Hong Kong Med J 2014 Oct;20(5):407–12 | Epub 20 Jun 2014
DOI: 10.12809/hkmj144211
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Three-year experience of using venovenous extracorporeal membrane oxygenation for patients with severe respiratory failure
George WY Ng, FHKAM (Medicine), MPH (HKU);
Anne KH Leung, MB, ChB, FHKAM (Anaesthesiology);
KC Sin, MB, ChB, FHKAM (Medicine);
SY Au, MB, BS, FHKCP;
Stanley CH Chan, MB, BS, FHKCA;
Osburga PK Chan, MB, BS, FHKAM (Medicine);
Helen HL Wu, MB, BS, FHKAM (Medicine)
Department of Intensive Care, Queen Elizabeth Hospital, 30 Gascoigne
Road, Kowloon, Hong Kong
Corresponding author: Dr George WY Ng (georgeng@ha.org.hk)
Abstract
Objective: To present the 3-year experience of using
venovenous extracorporeal membrane oxygenation
for patients with severe respiratory failure in a single
centre in Hong Kong.
Design: Case series.
Setting: A 19-bed Intensive Care Unit of a tertiary
hospital in Hong Kong.
Patients: All patients who were managed with
venovenous extracorporeal membrane oxygenation
from 1 July 2010 to 30 June 2013 in the Intensive
Care Unit.
Results: Overall, 31 patients (mean age, 42.2 years,
standard deviation, 14.1 years; 21 males) received
venovenous extracorporeal membrane oxygenation
for the treatment of severe respiratory failure. Of
these, 90.3% (28 patients) presented with pneumonia
as the cause of the respiratory failure, and 22 of
them had identifiable causes. A total of nine (29.0%)
patients were diagnosed to have H1N1 infection.
The median Murray score was 3.5 (interquartile
range, 3.0-3.5); the median duration of venovenous
extracorporeal membrane oxygenation support
was 5.0 (2.8-8.6) days; and the median duration of
mechanical ventilator support was 18.2 (7.8-27.9)
days. The overall intensive care unit mortality was
19.4% (n=6). The overall in-hospital mortality and
the 28-day mortality were both 22.6% (n=7). Among the 22 patients who had identifiable infective causes,
those suffering from viral infection had lower
intensive care unit and hospital mortality than those
who had bacterial infection (8.3% vs 20.0%). All the
H1N1 patients survived. Complications related to
extracorporeal membrane oxygenation included
severe bleeding (n=2; 6.5%) and mechanical
complications of the circuits (n=3; 9.7%).
Conclusions: Venovenous extracorporeal
membrane oxygenation is an effective adjunctive
therapy and can be used as a life-saving procedure
for carefully selected patients with severe acute
respiratory distress syndrome when the limits of
standard therapy have been reached.
New knowledge added by this
study
- Venovenous extracorporeal membrane oxygenation (ECMO) has become a reliable respiratory support for patients with severe respiratory failure due to acute respiratory distress syndrome and severe hypoxaemia despite the use of conventional therapy.
- Use of venovenous ECMO allows protective ventilation and reduces ventilator-induced lung injury.
- H1N1 patients had a very good survival outcome when they received ECMO therapy.
- ECMO is available in specialised centres in Hong Kong. Patients with severe acute respiratory distress syndrome, particularly after H1N1 pneumonia, will be good candidates for receiving ECMO treatment.
- ECMO therapy is safe but associated with complications.
Introduction
Acute respiratory distress syndrome (ARDS), after
severe viral or bacterial infection, is a common
cause of severe respiratory failure in the Intensive
Care Unit (ICU). The syndrome is defined as acute onset of hypoxaemic respiratory failure, which is
accompanied by bilateral infiltrates of chest, and
occurs due to non-cardiogenic cause.1 2 Despite
vigorous researches on pharmacological treatment
and ventilator strategy in recent decades, ARDS with profound hypoxaemia continues to be associated
with high mortality rate. In 2009, the conventional
ventilatory support versus extracorporeal membrane
oxygenation (ECMO) for severe adult respiratory
failure (CESAR) trial, conducted by Peek et al3 in
the UK, showed a significant survival advantage
with the use of ECMO for patients with severe
ARDS. Extracorporeal membrane oxygenation is
a life-support technology with a history of more
than 40 years.4 With the evolution of technology,
the procedure has become simpler, safer, and
more reliable. Since 2010, the Queen Elizabeth
Hospital (QEH) in Hong Kong has started providing
venovenous (v-v) ECMO to selected patients with
severe respiratory failure due to severe
ARDS and profound hypoxaemia.
Methods
This was a retrospective observational study
performed in a 19-bed ICU of a tertiary hospital
in Hong Kong. Eligible patients needed to have
potentially reversible causes for respiratory failure,
refractory respiratory failure despite maximum
conventional ventilator support, and Murray score
of 3.0 or higher (Murray score4 is calculated by:
PaO2/FiO2 ratio, positive end-expiratory pressure [PEEP], lung compliance, chest radiographic appearance).
Patients with acute-status asthmaticus and refractory
respiratory failure were also selected as candidates
for ECMO despite having Murray score of lower than
3.0. Patients were excluded for ECMO therapy if
they had intracranial bleeding; severe, irreversible
brain damage; or were older than 70 years.
Extracorporeal membrane oxygenation retrieval
The QEH ECMO team supports eligible patients
from other ICUs that do not have ECMO service.
The QEH ECMO retrieval team puts eligible patients
in the referring hospitals on ECMO circuit, and then
escorts them to QEH. The team consists of two
intensivists and two intensive care nurses.
Technique of extracorporeal membrane oxygenation setup
Access catheters (Maquet HLS, Germany; BIOLINE
coating) were inserted in either the right or left
femoral vein, and return catheters were inserted
in the right internal jugular vein. All cannulation
procedures were performed at the bedside, by ICU
specialists, with Seldinger technique and ultrasound
guidance. The size of the cannulas was chosen
according to the body weight of patients. The default
size was 19 Fr for return catheter and 23 Fr for access
catheter. The jugular-femoral approach for return
(19 Fr) and access (23 Fr) catheter cannulation
was adopted for all patients. The catheters were
connected to the ECMO machine (either Rotaflow:
BE-PLS 12050–Quadrox PLS [Jostra], or Cardiohelp:
HLS module advanced 7.0).
Extracorporeal membrane oxygenation care
As per our ICU ECMO protocol, ECMO nurses
and ECMO specialists have to provide special
regular monitoring of coagulation status, circuit
conditions, perfusion status, and neurological status.
Accordingly, unfractionated heparin infusion is the
default and only anticoagulant used. Anticoagulation
is monitored at the bedside with a target-activated
clotting time of 180-220 seconds. Activated clotting
time is measured every 4 hours. We maintain a
platelet count of 100 x 109 /L, international normalised
ratio of <1.5, and haemoglobin level of >120 g/L.
The ECMO nurses need to check the following
every 4 hours: presence of clot in the oxygenator
membrane, any colour difference between the access
and return catheters, and oxygenator membrane
pressure gradient. The post-oxygenator partial
pressure of oxygen and free-haemoglobin level are
checked daily.
Other routine care
We use benzodiazepine and narcotics for sedation. Pupil size, sedation score, and conscious status are
assessed every 4 hours. Propofol is not recommended
due to the potential interaction with oxygenator
membrane. Enteral nutrition is used when possible,
and as early as possible, according to our ICU feeding
protocol. Fluid balance is maintained with diuretics
and continuous v-v haemofiltration, as clinically
indicated.
Ventilation strategy
Once the ECMO support is started, we change the
ventilator setting so as to allow ‘lung rest’ (ie FiO2
0.4, PEEP 10 cm H2O, tidal volume 4 mL/kg, rate 10
cycles/min) with an inspiratory/expiratory ratio of
1:1.3.
Renal replacement therapy
Continuous v-v haemofiltration is used for patients
with acute kidney injury, excessive fluid gain, and
metabolic acidosis. The venous and arterial lines are
connected at post-pump to minimise the risk of air
embolism.
Decannulation
Heparin infusion is stopped 30 minutes before
decannulation. Decannulation is performed at the bedside with two-team approach. Both jugular and
femoral catheters are removed simultaneously.
Direct pressure is then applied to the sites for at least
15 minutes.
Statistical analysis
Normally distributed data were expressed as mean
± standard deviation (SD). Independent t test
was used for comparison of means. Data, if not
normally distributed, were expressed as median and
interquartile range (IQR). Mann Whitney U test was
used for comparison of medians. Categorical data
were analysed using Fisher’s exact test. Statistical
analysis was performed using the Statistical Package
for the Social Sciences (Windows version 17; SPSS
Inc, Chicago [IL], US). P values of <0.05 were
considered statistically significant.
Ethics review
This proposal was reviewed and approved by the
Research Ethics Committee of the Kowloon Central
Cluster/Kowloon East Cluster (Kowloon Central/
Kowloon East; REC [KC/KE]).
Results
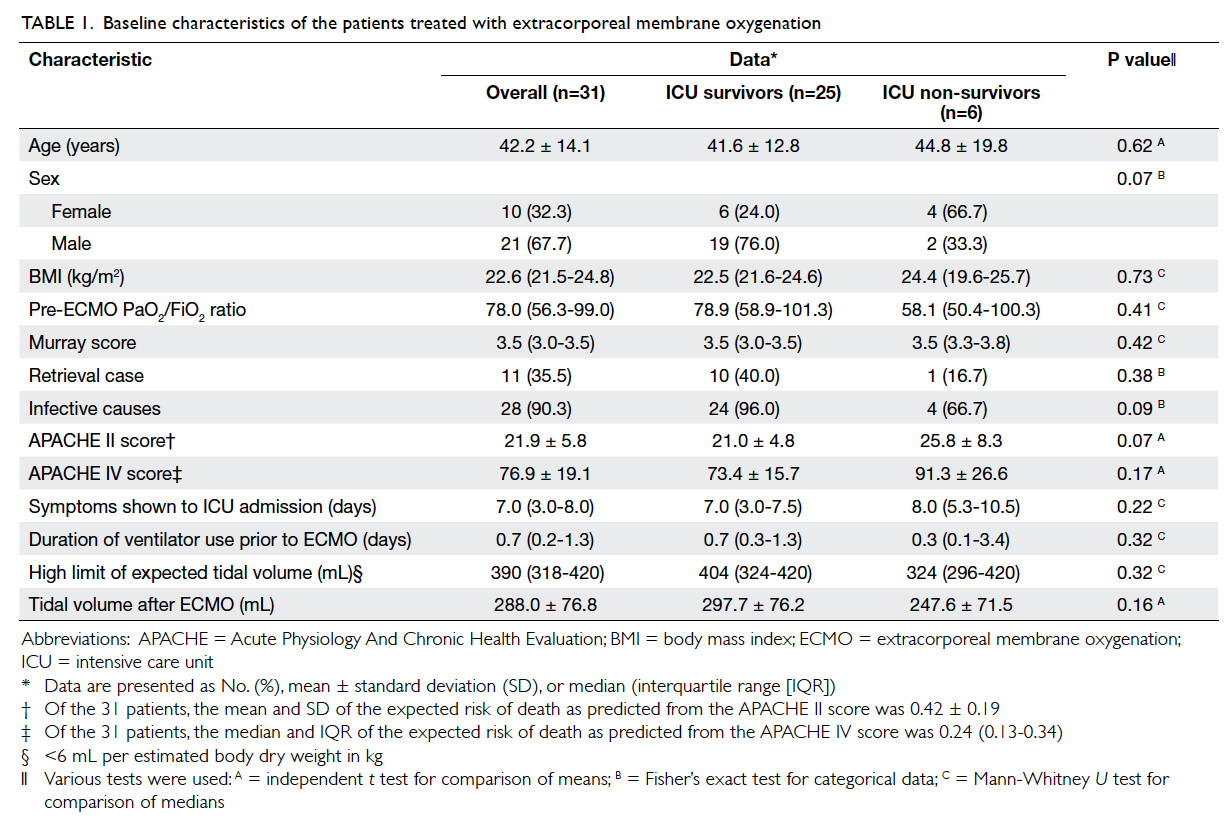
Between 1 July 2010 and 30 June 2013, 31 patients (mean ± SD, 42.2 ± 14.1 years; 21 males) received v-v ECMO
for the treatment of severe respiratory failure and
ARDS. The median body mass index was 22.6 (IQR,
21.5-24.8) kg/m2. The median Murray score was 3.5
(IQR, 3.0-3.5). A total of 11 cases were retrieved from
other acute hospitals. The median time required
for patients to arrive at the ICU was 7.0 (IQR, 3.0-8.0)
days (Table 1). The mean duration of mechanical
ventilation before starting ECMO treatment was 1.6
± 2.7 days.
Male gender and younger age were associated
with better survival rate, although they did not attain
statistical significance. Survivors and non-survivors
had similar Murray scores. Survivors had a higher
pre-ECMO PaO2/FiO2 ratio, lower APACHE (Acute
Physiology And Chronic Health Evaluation) II and
APACHE IV scores, and shorter time for symptoms
to ICU admission versus the non-survivors, but
none of the differences was statistically significant
(Table 1). Of the 31 patients who presented with
respiratory failure, 28 (90.3%) were diagnosed to have pneumonia, one had severe smoke inhalation injury,
and two had status asthmaticus; 22 of the 28 pneumonia
patients had identifiable laboratory causes (Table 2).
Patients suffering from viral infection as primary
cause of respiratory failure (1 dead/11 alive) had
better ICU survival than those suffering from
bacterial infection (2 dead/8 alive); however, the
difference was not statistically significant (92% vs
80%, P=0.57, Fisher’s exact test; Table 2). Overall,
nine (29.0%) patients were diagnosed to have H1N1
infection, either by polymerase chain reaction or
serology or both. Patients with H1N1 as the cause
of respiratory failure had excellent survival outcome
(100%; Table 3).
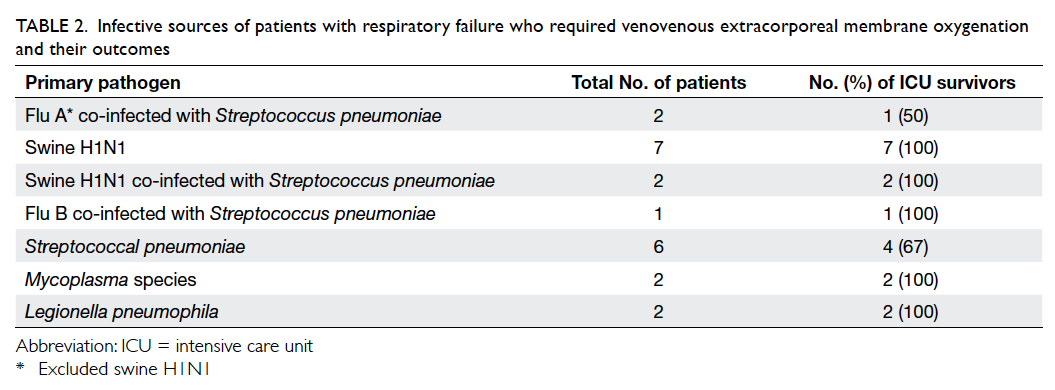
Table 2. Infective sources of patients with respiratory failure who required venovenous extracorporeal membrane oxygenation and their outcomes
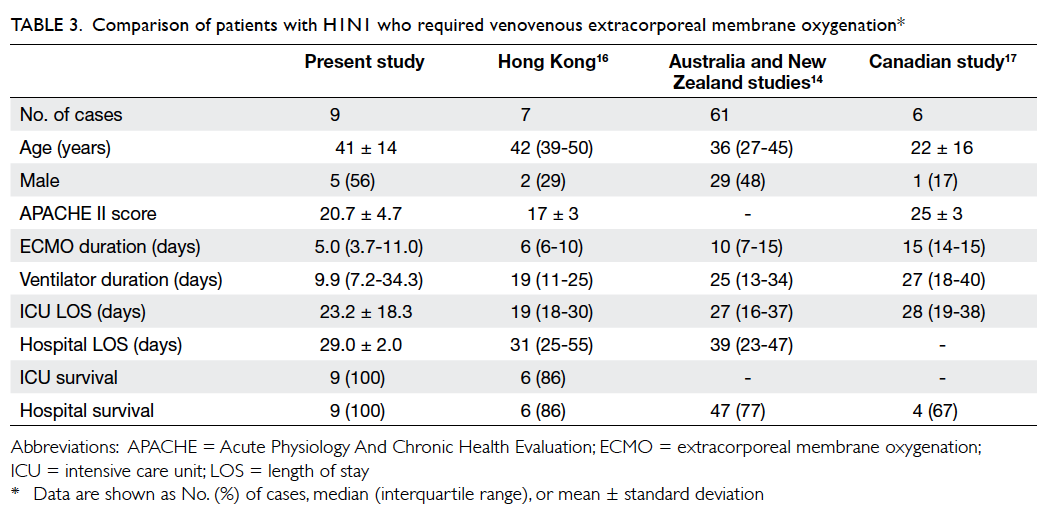
Table 3. Comparison of patients with H1N1 who required venovenous extracorporeal membrane oxygenation
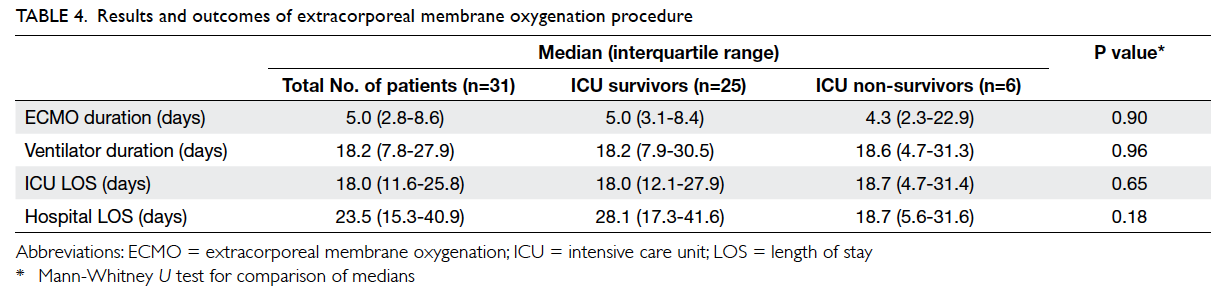
The median (IQR) duration of ECMO therapy
was 5.0 (2.8-8.6) days. The median length of ICU
stay was 18.0 (11.6-25.8) days, and median length
of hospital stay was 23.5 (15.3-40.9) days. A total of
25 (80.6%) patients survived ICU discharge and 24
(77.4%) patients survived hospital discharge and had
28-day survival (Table 4).
On logistic regression analysis, APACHE
II score was the only significant factor that could
predict hospital mortality.
Of the 31 patients, two (6.5%) patients
developed severe haemorrhage (haemothorax
[n=1] and cerebral bleeding [n=1]) and three (9.7%)
patients developed mechanical complications of
the circuits (clotted membrane [n=1], suspected
oxygenator failure [n=1], and vascular injury [n=1]).
Discussion
The first successful ECMO treatment case was
reported in 1972.5 However, two randomised
controlled trials6 7 that were published several years
after this reported case failed to show any significant
advantage with ECMO. The use of ECMO in adult
patients remained limited until publication of the
CESAR trial in 2009,3 which showed significant
advantages with ECMO in terms of survival for
patients with severe respiratory failure and ARDS
after H1N1 pandemic.
Our patients, who were managed with v-v
ECMO for severe respiratory failure, had ICU
mortality and hospital mortality of 19.4% and 22.6%,
respectively. Most of them (n=29; 93.6%) had severe
ARDS that failed conventional treatment. Our
results (7 dead/24 alive) compared favourably with
the ECLS (Extracorporeal Life Support) Registry
Report,8 in which the hospital mortality was reported
to be 44% (2283 dead/2905 alive; P=0.018 by Fisher’s
exact test). Mortality of ARDS, before 1990s, was
higher than 50%.9 10 Mechanical ventilator is the
cornerstone of treatment for ARDS. Although it can
support lung ventilation, inappropriate use can lead
to lung damage including excessive transpulmonary
pressure (barotrauma), excessive lung volume
inside alveoli (volutrauma), and shearing stress
during repetitive opening and closing of alveoli
(atelectrauma).11 Moreover, the damage caused by
mechanical ventilation is not limited to the lungs.
Lung trauma can trigger systemic inflammatory
response (biotrauma) that involves other distal
organs leading to multiorgan damage. To date,
the only strategy that can improve survival is lung
protective strategy (≤6 mL/kg of predicted body weight; plateau pressure ≤30 cm H2O).12 13 With
the use of lung protective strategy and ECMO
treatment, recent publications reported a mortality
of approximately 20% to 40%.3 14 Lung protective
strategy was the most evidence-based approach
in ARDS management. Extracorporeal membrane
oxygenation use in ARDS patients can ensure the
effective application of low tidal volume and plateau
pressure strategy.
In our report, the mean tidal volume after
ECMO therapy was 288.0 ± 76.8 mL, which was
within the higher limit of the expected tidal volume
(390 mL) according to the lung protective strategy
(Table 1). The ICU mortality and hospital mortality
rates in our cases were 19.4% and 22.6%, respectively.
These figures are favourable when compared with
patients who receive only lung protective strategy.13
In fact, ICU doctors often face challenges to comply
with the lung protective strategy in real situation.
The presence of stiff lung and hypercarbia in severe
ARDS patients may make it difficult for ICU doctors
to set low tidal volume and transpulmonary pressure.
The use of ECMO, however, can overcome these
challenges. Extracorporeal membrane oxygenation
can allow both CO2 removal and oxygenation with
an independent circuit that bypasses the sick
lungs. This permits complete lung rest with the lung
protective strategy.
H1N1 infection is widely reported to have
better survival rate and shorter duration of ECMO
support, mechanical ventilator days, and length of
ICU stay. According to the ELSO (Extracorporeal
Life Support Organization) registry (as dated to 13
April 2011), the H1N1 survival rate was 76.8% (66
dead/218 alive) in patients older than 20 years.15
In our study, all nine H1N1 swine flu patients
survived (Table 2). H1N1 patients in Hong Kong
had more favourable outcomes compared with
those in Australia and Canada (Table 3).14 16 17 These
outcomes included shorter ECMO duration, shorter
ventilator days, and shorter ICU and hospital length
of stay. Future study shall explore other factors
that affect outcomes including duration of inter-hospital
transportation, manpower availability, and
use of pharmacological treatment. In our centre, all H1N1 patients received N-acetylcysteine (NAC)
intravenous infusion together with oseltamivir
from day 0 of ICU admission. The effect of NAC,
an antioxidant18 19 20 21 as adjunct therapy in treating
severe H1N1 respiratory infection, deserves further
exploration in future.
In our study, vascular injury was the single
complication that was related to the procedure. We
encountered one oxygenator-related thrombosis
and one suspected oxygenator failure. In one case,
we postulated that the cause of thrombosis was
hypercoagulopathy related to mycoplasma infection.
Another case had contra-indication to heparin due
to active bleeding. One patient was diagnosed with
intracerebral bleeding after initiation of ECMO
therapy. The bleeding was probably related to the
patient’s own brain pathology. The patient was
diagnosed with haematological lymphoproliferative
disease that probably infiltrated the brain and caused
death, as suggested by the postmortem examination.
Limitations
Our report had several limitations. As ECMO
therapy is relatively new in our centre, we have a
limited number of cases. This study was a retrospective
review of a single-centre experience. All patients who
received ECMO therapy were carefully selected, and
we did not have a control group to demonstrate the
superiority of ECMO therapy. We only considered
mortality as our main outcome and did not follow-up
the long-term morbidity of the survivors. Future
study with ECMO shall consider outcomes that
cover physical, functional, and neuropsychological
aspects.
Conclusions
Venovenous ECMO is an effective adjunctive
therapy, useful as a life-saving procedure for carefully
selected severe ARDS patients when the limits of
standard therapy have been reached.
References
1. Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818-24. CrossRef
2. ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012;307:2526-33.
3. Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. CrossRef
4. Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet 1967;2:319-23. CrossRef
5. Hill JD, De Leval MR, Fallat RJ, et al. Acute respiratory insufficiency. Treatment with prolonged extracorporeal oxygenation. J Thorac Cardiovasc Surg 1972;64:551-62.
6. Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 1994;149:295-305. CrossRef
7. Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979;242:2193-6. CrossRef
8. Extracorporeal Life Support Organization. ECLS Registry Report. International Summary. Jul 2013.
9. Villar J, Slutsky AS. Is the outcome from acute respiratory distress syndrome improving? Curr Opin Crit Care 1996;2:79-87. CrossRef
10. Phua J, Badia JR, Adhikari NK, et al. Has mortality from acute respiratory distress syndrome decreased over time? A systematic review. Am J Respir Crit Care Med 2009;179:220-7. CrossRef
11. Tremblay LN, Slutsky AS. Ventilator-induced lung injury: from the bench to the bedside. Intensive Care Med 2006;32:24-33. CrossRef
12. Hickling KG, Walsh J, Henderson S, Jackson R. Low mortality rate in adult respiratory distress syndrome using low-volume pressure-limited ventilation with permissive hypercapnia: a prospective study. Crit Care Med 1994;22:1568-78. CrossRef
13. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301-8. CrossRef
14. Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators, Davies A, Jones D, Bailey M, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009;302:1888-95. CrossRef
15. Extracorporeal Life Support Organization. H1N1 ECMO Registry (as of April 13, 2011). ECLS Registry Report.
16. Chan KK, Lee KL, Lam PK, Law KI, Joynt GM, Yan WW. Hong Kong's experience on the use of extracorporeal membrane oxygenation for the treatment of influenza A (H1N1). Hong Kong Med J 2010;16:447-54.
17. Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 2009;302:1872-9. CrossRef
18. Nimmerjahn F, Dudziak D, Dirmeier U, et al. Active NF-kappaB signalling is a prerequisite for influenza virus infection. J Gen Virol 2004;85:2347-56. CrossRef
19. Geiler J, Michaelis M, Naczk P, et al. N-acetyl-L-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with highly pathogenic H5N1 influenza A virus. Biochem Pharmacol 2010;79:413-20. CrossRef
20. Prescott LF, Donovan JW, Jarvie DR, Proudfoot AT. The disposition and kinetics of intravenous N-acetylcysteine in patients with paracetamol overdosage. Eur J Clin Pharmacol 1989;37:501-6. CrossRef
21. Garozzo A, Tempera G, Ungheri D, Timpanaro R, Castro A. N-acetylcysteine synergizes with oseltamivir in protecting mice from lethal influenza infection. Int J Immunopathol Pharmacol 2007;20:349-54.