Hong Kong Med J 2015 Feb;21(1):5–9 | Epub 2 Jan 2015
DOI: 10.12809/hkmj144376
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Disease spectrum and treatment patterns in a local male infertility clinic
KL Ho, FRCSEd (Urol), FHKAM (Surgery); James HL Tsu, FRCSEd (Urol), FHKAM (Surgery); PC Tam, FRCSEd (Urol), FHKAM (Surgery); MK Yiu, FRCS (Edin), FHKAM (Surgery)
Division of Urology, Department of Surgery, The University of Hong Kong,
Queen Mary Hospital, Pokfulam, Hong Kong
Corresponding author: Dr KL Ho (hokwanlun@gmail.com)
Abstract
Objective: To review disease spectrum and
treatment patterns in a local male infertility clinic.
Design: Case series.
Setting: Male infertility clinic in a teaching hospital
in Hong Kong.
Patients: Patients who were seen as new cases in
a local male infertility clinic between January 2008
and December 2012.
Intervention: Infertility assessment and counselling
on treatment options.
Main outcome measures: Disease spectrum and
treatment patterns.
Results: A total of 387 new patients were assessed
in the male infertility clinic. The mean age of the
patients and their female partners was 37.2 and
32.1 years, respectively. The median duration of
infertility was 3 years. Among the patients, 36.2%
had azoospermia, 8.0% had congenital absence
of vas deferens, and 48.3% of patients had other
abnormalities in semen parameters. The commonest
causes of male infertility were unknown (idiopathic),
clinically significant varicoceles, congenital absence
of vas deferens, mumps after puberty, and erectile or
ejaculatory dysfunction. Overall, 66.1% of patients
chose assisted reproductive treatment and 12.4% of
patients preferred surgical correction of reversible
male infertility conditions. Altogether 36.7% of
patients required either surgical sperm retrieval or
correction of male infertility conditions.
Conclusions: The present study provided important
local data on the disease spectrum and treatment
patterns in a male infertility clinic. The incidences of
azoospermia and congenital absence of vas deferens
were much higher than those reported in the
contemporary literature. A significant proportion of
patients required either surgical sperm retrieval or
correction of reversible male infertility conditions.
New knowledge added by this
study
- The present study provided important local data on the disease spectrum and treatment patterns in a male infertility clinic.
- The incidences of azoospermia and congenital absence of vas deferens in the present study were much higher than those reported in the contemporary literature.
- The present study may help to increase public awareness of the contribution of male factors in infertility assessment and treatment.
- The study provides a background for future research into azoospermia and congenital absence of vas deferens in the locality.
Introduction
Infertility is defined by the inability to conceive after
1 year of regular unprotected sexual intercourse, and
it affects 15% of couples worldwide.1 Male factors
contribute to about 50% of infertile couples. Clinically
significant varicocele is present in 40% of infertile
men and is the commonest surgically reversible
condition. As many as 10% to 15% of infertile men
have azoospermia.2 According to the report of the
Council on Human Reproductive Technology in 2012,
male factor infertility contributes to 50% of women
receiving reproductive technology treatment.3 Local
data on male factor infertility, however, have been
scarce. The objective of the present article was to
review the disease spectrum and treatment patterns
in a local male infertility clinic.
Methods
All consecutive new patients seen in a local teaching
hospital (Queen Mary Hospital) male infertility
clinic from January 2008 to December 2012 were
included in this retrospective study. The clinical
records were reviewed and the demographics of the
patients and their female partners, aetiologies of
male factor infertility, semen analyses, and treatment
were analysed.
All patients underwent two separate semen
analyses and hormonal profiles (including morning
serum testosterone, and follicle-stimulating and
luteinising hormones) before being seen in the clinic.
A detailed urological and reproductive history was
taken, followed by a focused physical examination.
The fertility history was ascertained and female
factors of age and gynaecological history
were taken into consideration. Clinical diagnoses
were made and possible aetiologies were postulated
based on the above information. The patients’ semen
results were classified as azoospermia (no sperms
were identified after examination of the post-centrifugation
pellet), abnormal (in concentration,
motility, morphology, or any combination according
to the contemporary World Health Organization
standards4), or normal. The exact analysis of semen
parameters was beyond the scope of the present
study. Patients with azoospermia were classified
clinically as having obstructive (normal-sized testes
and hormonal profiles) and non-obstructive (small
testes and elevated follicle-stimulating hormones)
disorder. Genetic studies, including karyotyping
and Y chromosome microdeletion, were offered to
patients with non-obstructive azoospermia or severe
oligospermia. Only grade 2 (palpable) or 3 (visible)
varicoceles when standing were considered clinically
significant in the assessment. Diagnosis of congenital
absence of vas deferens was made by physical
examination and occasionally supplemented with
transrectal ultrasound for unclear cases. For patients
with a history of mumps after puberty and no other
identifiable causes of male infertility, mumps was
quoted as the main cause. Depending on the clinical
scenarios, the couples were counselled on different
treatment options, including surgical or assisted
reproductive treatments (ART), donor insemination,
adoption, and conservative treatment.
Results
From January 2008 to December 2012, 387 patients
had been seen in the male infertility clinic as new
cases. The mean age of the patients and their female
partners was 37.2 and 32.1 years, respectively. The
median duration of infertility was 3 years. Of the
patients, 140 (36.2%) had azoospermia, of whom 67
and 71 patients had obstructive and non-obstructive
causes, respectively, and two had both components
of azoospermia. A total of 187 (48.3%) patients had
abnormalities in one or more semen parameters
(Table 1).
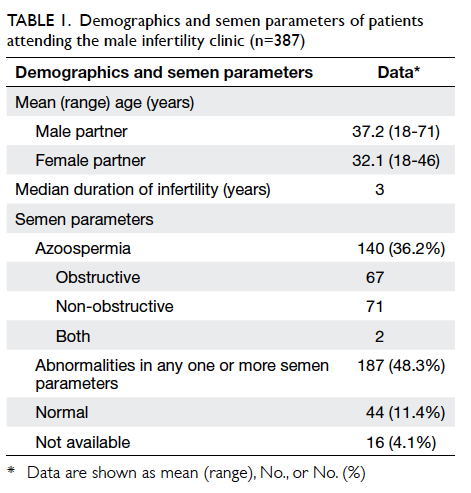
Table 1. Demographics and semen parameters of patients attending the male infertility clinic (n=387)
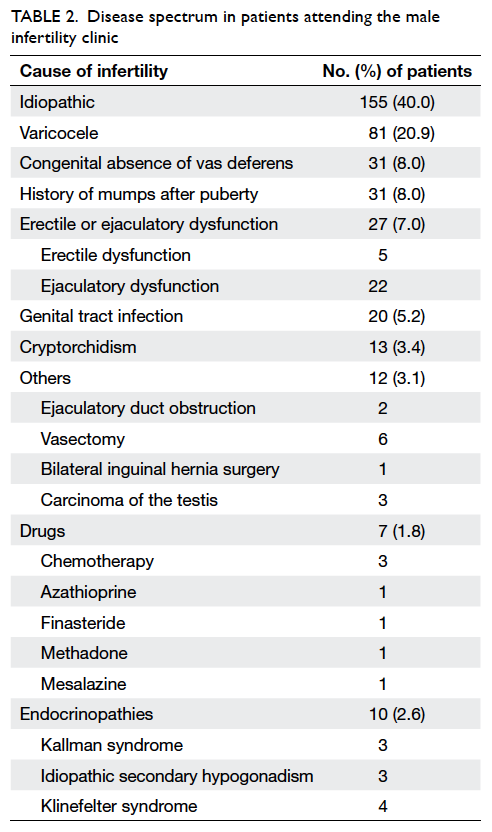
The commonest causes of male factor infertility
were unknown (idiopathic), clinically significant varicoceles,
congenital absence of vas deferens, mumps after
puberty, and erectile or ejaculatory dysfunction
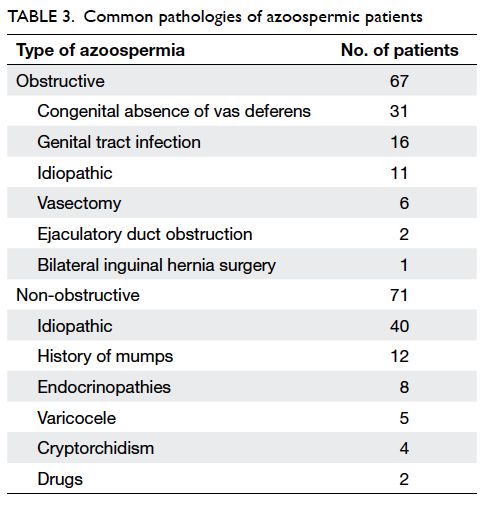
(Table 2). For patients with obstructive azoospermia,
common pathologies included congenital absence of
vas deferens and genital tract infection. For patients
with non-obstructive azoospermia, no causes were
identified in most patients. A history of mumps and
endocrinopathies were implicated in some non-obstructive
azoospermic patients (Table 3).
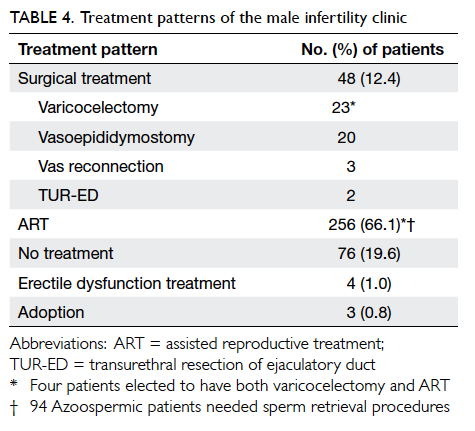
Most patients (66.1%) sought ART. Of these
patients, 94 azoospermic patients (56 patients with
non-obstructive azoospermia, 37 with obstructive
azoospermia, and one with both components)
required sperm retrieval procedures. Besides, 12.4% patients chose surgical treatments for
reversible causes of male infertility. The procedures
included varicocelectomy, vas reconnection, and
vasoepididymostomy. Four patients with varicoceles
and severe oligospermia or azoospermia proceeded
to varicocelectomy and employed ART as backup
treatment. Also, 19.6% patients elected to have
no further treatment of infertility (Table 4).
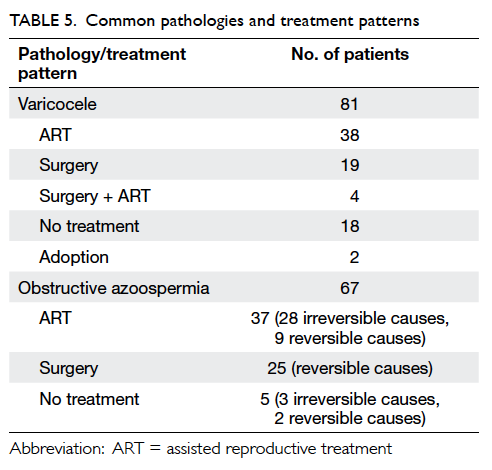
Of 81 patients who had clinically significant
varicoceles and abnormal semen parameters, 23
proceeded to surgery and 38 chose ART. Of 67 patients
who had obstructive azoospermia, 37 proceeded to
ART; 28 of these patients had congenital absence of
vas deferens that was irreversible, requiring sperm
retrieval and ART. For the other patients with
reversible causes of obstructive azoospermia, nine
preferred sperm retrieval and ART, while 25 elected
to have surgical treatments (Table 5).
Discussion
Infertility has remained a worldwide problem in the
past two decades.5 Traditionally, the female partner
has shouldered the major burden of infertility
assessment and treatment. With recent advances
in male infertility treatment6 and increasing public
awareness, there is a growing demand for assessment
and treatment of men with fertility issues.
When the infertility clinic at Queen Mary
Hospital was first established, it consisted of a joint
clinic assessment both by urologists specialising
in male infertility treatment and by gynaecologists
specialising in ART. Due to the long waiting list
at the conjoint clinic, the male infertility clinic
has since separated out. All infertile couples with
clinically suspected male infertility factors—such as
gross abnormalities in semen parameters, or erectile
or ejaculatory dysfunction—are referred to the male
infertility clinic for prompt assessment.
The median duration of infertility in this study
was 3 years before the infertile couples attended
for assessment. Upon referral, a large proportion
(36.2%) of patients had azoospermia. This figure was
much higher than the commonly quoted figures in
the current literature, where azoospermia was found
in 1% of all men and 10% to 15% of infertile men.7 8 9
The much higher figure in the present study was
probably related to referral bias. Male partners with
milder forms of abnormalities in semen parameters
might not have been referred for assessment.
These couples with an azoospermic male partner
would have benefited from earlier intervention
instead of wasting precious time attempting natural
conception. The present study illustrates the importance
of a premarital, or at least a pre-pregnancy,
checkup. Simple semen analysis would have identified
male partners with azoospermia or severe deficits
of semen parameters for early assessment, fertility
treatment, counselling, and potential intervention.
Besides, 11.4% of patients had normal
semen parameters and fell into the category of
unexplained infertility.10 After common female
factors have been ruled out, there are still many
possible causes of infertility, ranging from the
couple’s miscomprehension of the female fertility
window and coital behaviours to abnormal sperm
function.11
Clinically significant varicocele was the
commonest identifiable cause of male infertility
in the present study. This was concurrent with the
contemporary literature.12 Controversies over the
best treatment of varicoceles in infertile couples have
been met with a meta-analysis13 and randomised
controlled trials14 15 favouring surgery in terms of
pregnancy outcomes. Varicocelectomy is offered to
patients according to the criteria of the American
Society for Reproductive Medicine,12 namely,
documented history of infertility, grade 2 or above
varicocele, abnormalities in semen parameters,
and reversible female factors. In our institution,
the microsurgical subinguinal approach is used for
its lower risks of recurrence and hydrocele.16 In the
present study, 38 patients with varicoceles chose
ART, while 23 patients chose surgery. The decision
to proceed to ART versus varicocelectomy was made
after thorough counselling of the involved couples.
Factors considered included the female partner’s age,
semen quality, risks of surgery versus ART, expertise
of the surgeons and ART centre staff, and the
respective treatment outcome audits. Both male and
female partners were strongly encouraged to attend
counselling together and arrive at the decision that is
most agreeable to both parties.
A significant proportion of patients in the study
had azoospermia, of which 47.9% had obstructive
causes. Congenital absence of vas deferens was the
commonest cause of obstructive azoospermia, which
constituted 8.0% of the study population. This was
higher than the 1% to 2% of infertile men reported in
the literature.7 9 The incidence of congenital absence of
vas deferens is not well reported in Chinese men with
infertility. One of the reasons for the high incidence
in this study could be referral bias, which led to a
very high incidence of azoospermia in our study
population. Hence, the proportional percentage of
congenital absence of vas deferens was much higher
than is usually quoted. Sperm retrieval and ART
was offered as the only solutions for childbearing.
In Caucasians, congenital absence of vas deferens
is associated with cystic fibrosis transmembrane
conductance regulator (CFTR) gene mutations of
cystic fibrosis,17 and routine genetic study is offered
to patients and their female partners. Recent data
in Chinese patients with congenital absence of vas
deferens showed different CFTR gene mutations,18
which might lead to the development of a mild
genital form of cystic fibrosis. Cystic fibrosis is very
rare in Asian populations, but genetic counselling
for patients with congenital absence of vas deferens
is still advised by most authorities. Unfortunately,
genetic study is not available at Queen Mary
Hospital, and the huge number of possible CFTR
gene mutations (>1500) makes targeted examination
in Chinese patients a big challenge.
Of 256 patients who proceeded to ART, 94
with azoospermia needed some sort of sperm
retrieval procedures. For patients with congenital
absence of vas deferens and markedly distended
epididymal tubules, percutaneous epididymal sperm
aspiration provided a simple and reliable method
of sperm retrieval. For patients with obstructive
azoospermia secondary to infection or idiopathic
causes, microsurgical epididymal sperm aspiration
was employed to retrieve the maximum number
of sperms with the least blood contamination.
Sometimes extensive adhesiolysis needed to be
performed to expose distended epididymal tubules.
Non-obstructive azoospermia was the most difficult
condition to treat. Conventional testicular sperm
extraction (TESE) involves multiple random testis
biopsies and can fail to find focal seminiferous tubules
harbouring active spermatogenesis. This method
involves excision of more testicular tissues and is
associated with more postoperative intra-testicular
haematoma and scarring.19 Microdissection
TESE (MicroTESE) involves identifying the
spermatogenically active regions of the testes by
direct examination of larger seminiferous tubules
under high magnification.20 This method is targeted
and involves retrieval of the maximum number of
sperms with the least testicular tissue loss. However,
MicroTESE is time-consuming and involves a steep
learning curve.21 Even in the hands of experts, the
procedure takes an average of 1.8 and 2.7 hours for
successful and unsuccessful cases, respectively.
Of 36 patients with reversible causes of
obstructive azoospermia (Table 5), 25 (69.4%)
chose surgical treatment, nine proceeded to ART, and
two preferred conservative treatment. In the era
of ART, surgical treatment remains a valuable
armamentarium in the management of reversible
obstructive azoospermia.1 At Queen Mary Hospital,
microsurgical intussusception vasoepididymostomy
was offered to patients with epididymal obstruction
secondary to infection or idiopathic causes.22 The
choice between surgical treatment and ART was
made after thorough consideration of the female
partner’s age, surgical expertise and ART success
rates, and the infertile couple’s wishes.
Within this study population, 142 (36.7%)
patients needed either surgical sperm retrieval
and ART or surgical correction of reversible male
infertility conditions.
Conclusions
The present study describes the disease spectrum
of a local male infertility clinic. The incidence of
azoospermia and congenital absence of vas deferens
was much higher than that described in the current
literature. There was high demand for sperm
retrieval or surgical correction services in this group
of patients.
References
1. Lee R, Li PS, Schlegel PN, Goldstein M. Reassessing
reconstruction in the management of obstructive
azoospermia: reconstruction or sperm acquisition? Urol
Clin North Am 2008;35:289-301. CrossRef
2. Practice Committee of American Society for Reproductive
Medicine in collaboration with Society for Male
Reproduction and Urology. The management of infertility
due to obstructive azoospermia. Fertil Steril 2008;90(5
Suppl):S121-4. CrossRef
3. Infertility diagnosis by age of patients receiving RT
procedures (other than DI and AIH) in 2012. Council on
Human Reproductive Technology. Available from: http://www.chrt.org.hk/english/publications/files/table17_2012.pdf. Accessed Aug 2014.
4. Cooper TG, Noonan E, von Eckardstein S, et al. World
Health Organization reference values for human semen
characteristics. Hum Reprod Update 2010;16:231-45. CrossRef
5. Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel
S, Stevens GA. National, regional, and global trends in
infertility prevalence since 1990: a systematic analysis of
277 health surveys. PLoS Med 2012;9:e1001356. CrossRef
6. Male Infertility Best Practice Policy Committee of the
American Urological Association; Practice Committee of
the American Society for Reproductive Medicine. Report
on optimal evaluation of the infertile male. Fertil Steril
2006;86(5 Suppl 1):S202-9. CrossRef
7. Wosnitzer M, Goldstein M, Hardy MP. Review of
azoospermia. Spermatogenesis 2014;4:e28218. CrossRef
8. Berookhim BM, Schlegel PN. Azoospermia due to
spermatogenic failure. Urol Clin North Am 2014;41:97-113. CrossRef
9. Wosnitzer MS, Goldstein M. Obstructive azoospermia.
Urol Clin North Am 2014;41:83-95. CrossRef
10. Hamada A, Esteves SC, Agarwal A. Unexplained male
infertility—looking beyond routine semen analysis. Eur
Urol Rev 2012;7:90-6.
11. Agarwal A, Bragais FM, Sabanegh E. Assessing sperm
function. Urol Clin North Am 2008;35:157-71, vii. CrossRef
12. Practice Committee of American Society for Reproductive
Medicine. Report on varicocele and infertility. Fertil Steril
2008;90(5 Suppl):S247-9. CrossRef
13. Marmar JL, Agarwal A, Prabakaran S, et al. Reassessing the
value of varicocelectomy as a treatment for male subfertility
with a new meta-analysis. Fertil Steril 2007;88:639-48. CrossRef
14. Abdel-Meguid TA, Al-Sayyad A, Tayib A, Farsi HM. Does
varicocele repair improve male infertility? An evidence-based
perspective from a randomized, controlled trial. Eur
Urol 2011;59:455-61. CrossRef
15. Mansour Ghanaie M, Asgari SA, Dadrass N, Allahkhah A,
Iran-Pour E, Safarinejad MR. Effects of varicocele repair
on spontaneous first trimester miscarriage: a randomized
clinical trial. Urol J 2012;9:505-13.
16. Leung L, Ho KL, Tam PC, Yiu MK. Subinguinal
microsurgical varicocelectomy for male factor subfertility:
ten-year experience. Hong Kong Med J 2013;19:334-40. CrossRef
17. Esteves SC, Miyaoka R, Agarwal A. Sperm retrieval
techniques for assisted reproduction. Int Braz J Urol
2011;37:570-83. CrossRef
18. Lu S, Yang X, Cui Y, et al. Different cystic fibrosis transmembrane conductance regulator mutations in Chinese men with congenital bilateral absence of vas deferens and other acquired obstructive azoospermia. Urology 2013;82:824-8. CrossRef
19. Okada H, Dobashi M, Yamazaki T, et al. Conventional
versus microdissection testicular sperm extraction for
nonobstructive azoospermia. J Urol 2002;168:1063-7. CrossRef
20. Schlegel PN. Testicular sperm extraction: microdissection
improves sperm yield with minimal tissue excision. Hum
Reprod 1999;14:131-5. CrossRef
21. Dabaja AA, Schlegel PN. Microdissection testicular sperm
extraction: an update. Asian J Androl 2013;15:35-9. CrossRef
22. Ho KL, Wong MH, Tam PC. Microsurgical
vasoepididymostomy for obstructive azoospermia. Hong
Kong Med J 2009;15:452-7.
Find HKMJ in MEDLINE: