj133925
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
From observation to aetiology: a case report of a twin fetus-in-fetu and a revisit of the known rarity
Kristine KY Pang, MB, ChB, MRCSEd1;
Nicholas SY Chao, FCSHK, FHKAM (Surgery)1;
TK Tsang, FHKAM (Radiology)2;
Betty YT Lau, FHKAM (Obstetrics and Gynaecology)3;
KY Leung, FHKAM (Obstetrics and Gynaecology)3;
SH Ting, MB, BS4;
Michael WY Leung, FCSHK, FHKAM (Surgery)1;
Kelvin KW Liu, FCSHK, FHKAM (Surgery)5;
1 Division of Paediatric Surgery, Department of Surgery, Queen Elizabeth Hospital, Jordan, Hong Kong
2 Department of Radiology and Imaging, Queen Elizabeth Hospital,
Jordan, Hong Kong
3 Department of Obstetrics and Gynaecology, Queen Elizabeth Hospital,
Jordan, Hong Kong
4 Department of Pathology, Queen Elizabeth Hospital, Jordan, Hong Kong
5 Division of Paediatric Surgery, Department of Surgery, United Christian
Hospital, Kwun Tong, Hong Kong
Corresponding author: Dr Nicholas SY Chao (nickchao@yahoo.com)
Abstract
A baby girl presented with an antenatal diagnosis
of a retroperitoneal tumour. Postnatal imaging
suggested that this mass contained two fetiform
structures with spine and long bone formation. This
teratomatous mass was completely excised at 3 weeks
of age. Histology was consistent with twin fetuses-in-fetu, revealing two fetiform masses each with an
umbilical cord connecting to a common placenta-like
mass. Despite a difference in the weight of the
twin fetuses-in-fetu, the level of organogenesis was
identical and corresponded to fetuses of 10 weeks of
gestation. Each mass had four limbs, intact skin, rib
cage, intestines, anus, ambiguous genitalia, primitive
brain tissue and a spine with ganglion cells in the
cord. Although considered a mature teratoma in the
current World Health Organization classification,
the theory of formation from multiple pregnancies
has been commonly implied in more recent
literature. The true aetiology of this rare condition
remains unclear.
Introduction
Fetus-in-fetu is a rare condition with an estimated
incidence of 1 in 500 000 births.1 It was a descriptive
term attributed to Meckel circa 1800. The key
feature entails well-organised fetal structures in
macroscopic pathology, with vertebral columns and,
commonly, long bones of the limbs. Variable degree
of organogenesis for the lung, liver, intestines, and
genitalia has been commonly reported. Although
grouped under the entity of teratoma and considered
the well-differentiated end of the neoplastic
spectrum in the current World Health Organization
(WHO) classification,2 the true aetiology remains
unclear. The theory of formation from monozygotic
twins has been commonly implied in the literature.3 4 5
The commonest presentation of this condition
was a painless mass lesion with or without pressure
symptoms. Prenatal diagnosis was made in nine
out of the 88 cases collectively reported by Hoeffel
et al.3 We, hereby, report the case of a twin fetus-in-fetu presenting on antenatal ultrasound, and its
histopathology.
Case report
Clinical course
A Chinese baby girl was admitted to our neonatal
unit on the day of birth for antenatal diagnosis of a
retroperitoneal mass in November 2010. This was
a singleton pregnancy from natural conception,
with allegedly normal antenatal ultrasound in early
gestation. There were no additional morphology
scans during second trimester ultrasound as the
mother was a resident of mainland China where
she received her obstetric care. Detailed antenatal
ultrasound at 37 weeks of maturity showed a 32 mm
x 30 mm x 30 mm mass in the left retroperitoneal
region of the fetus. There were no other apparent
abnormalities, or complicating intestinal or urinary
obstruction. The initial differential diagnoses
included congenital adrenal tumour and adrenal
haemorrhage.
The birth weight of the baby was 4.07 kg.
Physical examination showed fullness in the left
flank. Targeted ultrasound of the retroperitoneal
mass was performed immediately after birth. It
showed cystic and solid components with areas of
ossification within the mass which were suggestive of
a teratoma. Abdominal X-ray showed neither dilated
bowel nor calcification. Alpha fetoprotein and beta
human chorionic gonadotropin levels measured
on day 2 of life were normal for age. Clinically, the
patient had no evidence of intestinal obstruction and
tolerated full feeding soon after birth.
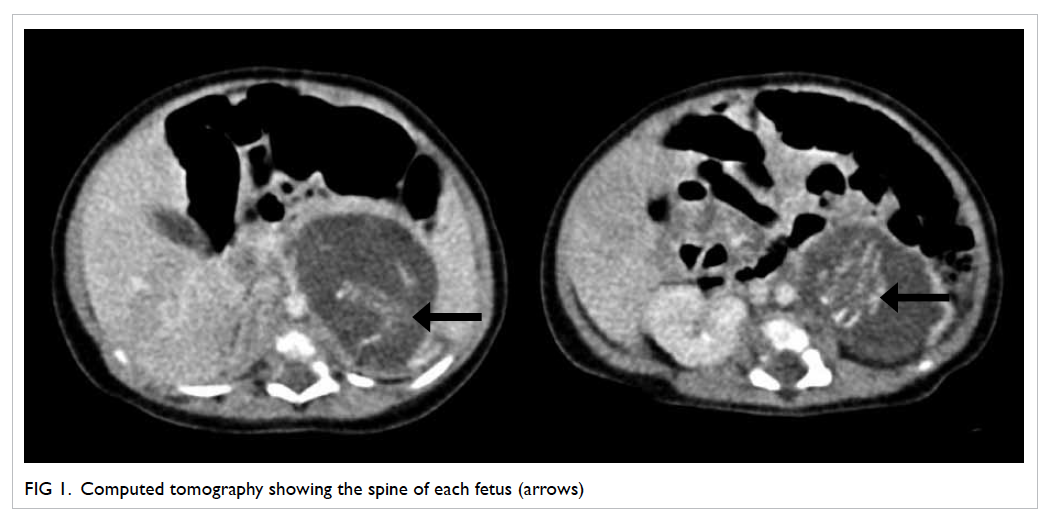
A detailed ultrasound of the abdominal region
was performed on day 4 and computed tomography
on day 7 (Fig 1). These showed a complex cystic
mass between the spleen and the left kidney, with
a maximal diameter of 47 mm. Within this single
thin-walled cyst, there were two heterogeneous
solid masses. Each mass contained a well-ossified
spine and two ossified long bones at the caudal end,
resembling the configuration of fetal femurs; no
cardiac or cranial structures were identifiable.
To rule out the likelihood of neuroblastoma,
urine catecholamine profile was performed which
turned out to be normal. While imaging pointed to a
likely fetus-in-fetu, the remote possibility of a mature
teratoma could not be completely ruled out. Thus, a
decision was made to perform an early excision of
the mass.
Elective laparotomy was performed on day
14. Mobilisation of the colon at the splenic flexure
revealed a retroperitoneal mass between the left
kidney and left adrenal gland that was supplied by
multiple, small feeding vessels from the aorta and
left renal artery. After flush-dividing all investing
vessels, the mass was resected with an intact capsule.
The baby made good recovery from the operation
and was discharged uneventfully on postoperative
day 22.
Histopathology
Pathological section showed two fetiform masses,
each with an umbilical cord connecting to a single
placenta-like mass (Fig 2). The lengths of the fetuses
were 37 mm and 35 mm, respectively. The larger
mass contained better developed fetal structures
and weighed 14.2 g, while the smaller mass weighed
9.3 g.
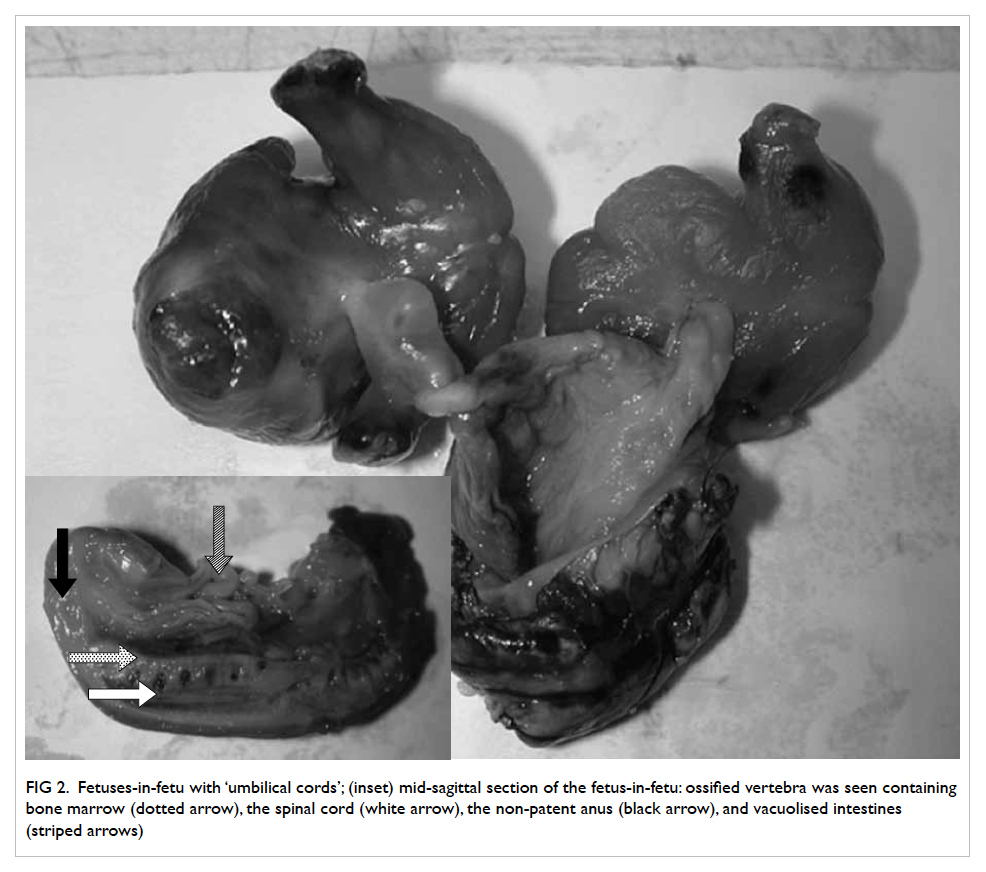
Figure 2. Fetuses-in-fetu with ‘umbilical cords’; (inset) mid-sagittal section of the fetus-in-fetu: ossified vertebra was seen containing bone marrow (dotted arrow), the spinal cord (white arrow), the non-patent anus (black arrow), and vacuolised intestines (striped arrows)
Within each of the ‘fetuses’, vacuolised
intestines could be seen in the abdominal cavity
but were leading to a non-patent anus (Fig 2, inset).
Ambiguous external genitalia were identified in both
fetuses. At the cranial end, there was no skull and no
skin coverage. The rest of the fetus was covered by
intact skin.
Regarding the skeletal formation, there was an
ossified segmented spine in each fetus. The spinal
cord was identified posterior to the vertebral bodies.
A well-developed rib cage with bone and cartilage
could be seen in the thoracic region. The pelvic bone
and the long bones of the lower limbs were ossified
with marrow formation in the centre. Two long
bones could be identified in the forearm of the larger
fetus. Metatarsals could be identified in both fetuses.
Microscopic examination revealed striated
muscles, bones, and cartilages in the limbs. Ganglion
cells were present in the spinal cord. The fetuses were
covered by organised skin tissue and appendages.
Respiratory mucosa was identified in the thoracic
region. The intestines in the abdominal cavity were
lined by intestinal mucosa. There was disorganised
primitive brain tissue in the cranial end of both
fetuses.
Discussion
Fetus-in-fetu is rare, with less than 200 cases reported
in the literature. Hui et al6 reported, formally,
the first regional case only in 2007. Despite the
detailed description in literature, its aetiology and
relationship with teratoma remains controversial.
Fetus-in-fetu is currently classified as a
variant of mature teratoma. Previous case reports
of recurrence after resection with malignant
transformation also support this classification,
whereby fetus-in-fetu should be the mature end of
the spectrum of teratoma.7 However, the theory of
monozygotic diamniotic twins has been increasingly
proposed in the recent literature.3 8 Despite the gaining popularity, there is, as yet no concrete
evidence to confirm this relationship. Blood group
typing, karyotyping, and DNA analysis, when
performed in the previously reported cases, always
showed identical findings between the fetuses and
their hosts. This finding is, however, compatible with
both monozygotic multiple pregnancy theory and
teratoma theory.
If we consider fetus-in-fetu a result of multiple
pregnancy with initial normal embryological
development, principles of embryological
assessment may be considered. By diagnostic
criteria, all fetuses-in-fetu possess vertebrae and,
therefore, such an embryo should have reached the
age of 24 to 25 days, corresponding to a gestational
age of 5 weeks. In our case, and indeed in most other
reported cases, the caudal neuropores were also
closed. This further aged the estimated gestation to 6
weeks before the development was arrested in these
presumed parasitic twins. In our case, since digital
rays were clearly identified in the hands and feet, the
embryonic age should be at least 44 to 46 days, which
is 8 weeks by gestation.9 If we consider the abundant
length of small bowel within the abdominal cavity,
the gestational age had likely reached 10 weeks for
both of these twin fetuses.
In conventional embryological assessment
of aborted products of gestation, size of the fetus
can sometimes be smaller than the normal size for
embryological age, since the fetuses often undergo a
certain period of growth restriction before the actual
death. On the other hand, direct measurement of
the specimen size may be slightly larger than the
ultrasound assessment due to flattening of the tissue
after its passage through the cervix.10 Interestingly, in
the reported literature, a poor correlation has been
observed between the level of organogenesis and
size of the parasitic fetus. In a review of 87 cases by
Hoeffel et al,3 there were 10 reported parasitic fetuses
weighing over 500 g. The level of organogenesis in
these fetuses, however, was immature compared to
that of a normal 500 g fetus or newborn.3 If these
parasitic fetuses were once products of multiple
pregnancies, an interesting conclusion would be that
these ‘fetuses’ continued to grow in size after their
arrest in development or, theoretically, the ‘death’ of
fetuses, although the ‘death’ of such fetuses is always
difficult to define in view of their ‘acardiac’ nature.
With increasing application of assisted
reproductive technology, a higher proportion of
multiple pregnancies can now be monitored with
ultrasound from early gestation. However, to date,
there is no longitudinal observation of the evolution
of fetus-in-fetu from multiple pregnancies, nor have
there been any reported cases arising from assisted
pregnancy. Whilst the earliest antenatal diagnosis of
this condition in literature was 16 weeks’ gestation,11
no sequential monitoring of the antenatal history of
fetus-in-fetu has been published.
In our case, both the twin parasitic fetuses
had body weights, sizes, and fetal structures that
corresponded well with a gestational age of 10 weeks.
A normal ultrasound during the early antenatal
period rather suggests that they might have been
tiny parasitic fetuses that had grown slowly with
the ‘patient’ and reached their significant sizes at
term, instead of the popular theory of early normal
development followed by parasitic inclusion and
arrest of growth. Although with limited antenatal
documentation, our case report does not support
the popular monozygotic multiple pregnancy theory,
and favours, by default, the traditional classification
into a teratoma.
Conclusion
Less than 200 cases of twin fetus-in-fetu have been
reported worldwide, and, to date, this was only the
second regional case report. Although classified by
WHO as a variant of mature teratoma, the theory
of demised multiple pregnancy has gained much
support recently. More evidence is needed to confirm
either theory. The widespread use of antenatal
ultrasound in early gestation may provide more
concrete evidence from longitudinal observation
and give light to the aetiology of this intriguing
condition.
References
1. Grant R, Pearn JH. Foetus-in-foetu. Med J Aust
1969;1:1016-20.
2. Scully RE, Young RH, Clement PB. Atlas of tumor
pathology, 3rd series, fascicle 23. Washington, DC: Armed
Forces Institute of Pathology; 1998: ch13.
3. Hoeffel CC, Nguyen KQ, Phan HT, et al. Fetus in fetu: a case
report and literature review. Pediatrics 2000;105:1335-44. CrossRef
4. Lewis RH. Foetus in foetu and retroperitoneal teratoma.
Arch Dis Child 1961;36:220-6. CrossRef
5. Thrakral CL, Maji DC, Sajwani MJ. Fetus-in-fetu: a
case report and review of the literature. J Pediatr Surg
1998;33:1432-4. CrossRef
6. Hui PW, Lam TP, Chan KL, Lee CP. Fetus in fetu—from
prenatal ultrasound and MRI diagnosis to postnatal
confirmation. Prenat Diagn 2007;27:657-61. CrossRef
7. Hopkins KL, Dickson PK, Ball TI, Ricketts RR, O’Shea PA,
Abramowsky CR. Fetus-in-fetu with malignant recurrence.
J Pediatr Surg 1997;32:1476-9. CrossRef
8. Mohan H, Chhabra S, Handa U. Fetus-in-fetu: a rare entity.
Fetal Diagn Ther 2007;22:195-7. CrossRef
9. O’Rahilly R, Müller F. Developmental stages in human
embryos. Carnegie Institute of Washington; 1987:
Publication no. 637.
10. Harkness LN, Rodger M, Baird DT. Morphological and
molecular characteristics of living human fetuses between
Carnegie stages 7 and 23: ultrasound scanning and direct
measurements. Hum Reprod Update 1996;3:25-33. CrossRef
11. Khatib MO, Deschamps F, Couture A, Giacalone PL,
Boulot P. Early prenatal ultrasonographic diagnosis of fetus
in fetu [in French]. J Gynecol Obstet Biol Reprod (Paris)
1998;27:438-40.
Find HKMJ in MEDLINE: