Hong Kong Med J 2014;20:241–50 | Number 3, June 2014 | Epub 23 May 2014
DOI: 10.12809/hkmj134167
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Current status of robot-assisted surgery
Ada TL Ng, FRCSEd (Urology), FHKAM (Surgery);
PC Tam, FRCSEd (Urology), FHKAM (Surgery)
Department of Surgery, The University of Hong Kong, Queen Mary
Hospital, Pokfulam, Hong Kong
Corresponding author: Dr PC Tam (dr.tampochor@gmail.com)
Abstract
The introduction of robot-assisted surgery, and
specifically the da Vinci Surgical System, is one
of the biggest breakthroughs in surgery since the
introduction of anaesthesia, and represents the
most significant advancement in minimally invasive
surgery of this decade. One of the first surgical uses
of the robot was in orthopaedics, neurosurgery, and
cardiac surgery. However, it was the use in urology,
and particularly in prostate surgery, that led to its
widespread popularity. Robotic surgery, is also widely
used in other surgical specialties including general
surgery, gynaecology, and head and neck surgery. In
this article, we reviewed the current applications of
robot-assisted surgery in different surgical specialties
with an emphasis on urology. Clinical results as
compared with traditional open and/or laparoscopic
surgery and a glimpse into the future development
of robotics were also discussed. A short introduction of the emerging areas of robotic surgery were also
briefly reviewed. Despite the increasing popularity
of robotic surgery, except in robot-assisted radical
prostatectomy, there is no unequivocal evidence to
show its superiority over traditional laparoscopic
surgery in other surgical procedures. Further trials
are eagerly awaited to ascertain the long-term results
and potential benefits of robotic surgery.
Introduction
The introduction of robot-assisted surgery, and
specifically the da Vinci Surgical System, is one
of the biggest breakthroughs in surgery since the
introduction of anaesthesia, and represents the
most significant advancement in minimally invasive
surgery of this decade. One of the first surgical uses
of the robot was in orthopaedics, neurosurgery, and
cardiac surgery. However, it was the use in urology,
and particularly in prostate surgery, that led to its
widespread popularity.1 Robotic surgery is also
widely used in other surgical specialties including
general surgery, gynaecology, and head and neck
surgery.
Urology has long been adoptive to advances
in technology. It is not surprising that soon after
robotic technology was first applied to medical
science, it was well received by the urology
community. Robotic surgery has applications in
many aspects of urological surgery. Since 1998, there
have been over 4000 peer-reviewed publications
in various specialties on the da Vinci Surgery, of
which 46% pertain to urology, 17% to cardiothoracic
surgery, 13% to general surgery, 8% to gynaecology,
7% to general surgical topics (including outcomes,
trends, and cost-effectiveness for different types of
robotic surgery), 4% to paediatric surgery, and 2% to
otorhinolaryngology.2
Literature review of current applications of robotics in different surgical specialties with
an emphasis on urology was performed. Clinical
results as compared with traditional open and/or
laparoscopic surgery and a glimpse into the future
development of robotics will be discussed. A short
introduction on emerging areas of robotic surgery
will also be briefly reviewed.
History of the surgical robot
The world’s first surgical robot, ‘Arthrobot’, was
born in 1983 and was designed to assist orthopaedic
procedures. In 1985, PUMA 560 (Unimate, New
Jersey, US) was used to precisely place a needle for
computed tomography–guided brain biopsy. This
was followed in 1988 by ROBODOC (Integrated
Surgical Systems, Delaware, US), a system used in
total hip arthroplasty to allow precise preoperative
planning, and to mill out precise fittings in the femur
for hip replacement. The first application in urology
occurred in 1988 at Imperial College (London, UK)
with the use of the PROBOT in clinical trials to
perform transurethral surgery. In 1993, Computer
Motion, Inc (Santa Barbara [CA], US)—the original
leading medical robots supplier—released AESOP
(Automated Endoscopic System for Optimal
Positioning), a robotic arm to assist in laparoscopic
camera holding and positioning. The CyberKnife
(Accuray, Sunnyvale [CA], US) was introduced in
1994 for stereotactic radiosurgery in neurosurgery. The year 1998 was a significant landmark, with
the introduction of ZEUS Robotic Surgical System
(Computer Motion, Inc) and the da Vinci Surgical
System (Intuitive Surgical, Inc, Sunnyvale [CA], US).
Both systems comprised a surgical control centre
and robotic arms. The first da Vinci robotic surgical
procedure was a robot-assisted heart bypass, and
it took place in Germany in 1998.3 In 2000, the da
Vinci robot was given approval by the US Food and
Drug Administration (FDA) for use in laparoscopic
procedures. The first reported robot-assisted radical
prostatectomy (RARP) took place in Paris, France,
in the same year.4 Intuitive Surgical, Inc took over
Computer Motion, Inc in 2003 and is now the
sole company marketing robotic surgical devices.
Other companies such as Olympus and Samsung
are developing new robotic surgical systems, with a
promise of lower cost and more compact machines.
The da Vinci Surgical System
The da Vinci Surgical System comprises three
components: a surgeon’s console, a patient-side
robotic cart with four robotic arms manipulated by
the surgeon (one to control the camera and three
to manipulate instruments), and a high-definition
three-dimensional (3D) vision system. Articulating
surgical instruments are mounted on the robotic
arms which are introduced into the body through
cannulas.2 The US FDA approved the system for
general laparoscopic surgery (gallbladder diseases
and reflux) in July 2000, for urological procedures
in 2001, for mitral valve repair surgery in November
2002, and for gynaecological conditions in 2005.
Advantages and cost-effectiveness
of the robotic surgery system
Robotic surgery by the da Vinci Surgical System
(Intuitive Surgical, Inc) has been popularised by its widespread usage in radical prostatectomy (RP).
The robotic system overcomes the limitations of
the standard laparoscopic approach and allows for
precise dissection in a confined space and hence the
increasing application of robot-assisted laparoscopic
prostatectomy in expert centres. These advantages
include stable operator-controlled camera, high-definition
3D magnified view of 10 to 12 times,
articulating instruments with seven degrees of
freedom, motion scaling, and tremor filtration.
Moreover, carbon dioxide insufflation during the
procedure helps in reduction of venous ooze, thus
leading to improved visualisation and reduced
blood loss.5 Across different specialties, the majority
of robotic surgeries have been associated with a
decreased length of stay, and fewer complications
including a lower transfusion rate and in-hospital
death rate.6 However, robot-assisted laparoscopic
surgery is costlier than laparoscopic surgery and
open surgery.
An analysis of new technology and health
care costs of 20 different robot-assisted surgeries
published in the New England Journal of Medicine
in 20107 showed that the use of the robot added 13%
(US$3200) to the total average cost of a procedure in
2007. However, there were no large-scale randomised
trials to definitely show that robot-assisted surgery
was superior to other procedures.7
Additional studies are needed to better
delineate the comparative and cost-effectiveness
of robot-assisted laparoscopic surgery relative to
laparoscopic surgery and open surgery. Robotic
surgery provides similar postoperative outcomes
to laparoscopic surgery but has a reduced learning
curve. Although costs are currently high, increased
competition from manufacturers and wider
dissemination of the technology may drive costs
down. Further trials are needed to evaluate long-term
outcomes in order to fully evaluate the value of
robots in surgical procedures.8
Application in urology
There has been a continuous expansion of robot-assisted
surgery for both upper and lower urinary
tract diseases in urology. This is especially true in
robotic prostatectomy, where the initial reports of
robotic prostatectomy by Menon et al9 led to an
exponential growth of robotic surgery in clinically
localised prostate cancer. More recently, there
has been an increasing number of robotic renal
surgeries10 and robotic cystectomy in centres of
excellence.11
Robotic radical prostatectomy
Prostate cancer is the most common solid organ
malignancy in men in the US, and the second
leading cause of cancer death. It is the second most common cancer in the world, with a world age-standardised
rate of 28 per 100 000 males.12 There is
a rapidly increasing incidence of prostate cancer in
Asian countries due to a more westernised lifestyle.13
In Hong Kong, prostate cancer is the third most
common cancer, accounting for 10.7% of all male
malignancies; it is the fifth major cause of cancer
death, responsible for 4.1% of all cancer deaths in
Hong Kong.14
Radical prostatectomy is a standard treatment
option for localised carcinoma of the prostate, with
a demonstrated survival advantage when compared
with watchful waiting in the randomised controlled
trial SPCG-4 (Scandinavian Prostate Cancer Group
Study No. 4).15 Radical prostatectomy showed a
significant relative risk reduction in cancer-specific
mortality as compared with watchful waiting—44%
decrease at 10 years, 35% at 12 years, and 38% at 15
years.15 16
However, open RP is associated with high
morbidity rates. Schuessler et al17 introduced
laparoscopic RP in 1997 with the aim of reducing
morbidity. The advantages of laparoscopic
prostatectomy, as reported in initial expert series,
showed a lower mean blood loss and transfusion rate,
decreased mean hospital stay, and earlier removal of
the Foley catheter compared with results from open
prostatectomy series.18
However, the technical demands of
laparoscopic RP prevented its widespread use by
the average urologist, with a limited case load. The
introduction of the da Vinci Surgical System was a
breakthrough in minimally invasive prostatectomy.
Menon et al1 from the Vattikuti Urology Institute in
Detroit [MI], US are responsible for the development
and popularisation of RARP. This technique offers all the advantages of minimally invasive laparoscopic
prostatectomy with the added advantage of shorter
learning curve and improved ergonomics, leading
to the widespread use and acceptance of RARP
worldwide.
Ahlering et al19 studied the learning curve for
robotic prostatectomies, and found that the robotic
system might significantly shorten the learning
curve for an experienced open yet laparoscopy-naïve
surgeon. The learning curve for achieving 4-hour
proficiency has been shown to be 12 patients.19
Robot-assisted RP has overtaken open RP as
the most common surgical approach for RP ever
since the FDA approval in 2001, and is estimated to
account for approximately 80% of all RP procedures
in the US.20
However, the rise in robotic procedures was
initially not backed by any evidence on clinical
benefits. No randomised trial showed the benefits
of robotic surgery until the publication of the
nationwide series by Trinh et al.21 Data from this
series demonstrated superior adjusted perioperative
outcomes after RARP in virtually all examined
outcomes. Of 19 462 RPs, 61.1% were RARPs, 38.0%
were open RPs, and 0.9% were laparoscopic RPs. In
multivariable analyses, patients undergoing RARP
were less likely to receive a blood transfusion (odds
ratio [OR]=0.34; 95% confidence interval [CI], 0.28-
0.40), to experience an intra-operative complication
(OR=0.47; 95% CI, 0.31-0.71) or a postoperative
complication (OR=0.86; 95% CI, 0.77-0.96), and to
experience a prolonged length of stay (OR=0.28; 95%
CI, 0.26-0.30) [Table 121].
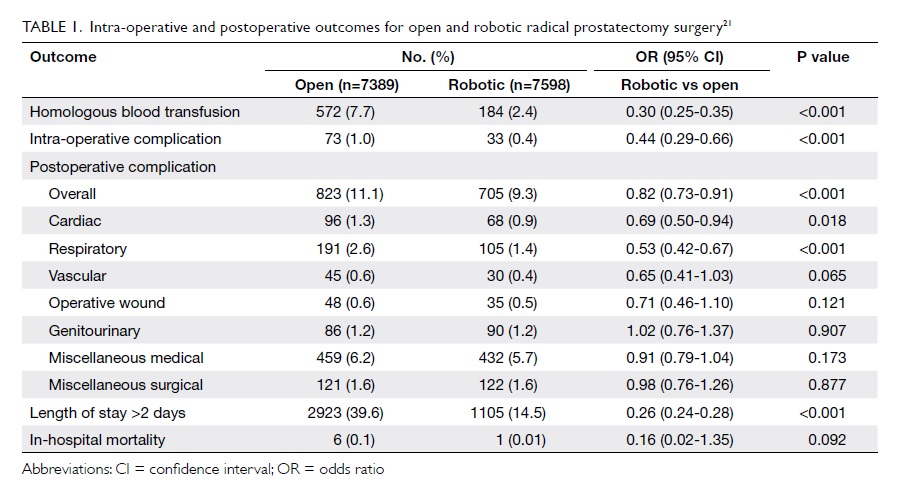
Table 1. Intra-operative and postoperative outcomes for open and robotic radical prostatectomy surgery21
A recent territory-wide review in Hong Kong22
showed that a total of 235 patients underwent
RARP between 2005 and 2009, with a 37.3% rate of trifecta (cancer cure, continence, and return of
sexual function) at 12 months, demonstrating the
feasibility, safety, and efficacy of RARP in low-to-intermediate
volume centres. In a series from a
high-volume centre, trifecta rates at 6 weeks, 3, 6, 12
and 18 months after RARP were 43%, 65%, 80%, 86%
and 91%, respectively.23
However, the majority of urologists in Hong
Kong are not from high-volume centres, thereby,
not being able to achieve these benchmark and
commendable results. Thus, it is now debated
whether robotic prostatectomy should be limited
to high-volume centres of excellence. A randomised
trial of open versus robot-assisted RP was
commenced in October 2010 in Australia.24 Overall,
200 men per treatment arm (400 men in total) are
being recruited after diagnosis and before treatment
through a major public hospital out-patient clinic
and randomised to robotic prostatectomy or open
prostatectomy. Clinical outcomes, quality-of-life
outcomes, and cost-effectiveness are being critically
and prospectively analysed to compare outcomes.24
To date, more than 250 patients have been recruited.
Results are eagerly awaited.25
Robotic partial nephrectomy
In the recent decade, there has been a stage and
size migration of renal tumours. Less than 10% of
new cases present with the classic triad of gross
haematuria, loin pain, and mass. The incidence of
small renal mass has increased by 3.7% per year
with widely available abdominal imaging such as
ultrasonography over the past decade.26 Numerous
studies have shown that renal insufficiency is
associated with increased cardiovascular events,
hospitalisation, and mortality,27 leading to increasing
role of renal-preserving strategies in the treatment
of localised renal cell carcinoma. Data from more
than 2000 patients who underwent surgery at
Memorial Sloan Kettering Cancer Center from 1989
to 2005 showed that radical nephrectomy was an
independent factor for new-onset chronic kidney
disease.28 According to the European Association
of Urology guidelines on renal tumour, nephron-sparing
surgery is the standard procedure for
solitary renal tumours measuring up to 7 cm in
diameter.29 Benefits of nephron-sparing surgery over
radical nephrectomy include equivalent oncological
outcome in tumours measuring less than 4 cm,
and probably up to 7 cm in diameter, avoidance of
overtreatment of benign lesions which account for
up to 20% of small renal masses, further treatment
options available if contralateral kidney recurrence
occurs, better quality of life, and decreased overall
mortality.30 Moreover, both procedures have
comparable survival rates.30
Open partial nephrectomy (OPN) currently
remains the standard procedure for partial nephrectomy. However, OPN is associated with
significant morbidity: the muscle-cutting flank
incision may involve removal of a lower rib, leading
to flank bulge, pain, paraesthesia, and hernia
formation. The introduction of laparoscopic partial
nephrectomy was aimed at reducing the morbidity
associated with OPN.
Laparoscopic partial nephrectomy offers
the advantages of shorter length of stay, decreased
operative blood loss, and a shorter operating time
versus OPN. However, it is associated with longer
warm ischaemic time, more postoperative urological
complications, and increased number of subsequent
procedures. State-of-the-art surgical expertise and
technique are prerequisites for laparoscopic partial
nephrectomy.31 Thus, the procedure is not routinely
performed in many centres in view of its prolonged
learning curve.
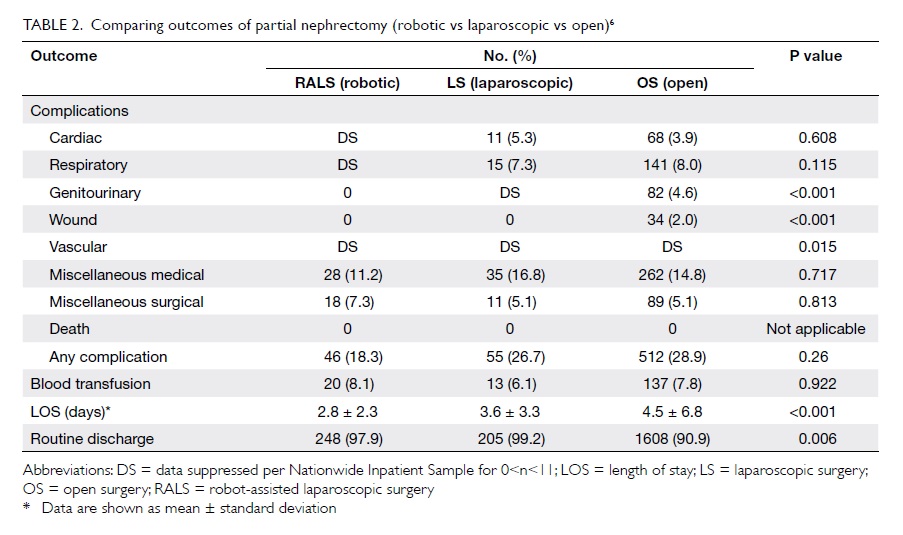
Robot-assisted partial nephrectomy shows
promise in bridging the gap between open and
laparoscopic approaches, providing similar
oncological results to radical nephrectomy and
improved morbidity with a shorter learning curve
than laparoscopic partial nephrectomy. Robot-assisted
partial nephrectomy has been shown to be
a safe and viable alternative to laparoscopic partial
nephrectomy in some published case series,32 33
providing equivalent early oncological outcomes to
laparoscopic partial nephrectomy, and the additional
advantages of decreased hospital stay, less intra-operative
blood loss, and shorter warm ischaemic
time averaging less than 20 minutes. Moreover,
operative parameters for robot-assisted partial
nephrectomy are less affected by tumour complexity
and surgical expertise of the surgeon as compared
with laparoscopic partial nephrectomy. A case series
published by our centre34 showed that robot-assisted
laparoscopic partial nephrectomy was technically
feasible, with the advantage of statistically significant
decreased warm ischaemic time (31 vs 40 minutes;
P=0.032; Table 26).
Robotic cystectomy
Radical cystectomy and pelvic lymph node
dissection are the standard treatment options for
muscle-invasive carcinoma of the bladder. However,
this procedure is associated with high morbidity of
up to 50% and mortality of up to 5%, even in centres
of excellence.35 Data from the Surgical Outcomes
Monitoring & Improvement Program Report of the
Hong Kong Hospital Authority showed that radical
cystectomy is a surgical procedure associated with
the highest morbidity and mortality among all
surgical operations in Hong Kong.36 From 2009 to
2010, the 30-day crude mortality rate was 9.7%, and
the 30-day crude morbidity rate was 65.3%.36
Laparoscopic cystectomy was introduced
with the aim of decreasing associated morbidity and length of hospital stay. The first laparoscopic
radical cystectomy was performed in 1992.37 Case
series performed at expert centres showed that
when compared with open surgery, laparoscopic
cystectomy resulted in a lower morbidity rate with
significantly lower intra-operative blood loss and
transfusion rates, lower pain scores, and allowing a
more rapid resumption of oral intake and a shorter
hospital stay.38 However, laparoscopic radical
cystectomy is technically challenging, with a steep
learning curve.
Robot-assisted radical cystectomy (RARC) was
introduced as an attempt to offset the high technical
skill required for laparoscopic cystectomy, and was
the first procedure performed in 2003 by Beecken et
al.39 A recent retrospective analysis40 on consecutive
series of patients undergoing radical cystectomy
(100 RARCs and 100 open radical cystectomies)
with curative intent over a 4-year period suggests
that patients undergoing RARC have perioperative
oncological outcomes comparable with open
radical cystectomies, and lower overall and major
complication (Clavien score ≥3) rates (35% vs 57%;
P=0.001 and 10% vs 22%; P=0.019, respectively), less
blood loss, and shorter hospital stay versus open
radical cystectomies. There were no significant
differences between the two groups for pathological
outcomes, including stage, number of nodes
harvested, or positive margin rates.40
Although the results for RARC are encouraging,
long-term functional and oncological control rates
are still unknown. Randomised, multi-institutional
comparisons of these techniques will be required
before widespread adoption of the procedure.
Other robotic applications in urological
surgery
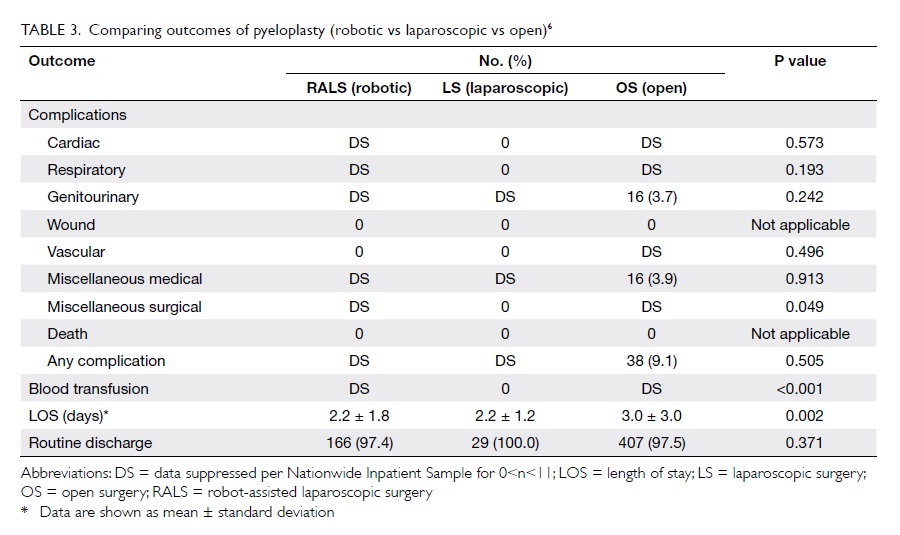
Reconstructive procedures including pyeloplasty,
ureteric reimplantation, appendicovesicostomy, and
augmentation enterocystoplasty are increasingly
performed with the assistance of the robot.41 Data
on pyeloplasty showed that the robotic approach
is associated with a lower transfusion rate and a
shorter length of stay as compared with the open and
laparoscopic approaches (Table 36).
Robot-assisted microsurgery is being utilised
to a greater degree in andrology including procedures
such as vasectomy reversal, subinguinal
varicocelectomy, targeted spermatic cord denervation
(for chronic orchialgia), and microsurgical testicular
sperm extraction.42
Application in gynaecology
The da Vinci Surgical System was approved for
use in gynaecological surgery in the US in 2005.
Applications of robotics in gynaecology include
hysterectomy, myomectomy, oophorectomy,
ovarian cystectomy, resection of endometriosis and
lymphadenectomy, with an increasing role of robotic
surgery in gynaecological oncology.
Endometrial carcinoma is the most common
malignancy of the female reproductive organs and
the consensus in the literature is that robotic surgery
is preferable to open surgery and is equivalent to
laparoscopy in many aspects.43 The robotic platform
offers distinct advantages in certain populations,
such as the morbidly obese, and is becoming a
commonly used procedure.43
Similarly, in cervical carcinoma, the published
data comparing robotic radical hysterectomy to
traditional laparoscopy or laparotomy showed that
the robotic approach produces more favourable
perioperative outcomes, including a lower blood loss,
shorter length of stay, and equivalent or lower rates
of intra-operative and postoperative complications.44
Hysterectomy for benign conditions is one
of the most commonly performed procedures
in women, with a one in nine chance of a woman
undergoing the procedure in her lifetime.45 Between
2007 and 2010, the utilisation of robot-assisted
hysterectomy for benign gynaecological disorders
increased substantially. However, robot-assisted and
laparoscopic hysterectomy had similar morbidity
profiles, offered little short-term benefit, but resulted
in substantially more costs.46 A 2012 Cochrane review
of robotic surgery for benign gynaecological diseases
showed that robotic surgery was not associated with
improved effectiveness or safety, but increased the
cost of the procedure substantially.47
The existing limited evidence shows that
robotic surgery does not benefit women with
gynaecological diseases in terms of effectiveness or
safety. Further well-designed randomised controlled
trials with complete reported data are required to
confirm or refute this conclusion.
Application in colorectal surgery
Laparoscopic colorectal surgery has become the
preferred standard of care in colorectal surgery and
has been proven to be as safe and effective as open
surgery, and associated with a lower blood loss and
shorter length of stay. Robotic technology aims to overcome some of the limitations of conventional
laparoscopic surgery. However, the role of robotics in
colorectal surgery remains controversial. Delaney et
al48 compared robotic versus traditional laparoscopic
colorectal surgery, and reported that robotic
colectomy was a feasible and safe procedure, but
involved greater costs and longer operating times.
In a comparative study between robotic versus
laparoscopic right hemicolectomy, deSouza et al49
reported that the robotic approach was safe and
feasible, but associated with longer operating times
and higher costs as compared with pure laparoscopic
approach. However, there were similar rates of
overall morbidity, lymph node dissection, blood loss,
conversion rate, and length of hospital stay in both
groups, showing no benefit of robotic approach for
right hemicolectomy over laparoscopic surgery.
The emerging role of robotic surgery in
colorectal conditions is in rectal pathologies,
especially in patients with a narrow pelvis. Total
mesorectum excision (TME) has been established
as a standard surgical technique in rectal cancer
surgery.50 Laparoscopic TME in a narrow pelvis
and locally advanced disease is a technically
demanding procedure, and it is associated with a
high conversion rate, high positive surgical margin,
and poor continence and erectile function.51 52
Robotic nerve-sparing TME was shown in
a randomised study to have significantly shorter
length of stay (6.9 days vs 8.7 days, P<0.001) with
similar mean operating time, conversion rate, and
specimen quality as compared with its counterpart
laparoscopic procedure.53 In another series by Kim
et al,54 robotic TME showed a shorter recovery time for erectile function as compared with laparoscopic
TME (6 vs 12 months). The authors postulated
that the precise identification of anatomical planes
and smaller neural components was facilitated by
magnified view and superior movement of wristed
robotic instruments.54
Recent studies48 49 50 51 52 53 54 55 have confirmed robotic
colorectal surgery to be feasible and oncologically
safe with potentially significant benefits in rectal
surgery. However, we await long-term results
concerning oncological outcome.
Application in general surgery
The application of robotics in general surgery has
been evolving, and the number of procedures has
been growing over the past decade, especially in
bariatric surgery, fundoplication, and hepatobiliary
surgery, although robotic approach is not routinely
employed for those procedures.
Bariatric procedures can be complex and
challenging in view of large patients, large livers,
thick abdominal walls and substantial visceral fat,
making exposure, dissection and reconstruction
difficult. The first robotic bariatric procedure was
an adjustable gastric banding procedure performed
by Belgian surgeons in September 1998.56 Since
then, the robotic approach has become an option
to standard laparoscopy. Robotic procedures in
bariatric surgery include robotic adjustable gastric
banding, robotic sleeve gastrectomy, robotic gastric
bypass, and biliopancreatic diversion with duodenal
switch.57 Robotic bariatric procedures appear to have
a decreased rate of gastro-intestinal leaks, lower risk
of needing follow-up surgery, and a lower conversion
rate to open surgery.58
Robotic Heller myotomy for achalasia has
been shown to result in fewer oesophageal tears, and
improved quality of life after surgery in studies as
compared with traditional laparoscopic surgery.59
Local data on the feasibility and safety of
robotic surgery for hepatocellular carcinoma
showed favourable short-term outcomes, including
hospital mortality and morbidity rates of 0% and
7.1%, respectively; the mean hospital stay was 6.2
days. The 2-year overall and disease-free survival
rates were 94% and 74%, respectively. However, the
long-term oncological results remain uncertain.60
Application in endocrine surgery
Thyroid surgery is traditionally performed via a
collar incision. However, with a large portion of
patients being young females, there is a demand for
avoiding the transverse cervical incision. This led to
the introduction of endoscopic techniques, with the
advantages of better cosmetic outcome and reduced
paraesthesia of the anterior neck.61 However, these
endoscopic techniques are technically demanding and time-consuming.
The introduction of the da Vinci Surgical
System has further revolutionised the surgical
management of thyroid diseases. Robotic surgery
overrides the drawbacks of endoscopic surgery,
being associated with better visualisation and
improved fine manipulation within the deep
and narrow cervical space. Better visualisation is
achieved through 10 to 12 times of magnification and
3D images, facilitating enhanced precise anatomical
dissection. Robotic thyroidectomy is also associated
with a shorter learning curve than endoscopic
thyroidectomy and causes less musculoskeletal
strain to the surgeon.62
The use of robots in thyroid surgery is rapidly
increasing. Results are promising in case series,
with more than 6000 procedures being performed
in Korea between 2007 and 2011.63 However,
randomised controlled trials comparing robotic with
conventional open or endoscopic surgery are needed
to assess the long-term oncological outcomes and
functional outcomes.63
Application in head and neck surgery
The use of robotics in the field of head and neck
surgery was adopted recently, with the first case
series published in 2006.64 Robotic surgery allows
transformation of open surgical management of
head and neck cancer to a transoral minimally
invasive approach. Robotic approach in head and
neck surgery has provided surgeons with the ability
to access anatomical locations that were previously
managed only via open techniques. This has resulted
in decreased overall morbidity and excellent
functional results with equivalent oncological
outcomes. Transoral robotic surgery provides access
to the oropharynx, hypopharynx, larynx, oral cavity,
parapharyngeal space, and skull base via the oral
aperture. It is useful in resection of the tumour and
in free-flap reconstruction.
The advantages of robotic surgery in patients
with head and neck cancer are access to anatomical
sites not accessible to conventional endoscopy,
absence of a neck incision, absence or decreased
duration of tracheotomy, absence or decreased
duration of nasogastric or gastric feeding tube, and
decreased length of hospital stay.65
Studies have shown that transoral robotic
surgery is a feasible option for surgical management
of head and neck tumours, which is associated with
reduced morbidity.65 66 However, long-term data are
required for oncological outcomes.
Application in cardiothoracic surgery
The first robotic cardiac procedure was performed in the US in 1999,67 and was one of the earliest
applications of robotic surgery. Robotic cardiac
surgical procedures have been performed to repair
and replace the mitral valve, bypass coronary arteries,
close atrial septal defects, implant left ventricular
pacing leads, and resect intracardiac tumours.
A US study compared robotic sternotomy and
thoracotomy approaches to mitral valve surgery
outcomes in more than 700 patients with mitral
valve disease over a 3-year period. The median
cardiopulmonary bypass time was 42 minutes
longer for robotic than complete sternotomy, 39
minutes longer than for partial sternotomy, and 11
minutes longer than for right mini-anterolateral
thoracotomy (P<0.0001). Moreover, the robotic
procedure was associated with a longer median
myocardial ischaemic time compared with
conventional procedures (P<0.0001). The quality
of mitral valve repair was similar among matched
groups. Neurological, pulmonary, and renal
complications were similar among groups. However,
the robotic approach was associated with the lowest
occurrences of atrial fibrillation and pleural effusion
and the shortest hospital stay (median 4.2 days); the
hospital stays with robotic surgery were 1.0, 1.6,
and 0.9 days shorter than for complete sternotomy,
partial sternotomy, and right mini-anterolateral
thoracotomy, respectively (P<0.001 for all
comparisons). This series showed that robotic repair
of posterior mitral valve leaflet prolapse is as safe
and effective as conventional approaches. Technical
complexity and longer operating times for robotic
repair are compensated for by lesser invasiveness
and shorter hospital stay.68
Robotic thoracic procedures include resection
of primary lung cancer, oesophageal tumours, thymic
diseases, and mediastinal tumours.69 Another US
series with 168 patients which compared patients
who underwent robotic pulmonary resection with
propensity-matched controls undergoing lobectomy
by rib- and nerve-sparing thoracotomy showed
that the robotic group had reduced morbidity
(27% vs 38%; P=0.05), lower mortality (0% vs 3.1%;
P=0.11), improved mental quality of life (53 vs 40;
P<0.001), and shorter hospital stay (2.0 vs 4.0 days;
P=0.02). Moreover, with the additional technical
modification of completely portal robotic lobectomy
with four arms, both the median operating time
(3.7 vs 1.9 hours; P<0.001) and conversion rates to
traditional thoracotomy (12/62 vs 1/106; P<0.001)
were lowered.69
Despite being one of the first specialties to
utilise the robotic technology, it is still unclear
whether the technical advantages bring about direct
merits for patients. Results have been mixed, with
no unequivocal evidence on benefits of the robotic
approach. Further evidence is awaited on the use of
robotics in the cardiothoracic field.
Future applications for robotics
Laparoendoscopic single-site surgery (LESS) and
natural orifice transluminal endoscopic surgery are
novel techniques that have the potential to further
minimise the invasiveness and morbidity of surgery.
However, the technical difficulty of the procedure is
increased with the need for specialised instruments.
Robotic technology is rapidly evolving, and with
the development of new robotic prototypes for
single-port surgery, it is expected that robotic-LESS will move forward with the goal of minimising
complications and improving outcomes.70
Conclusion
Robotic surgery with the da Vinci Surgical System
is increasingly being applied in a wide range of
surgical specialties, especially in urology. It aims to
improve outcomes as compared with open surgery,
and to overcome the limitations of laparoscopic/
thoracoscopic techniques. Despite the increasing
popularity of robotic surgery, except in RARP, there
is no unequivocal evidence to show the superiority of
robotic surgery over traditional laparoscopic surgery
in other surgical procedures. Cost-effectiveness
is also an issue due to the high installation and
maintenance costs. We eagerly await the introduction
of different robotic systems by competitors. Further
randomised studies are required to ascertain the
long-term results and potential benefits of robotic
surgery. We eagerly await the results of the ongoing
randomised trial of open versus robotic RP from
Australia.
Declaration
No conflicts of interest were declared by authors.
References
1. Menon M, Tewari A, Baize B, Guillonneau B, Vallancien G. Prospective comparison of radical retropubic prostatectomy and robot-assisted anatomic prostatectomy: the Vattikuti Urology Institute experience. Urology 2002;60:864-8. CrossRef
2. Intuitive Surgical company website. Available from: http://www.intuitivesurgical.com/company/media/publications/da-vinci-surgery-high-LOE-publications-en-031114.pdf. Accessed Apr 2014.
3. Pugin F, Bucher P, Morel P. History of robotic surgery: from AESOP® and ZEUS® to da Vinci®. J Visc Surg 2011;148(5 Suppl):e3-8. CrossRef
4. Abbou CC, Hoznek A, Salomon L, et al. Remote laparoscopic radical prostatectomy carried out with a robot. Report of a case [in French]. Prog Urol 2000;10:520-3.
5. Smith JA Jr, Herrell SD. Robotic-assisted laparoscopic prostatectomy: do minimally invasive approaches offer significant advantages? J Clin Oncol 2005;23:8170-5. CrossRef
6. Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Hu JC. Use, costs and comparative effectiveness of robotic assisted, laparoscopic and open urological surgery. J Urol 2012;187:1392-8. CrossRef
7. Barbash GI, Glied SA. New technology and health care costs—the case of robot-assisted surgery. N Engl J Med 2010;363:701-4. CrossRef
8. Ahmed K, Ibrahim A, Wang TT, et al. Assessing the cost effectiveness of robotics in urological surgery—a systematic review. BJU Int 2012;110:1544-56. CrossRef
9. Menon M, Shrivastava A, Tewari A, et al. Laparoscopic and robot assisted radical prostatectomy: establishment of a structured program and preliminary analysis of outcomes. J Urol 2002;168:945-9. CrossRef
10. Benway BM, Bhayani SB, Rogers CG, et al. Robot assisted partial nephrectomy versus laparoscopic partial nephrectomy for renal tumors: a multi-institutional analysis of perioperative outcomes. J Urol 2009;182:866-72. CrossRef
11. Dasgupta P, Rimington P, Murphy D, Elhage O, Challacombe B, Khan MS. Robotically assisted radical cystectomy. BJU Int 2008;101:1489-90. CrossRef
12. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2: cancer incidence and mortality worldwide. IARC CancerBase No. 10. Lyon, France: IARC Press; 2010.
13. Yip SK, Sim HG. Robotic radical prostatectomy in east Asia: development, surgical results and challenges. Curr Opin Urol 2010;20:80-5. CrossRef
14. Hospital Authority, Hong Kong. Hong Kong Cancer Registry. 2010. Available from: http://www3.ha.org.hk/cancereg/Summary%20of%20CanStat%202010.pdf. Accessed Apr 2014.
15. Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2005;352:1977-84. CrossRef
16. Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2011;364:1708-17. CrossRef
17. Schuessler WW, Schulam PG, Clayman RV, Kavoussi LR. Laparoscopic radical prostatectomy: initial short-term experience. Urology 1997;50:854-7. CrossRef
18. Guillonneau B, Vallancien G. Laparoscopic radical prostatectomy: initial experience and preliminary assessment after 65 operations. Prostate 1999;39:71-5. CrossRef
19. Ahlering TE, Skarecky D, Lee D, Clayman RV. Successful transfer of open surgical skills to a laparoscopic environment using a robotic interface: initial experience with laparoscopic radical prostatectomy. J Urol 2003;170:1738-41. CrossRef
20. Skarecky DW. Robotic-assisted radical prostatectomy after the first decade: surgical evolution or new paradigm. ISRN Urol 2013;2013:157379.
21. Trinh QD, Sammon J, Sun M, et al. Perioperative outcomes of robot-assisted radical prostatectomy compared with open radical prostatectomy: results from the nationwide inpatient sample. Eur Urol 2012;61:679-85. CrossRef
22. Yip KH, Yee CH, Ng CF, et al. Robot-assisted radical prostatectomy in Hong Kong: a review of 235 cases. J Endourol 2012;26:258-63. CrossRef
23. Patel VR, Coelho RF, Chauhan S, et al. Continence, potency and oncological outcomes after robotic-assisted radical prostatectomy: early trifecta results of a high-volume surgeon. BJU Int 2010;106:696-702. CrossRef
24. Gardiner RA, Coughlin GD, Yaxley JW, et al. A progress report on a prospective randomised trial of open and robotic prostatectomy. Eur Urol 2014;65:512-5. CrossRef
25. Gardiner RA, Yaxley J, Coughlin G, et al. A randomised trial of robotic and open prostatectomy in men with localised prostate cancer. BMC Cancer 2012;12:189. CrossRef
26. Hock LM, Lynch J, Balaji KC. Increasing incidence of all stages of kidney cancer in the last 2 decades in the United States: an analysis of surveillance, epidemiology and end results program data. J Urol 2002;167:57-60. CrossRef
27. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296-305. CrossRef
28. Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol 2006;7:735-40. CrossRef
29. European Association of Urology. Renal Cell Cancer Guidelines, 2013 update. Available from: http://www.uroweb.org/gls/pdf/10_Renal_Cell_Carcinoma_LR.pdf. Accessed Apr 2014.
30. Weight CJ, Larson BT, Fegany AF, et al. Nephrectomy induced chronic renal insufficiency is associated with increased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses. J Urol 2010;183:1317-23. CrossRef
31. Gill IS, Kavoussi LR, Lane BR, et al. Comparison of 1,800 laparoscopic and open partial nephrectomies for single renal tumors. J Urol 2007;178:41-6. CrossRef
32. Benway BM, Bhayani SB, Rogers CG, et al. Robotassisted partial nephrectomy versus laparoscopic partial nephrectomy for renal tumors: a multi-institutional analysis of perioperative outcomes. J Urol 2009;182:866-72. CrossRef
33. Khalifeh A, Autorino R, Hillyer SP, et al. Comparative outcomes and assessment of trifecta in 500 robotic and laparoscopic partial nephrectomies: a single surgeon experience. J Urol 2013;189:1236-42. CrossRef
34. Cho CL, Ho KL, Chu SS, Tam PC. Robot-assisted versus standard laparoscopic partial nephrectomy: comparison of perioperative outcomes from a single institution. Hong Kong Med J 2011;17:33-8.
35. Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol 2001;19:666-75.
36. Hospital Authority, Hong Kong. Surgical Outcomes Monitoring & Improvement Program (SOMIP) Report Volume 4;2012.
37. Parra RO, Andrus CH, Jones JP, Boullier JA. Laparoscopic cystectomy: initial report on a new treatment for the retained bladder. J Urol 1992;148:1140-4.
38. Guillotreau J, Gamé X, Mouzin M, et al. Radical cystectomy for bladder cancer: morbidity of laparoscopic versus open surgery. J Urol 2009;181:554-9. CrossRef
39. Beecken WD, Wolfram M, Engl T, et al. Robotic-assisted laparoscopic radical cystectomy and intra-abdominal formation of an orthotopic ileal neobladder. Eur Urol 2003;44:337-9. CrossRef
40. Kader AK, Richards KA, Krane LS, Pettus JA, Smith JJ, Hemal AK. Robot-assisted laparoscopic vs open radical cystectomy: comparison of complications and perioperative oncological outcomes in 200 patients. BJU Int 2013;112:E290-4. CrossRef
41. Gundeti MS, Kojima Y, Haga N, Kiriluk K. Robotic-assisted laparoscopic reconstructive surgery in the lower urinary tract. Curr Urol Rep 2013;14:333-41. CrossRef
42. Parekattil SJ, Gudeloglu A. Robotic assisted andrological surgery. Asian J Androl 2013;15:67-74. CrossRef
43. Gaia G, Holloway RW, Santoro L, Ahmad S, Di Silverio E, Spinillo A. Robotic-assisted hysterectomy for endometrial cancer compared with traditional laparoscopic and laparotomy approaches: a systematic review. Obstet Gynecol 2010;116:1422-31. CrossRef
44. Weinberg L, Rao S, Escobar PF. Robotic surgery in gynecology: an updated systematic review. Obstet Gynecol Int 2011;2011:852061.
45. ACOG Committee Opinion No. 444: choosing the route of hysterectomy for benign disease. Obstet Gynecol 2009;114:1156-8. CrossRef
46. Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. JAMA 2013;309:689-98. CrossRef
47. Liu H, Lu D, Wang L, Shi G, Song H, Clarke J. Robotic surgery for benign gynaecological disease. Cochrane Database Syst Rev 2012;(2):CD008978.
48. Delaney CP, Lynch AC, Senagore AJ, Fazio VW. Comparison of robotically performed and traditional laparoscopic colorectal surgery. Dis Colon Rectum 2003;46:1633-9. CrossRef
49. deSouza AL, Prasad LM, Park JJ, Marecik SJ, Blumetti J, Abcarian H. Robotic assistance in right hemicolectomy: is there a role? Dis Colon Rectum 2010;53:1000-6. CrossRef
50. Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg 1988;133:894-9.
51. Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 2005;365:1718-26. CrossRef
52. Kennedy GD, Heise C, Rajamanickam V, Harms B, Foley EF. Laparoscopy decreases postoperative complication rates after abdominal colectomy: results from the national surgical quality improvement program. Ann Surg 2009;249:596-601. CrossRef
53. Baik SH, Ko YT, Kang CM, et al. Robotic tumor-specific mesorectal excision of rectal cancer: short-term outcome of a pilot randomized trial. Surg Endosc 2008;22:1601-8. CrossRef
54. Kim JY, Kim NK, Lee KY, Hur H, Min BS, Kim JH. A comparative study of voiding and sexual function after total mesorectal excision with autonomic nerve preservation for rectal cancer: laparoscopic versus robotic surgery. Ann Surg Oncol 2012;19:2485-93. CrossRef
55. Liao G, Zhao Z, Lin S, et al. Robotic-assisted versus laparoscopic colorectal surgery: a meta-analysis of four randomized controlled trials. World J Surg Oncol 2014;12:122. CrossRef
56. Cadiere GB, Himpens J, Vertruyen M, Favretti F. The world’s first obesity surgery performed by a surgeon at a distance. Obes Surg 1999;9:206-9. CrossRef
57. Wilson EB, Sudan R. The evolution of robotic bariatric surgery. World J Surg 2013;37:2756-60. CrossRef
58. Hagen ME, Pugin F, Chassot G, et al. Reducing cost of surgery by avoiding complications: the model of robotic Roux-en-Y gastric bypass. Obes Surg 2012;22:52-61. CrossRef
59. Horgan S, Galvani C, Gorodner MV, et al. Robotic-assisted Heller myotomy versus laparoscopic Heller myotomy for the treatment of esophageal achalasia: multicenter study. J Gastrointest Surg 2005;9:1020-30. CrossRef
60. Lai EC, Yang GP, Tang CN. Robot-assisted laparoscopic liver resection for hepatocellular carcinoma: short-term outcome. Am J Surg 2013;205:697-702. CrossRef
61. Kang SW, Jeong JJ, Yun JS, et al. Gasless endoscopic thyroidectomy using trans-axillary approach; surgical outcome of 581 patients. Endocr J 2009;56:361-9. CrossRef
62. Lee J, Yun JH, Choi UJ, Kang SW, Jeong JJ, Chung WY. Robotic versus endoscopic thyroidectomy for thyroid cancers: a multi-institutional analysis of early postoperative outcomes and surgical learning curves. J Oncol 2012;2012:734541.
63. Lee J, Chung WY. Robotic thyroidectomy and neck dissection: past, present, and future. Cancer J 2013;19:151-61. CrossRef
64. O’Malley BW Jr, Weinstein GS, Snyder W, Hockstein NG. Transoral robotic surgery (TORS) for base of tongue neoplasms. Laryngoscope 2006;116:1465-72. CrossRef
65. Hans S, Delas B, Gorphe P, Ménard M, Brasnu D. Transoral robotic surgery in head and neck cancer. Eur Ann Otorhinolaryngol Head Neck Dis 2012;129:32-7. CrossRef
66. Van Abel KM, Moore EJ. The rise of transoral robotic surgery in the head and neck: emerging applications. Expert Rev Anticancer Ther 2012;12:373-80. CrossRef
67. Loulmet D, Carpentier A, d’Attellis N, et al. Endoscopic coronary artery bypass grafting with the aid of robotic assisted instruments. J Thorac Cardiovasc Surg 1999;118:4-10. CrossRef
68. Mihaljevic T, Jarrett CM, Gillinov AM, et al. Robotic repair of posterior mitral valve prolapse versus conventional approaches: potential realized. J Thorac Cardiovasc Surg 2011;141:72-80.e1-4.
69. Cerfolio RJ, Bryant AS, Skylizard L, Minnich DJ. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. CrossRef
70. Autorino R, Kaouk JH, Stolzenburg JU, et al. Current status and future directions of robotic single-site surgery: a systematic review. Eur Urol 2013;63:266-80. CrossRef