Hong Kong Med J 2014;20:234–40 | Number 3, June 2014 | Epub 9 May 2014
DOI: 10.12809/hkmj134159
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE
Thoracoscopic operations in children
CT Lau, MB, BS, MRCS1; Jessie
Leung, MB, BS, MRCS1; Theresa WC Hui, MB, BS, FHKAM
(Anaesthesiology)2; Kenneth KY Wong, FRCSEd, FHKAM
(Surgery)1
1 Department of Surgery,
LKS Faculty of Medicine, The University of Hong Kong, Pokfulam,
Hong Kong
2 Department of
Anaesthesiology, LKS Faculty of Medicine, The University of Hong
Kong, Pokfulam, Hong Kong
Corresponding author: Dr Kenneth KY Wong
(kkywong@hku.hk)
Abstract
Over the past two decades there has been
an exponential growth in the use of thoracoscopy in children.
Indeed, many advanced procedures—including lobectomy, repair of
tracheoesophageal fistula, excision of mediastinal tumours, and
diaphragmatic hernia repairs—can now be performed by this means
in advanced paediatric surgical centres in the world. This
review describes the historical perspectives and the current
state of thoracoscopic surgery, including potential benefits and
challenges, in children.
Introduction
Minimally invasive surgery is considered
one of the most important milestones in surgery in recent decades.
In this regard, operating in the thoracic cavity of children has
changed drastically from an open approach to a completely
thoracoscopic procedure in just a little over 30 years. In
paediatric patients, thoracoscopic procedures had once been
regarded as a ‘state of the art’ practice, but are now the
standard of care for many disease conditions in advanced
paediatric surgical centres. In this review, we describe their
development for children and their current status.
Historical perspective
The concept of thoracoscopy was first
introduced more than a hundred years ago by a Swedish physician,
Hans Christian Jacobaeus. In 1910, he reported his initial
experience after inserting a cystoscope into the pleural cavity to
perform lysis of a tuberculous pleural adhesion as part of the
treatment. But it was not until almost 70 years later in 1976,
when Rodgers and Talbert1
put thoracoscopy into first practical use for paediatric patients.
At this early stage, thoracoscopic procedures in children were
only limited to lung biopsies, evaluation of thoracic or pulmonary
lesions, and regional decortication of an empyema.2 Despite increasing recognition of its potential
advantages, it did not gain widespread acceptance or popularity
owing to technical and anaesthetic difficulties.
The first laparoscopic cholecystectomy in
1985 by Mühe3 was a turning
point that brought about a revolutionary change in this type of
surgery. This ensuing exponential growth in the development of
minimally invasive surgical procedures also stimulated the
technological advances pertaining to associated surgical
instruments, including the development of high-definition digital
cameras, smaller-calibre instruments, and new energy-delivering
devices. This meant that surgeries could be performed in smaller
children more safely and effectively, and in a minimally invasive
manner. The experience and skills gained from laparoscopic
surgeries, together with improvements in anaesthetic techniques,
enabled paediatric surgeons to venture into the thoracic cavity.
Advantages and difficulties
Cosmetic superiority is the most obvious
advantage provided by thoracoscopic operations (Fig
1). Smaller incisions not only meant that postoperatively
there could be much smaller and almost invisible surgical scars,
but more importantly the pain associated with traditional
thoracotomy was greatly reduced. As a result of such extreme
facility, some centres are now performing minor thoracoscopic
procedures on an out-patient basis.4
In addition, the significant decrease in overall wound lengths and
tension reduced the risks of wound infection and dehiscence,5 which were associated with shorter hospital
stays and earlier recovery.6
7
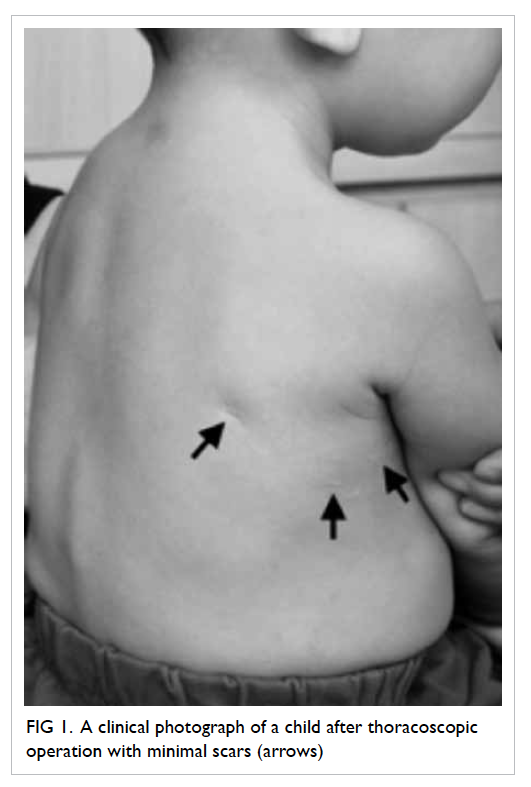
Figure 1. A clinical photograph of a child after thoracoscopic operation with minimal scars (arrows)
The most dreaded and well-known long-term
complications of thoracotomy are musculoskeletal. They include
chest wall deformities, rib fusion, shoulder girdle weakness and
scoliosis, and can occur in up to 30% of patients undergoing
thoracotomy.8 9 The mechanism underlying these problems is
related to the division of shoulder girdle muscles such as the
latissimus and serratus, and often resulted in girdle weakness.
Furthermore, the tensile forces created by thoracotomy wound
closure over the ipsilateral chest wall could distort the thoracic
cage as the child grows.10
In contrast, these complications are virtually non-existent in
patients who undergo thoracoscopic procedures.11
Thoracoscopic operations enable surgeons to
enjoy superior surgical visibility and precision. With the aid of
high-definition monitors and cameras, the smallest structures
including blood vessels and nerves can now be visualised under
magnification (Fig 2), which allowed surgeons to dissect
with greater precision and thus avoid unintentional injuries.
Another advantage of thoracoscopy is provided by telescopes with
viewing angles that enable easy evaluation of the whole thoracic
cavity and the entire lung surface from a limited port access. As
a result, even the most deep-seated areas and corners can now be
seen clearly, which was previously not possible during
conventional thoracotomies.
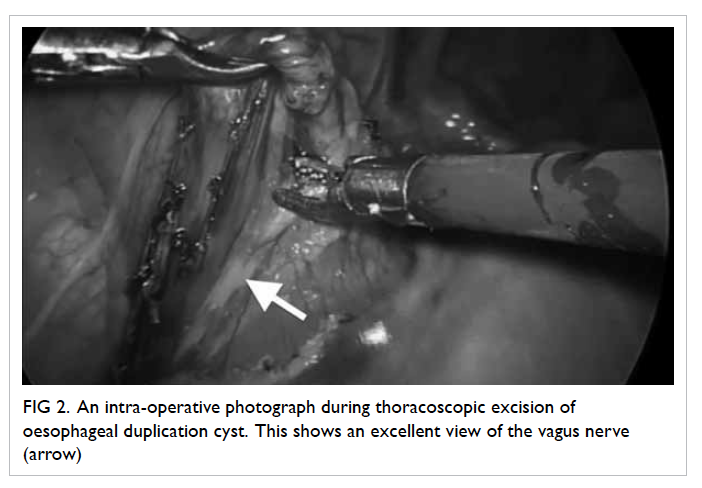
Figure 2. An intra-operative photograph during thoracoscopic excision of oesophageal duplication cyst. This shows an excellent view of the vagus nerve (arrow)
Everything comes at a price, and
thoracoscopic surgery is no exception. First, there are the
challenges encountered across the spectrum of minimally invasive
surgery in general, and include lack of three-dimensional vision,
reduced feedback from tactile sensation, and the protracted
learning curve for paediatric thoracoscopic surgeons. One reason
for the latter was the body size of our patients. Since a young
child with only half the height of an adult provides one-eighth
the working thoracoscopic space, the difficulties encountered in
manipulating instruments inside the thorax of a neonate are
obvious. Second, apart from the limitation of working space
(always a concern for paediatric surgeons), the ability to achieve
adequate single-lung ventilation was also a limitation. This was
partially solved by creating more space, as well as the
development of smaller instruments that allowed finer and more
ergonomically friendly movements. Third, the variation in body
size among paediatric patients also made the learning process
difficult. Surgeons had to adapt from a 3-kg neonate to a 70-kg
teenager, before they could truly master all the necessary skills,
which also imposed a significant effect on the length of the
learning curve.
Safe control of major vasculature and other
passages remains a major challenge even for experienced surgeons,
especially in the case of thoracoscopic lobectomy. Unlike adults,
in whom the endoscopic stapler can be employed to take control of
the pulmonary vessels and bronchi, this device often proves too
large to be used in children, as a 12-mm trocar port and at least
5 cm of intrathoracic space are required for it to open fully.12 New sealing devices—such as LigaSure
(Covidien, US), EnSeal (Ethicon, US), and Thunderbeat (Olympus,
Japan)—allow safe sealing of the main pulmonary vessels up to 7 mm
in diameter and thus they have replaced resorting to endoclips,
which may dislodge during dissection or obscure satisfactory
tissue dissection due to the space they occupy. These
energy-sealing devices also diminish technical difficulties during
the performance of complex lobectomies, as they are proven to be
safe and efficient in sealing off lung tissues and dividing
incomplete fissures.13
Nonetheless, a complete understanding of the three-dimensional
anatomical relationships and precision in tissue dissection is
still the key to success.
Anaesthetic aspects
Paediatric thoracoscopic surgery is not
only about surgical and technical refinements. Anaesthetic
techniques play a major role in achieving successful thoracoscopic
surgery. To create adequate thoracic space for efficient surgery
with good exposure, single-lung ventilation is a prerequisite in
the surgical management of many thoracic conditions. Unlike adults
in whom single-lung ventilation can be easily performed using a
double-lumen endotracheal tube, this is not feasible in young
children. The smallest double lumen tube is a 26F, and may even be
used for children younger than 8 years old. For even smaller
patients, standard endotracheal intubation together with insertion
of an endobronchial blocker in the ipsilateral bronchus of the
operated lung or selective intubation of the contralateral
bronchus with an endotracheal tube turn out to be the solution. An
endobronchial blocker is a catheter-like device with a balloon
attached to its tip for occlusion and contains a central stylet.
Depending on the size of the patient, under fibre-optic
bronchoscopic guidance, the endobronchial blocker is placed either
within or outside the lumen of the endotracheal tube and advanced
into the main stem bronchus of choice. The balloon is then
inflated to create bronchial occlusion under direct vision.
Problems with bronchial blockers include dislodgement of the
blocker balloon into the trachea with blockade of ventilation, and
overdistention of the balloon leading to damage of the airway.
With selective intubation of the contralateral main stem bronchus,
an uncuffed endotracheal tube around half to one size smaller than
the usual is selected for advancement into the main stem bronchus
under fibre-optic bronchoscopic guidance. Problems with selective
main stem intubation include difficulty providing adequate seal,
obstruction of the upper lobe bronchus, and inability to provide
suction for the operative lung.4
Both of these techniques have produced single-lung ventilation
with satisfactory result.14
After successful establishment of
single-lung ventilation, lung collapse can be enhanced further by
carbon dioxide insufflation into the thorax. This is particularly
helpful in the event the endobronchial tube is not totally
occlusive resulting in a degree of overflow ventilation. Carbon
dioxide infusion at low pressure (4 mm Hg) and low flow (1 L/min)
helps keep the lung compressed during the surgery and reduces the
risk of injury from using a retractor. Maintenance of this
low-setting environment requires the use of valved trocars.
The safety of single-lung ventilation in
paediatric patients had been a major concern. Although there was a
previous report on mucosal or bronchial injury during intubation,14 several recently
reported large series15 16 17 have demonstrated the safety and efficacy of
single-lung ventilation in children, without major complications
or mortality. Dingemann et al18
compared children having single-lung ventilation and those having
conventional two-lung ventilation. They found no statistically
significant difference between the groups in terms of the timing
of extubation, the rate of postoperative atelectasis or pneumonia,
and the length of intensive care unit stays.
Increased compression of the dependent lung
in the lateral decubitus position, surgical retraction and
single-lung ventilation with collapse of the operative lung can
aggravate ventilation-perfusion mismatch. Intra-operative
hypercapnia and acidosis associated with thoracoscopic procedures
have been well documented.19
20 21 It has been postulated that hypercapnia and
acidosis are caused by the use of carbon dioxide as the
insufflation agent, increasing carbon dioxide absorption into the
systemic circulation. Based on a pilot randomised controlled
trial, Bishay et al22 has
confirmed the presence of prolonged hypercapnia in thoracoscopic
surgery patients compared to those having open thoracotomy, but
the long-term consequence of this finding was unclear.
Selected conditions
Thus far, thoracoscopy has been reported to
be the surgical approach in more than 20 types of thoracic
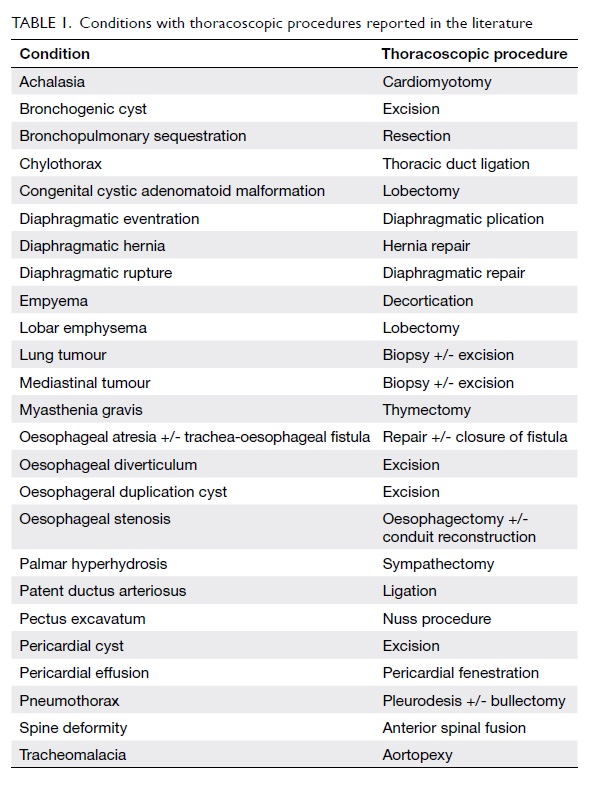
conditions in children and infants (Table 1). As there are neither absolute
contra-indication nor guidelines on which thoracic condition
should or should not be performed thoracoscopically, this means
that virtually all chest condition can be managed in this manner.
Thoracic empyema was the first condition in
which the thoracoscopic approach was deployed. Early thoracoscopic
decortication following the failure of initial conservative
treatment with chest tube drainage and antibiotics is now
recommended.23 In most
patients, primary spontaneous pneumothorax has been shown to be
related to underlying lung bullae.24
These can be managed by thoracoscopic bullectomy without the need
for prolonged chest tube drainage and hospitalisation, which is in
contrast to simple conservative management. Moreover, it has
evolved to become the standard treatment in many regional centres.
Likewise, thoracoscopic lung biopsy has been widely used as a
diagnostic tool in interstitial lung disease or for intrathoracic
tumour, and some centres even advocate these to be performed as
day-case procedures.25
The most commonly performed thoracoscopic
operation in young infants is for congenital cystic lung disease.
The condition consists of congenital cystic adenomatoid
malformations, bronchopulmonary sequestration, bronchogenic cysts,
and congenital lobar emphysemas. With the increasing use of
antenatal ultrasonography during routine follow-up, there has been
a significant increase in the reported incidence of this disease.
Thoracoscopic resection or lobectomy is usually recommended at 6
months of age, in view of the risks from frequent pneumonia and
the potential for future malignancies.
Centres with experience have now pushed
the application of paediatric thoracoscopic surgery
towards the treatment of neonatal conditions. Ever
since the first successful case of thoracoscopic repair
of oesophageal atresia in 1999,26 the procedure has been labelled as the
‘pinnacle of paediatric surgery’. Due to its difficulty, only a
few small series (including ours) have been published and the
initial results are encouraging.27
28 29 Repair of Bochdalek’s congenital
diaphragmatic hernia is also routinely managed using the
thoracoscopic approach. Due to the underlying pulmonary
hypoplasia, the thoracic cavity on the affected side provides
excellent working space, for which single-lung ventilation may not
be necessary and only very-low-pressure low-flow carbon dioxide
insufflation is all that is required.30
Table 2 7
18 20 21 24 27 28 29 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50
51
52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 provides a brief summary of the major studies
dealing with the aforementioned conditions.
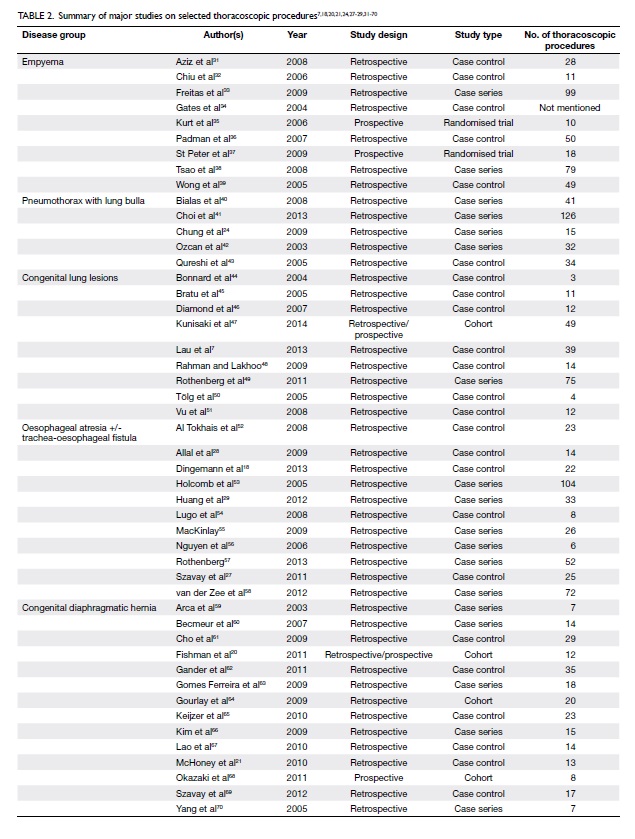
Table 2. Summary of major studies on selected thoracoscopic procedures7 18 20 21 24 27 28 29 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70
Conclusion
Thoracoscopic surgery in children has come
a long way since its inception. There is solid evidence supporting
its safety and applicability in routine clinical use. More
prospective studies are required to determine whether it offers
genuine advantages over traditional open surgery.
References
1. Rodgers BM, Talbert JL.
Thoracoscopy for diagnosis of intrathoracic lesions in children. J
Pediatr Surg 1976;11:703-8. CrossRef
2. Rodgers BM. Pediatric
thoracoscopy: where have we come and what have we learned? Ann
Thorac Surg 1993;56:704-7. CrossRef
3. Mühe E. Laparoscopic
cholecystectomy—late results [in German]. Langenbecks Arch Chir
Suppl Kongressbd 1991:416-23.
4. Rothenberg SS. Thoracoscopic
pulmonary surgery. Semin Pediatr Surg 2007;16:231-7. CrossRef
5. Blinman T. Incisions do not
simply sum. Surg Endosc 2010;24:1746-51. CrossRef
6. Nasr A, Bass J. Thoracoscopic vs
open resection of congenital lung lesions: a meta-analysis. J
Pediatr Surg 2012;47:857-61. CrossRef
7. Lau CT, Leung L, Chan IH, et al.
Thoracoscopic resection of congenital cystic lung lesions is
associated with better post-operative outcomes. Pediatr Surg Int
2013;29:341-5. CrossRef
8. Jaureguizar E, Vazquez J, Murcia
J, Diez Pardo JA. Morbid musculoskeletal sequelae of thoracotomy
for tracheoesophageal fistula. J Pediatr Surg 1985;20:511-4. CrossRef
9. Korovessis P, Papanastasiou D,
Dimas A, Karayannis A. Scoliosis by acquired rib fusion after
thoracotomy in infancy. Eur Spine J 1993;2:53-5. CrossRef
10. Blinman T, Ponsky T. Pediatric
minimally invasive surgery: laparoscopy and thoracoscopy in
infants and children. Pediatrics 2012;130:539-49. CrossRef
11. Lawal TA, Gosemann JH, Kuebler
JF, Glüer S, Ure BM. Thoracoscopy versus thoracotomy improves
midterm musculoskeletal status and cosmesis in infants and
children. Ann Thorac Surg 2009;87:224-8. CrossRef
12. Rothenberg SS. First decade’s
experience with thoracoscopic lobectomy in infants and children. J
Pediatr Surg 2008;43:40-4; discussion 45. CrossRef
13. Bignon H, Buela E,
Martinez-Ferro M. Which is the best vessel-sealing method for
pediatric thoracoscopic lobectomy? J Laparoendosc Adv Surg Tech A
2010;20:395-8. CrossRef
14. Ender J, Brodowsky M, Falk V,
et al. High-frequency jet ventilation as an alternative method
compared to conventional one-lung ventilation using double-lumen
tubes during minimally invasive coronary artery bypass graft
surgery. J Cardiothorac Vasc Anesth 2010;24:602-7. CrossRef
15. Bataineh ZA, Zoeller C,
Dingemann C, Osthaus A, Suempelmann R, Ure B. Our experience with
single lung ventilation in thoracoscopic paediatric surgery. Eur J
Pediatr Surg 2012;22:17-20. CrossRef
16. Gentili A, Lima M, De Rose R,
Pigna A, Codeluppi V, Baroncini S. Thoracoscopy in children:
anaesthesiological implications and case reports. Minerva
Anestesiol 2007;73:161-71.
17. Byon HJ, Lee JW, Kim JK, et
al. Anesthetic management of video-assisted thoracoscopic surgery
(VATS) in pediatric patients: the issue of safety in infant and
younger children. Korean J Anesthesiol 2010;59:99-103. CrossRef
18. Dingemann C, Zoeller C, Ure B.
Thoracoscopic repair of oesophageal atresia: results of a
selective approach. Eur J Pediatr Surg 2013;23:14-8.
19. Bliss D, Matar M, Krishnaswami
S. Should intraoperative hypercapnea or hypercarbia raise concern
in neonates undergoing thoracoscopic repair of diaphragmatic
hernia of Bochdalek? J Laparoendosc Adv Surg Tech A 2009;19 Suppl
1:S55-8. CrossRef
20. Fishman JR, Blackburn SC,
Jones NJ, et al. Does thoracoscopic congenital diaphragmatic
hernia repair cause a significant intraoperative acidosis when
compared to an open abdominal approach? J Pediatr Surg
2011;46:458-61. CrossRef
21. McHoney M, Giacomello L, Nah
SA, et al. Thoracoscopic repair of congenital diaphragmatic
hernia: intraoperative ventilation and recurrence. J Pediatr Surg
2010;45:355-9. CrossRef
22. Bishay M, Giacomello L,
Retrosi G, et al. Hypercapnia and acidosis during open and
thoracoscopic repair of congenital diaphragmatic hernia and
esophageal atresia: results of a pilot randomized controlled
trial. Ann Surg 2013;258:895-900. CrossRef
23. Islam S, Calkins CM, Goldin
AB, et al. The diagnosis and management of empyema in children: a
comprehensive review from the APSA Outcomes and Clinical Trials
Committee. J Pediatr Surg 2012;47:2101-10. CrossRef
24. Chung PH, Wong KK, Lan LC, Tam
PK. Thoracoscopic bullectomy for primary spontaneous pneumothorax
in pediatric patients. Pediatr Surg Int 2009;25:763-6. CrossRef
25. Rothenberg SS, Wagner JS,
Chang JH, Fan LL. The safety and efficacy of thoracoscopic lung
biopsy for diagnosis and treatment in infants and children. J
Pediatr Surg 1996;31:100-3; discussion 103-4. CrossRef
26. Rothenberg SS. Thoracoscopic
repair of tracheoesophageal fistula in newborns. J Pediatr Surg
2002;37:869-72. CrossRef
27. Szavay PO, Zundel S,
Blumenstock G, et al. Perioperative outcome of patients with
esophageal atresia and tracheo-esophageal fistula undergoing open
versus thoracoscopic surgery. J Laparoendosc Adv Surg Tech A
2011;21:439-43. CrossRef
28. Allal H, Pérez-Bertólez S,
Maillet O, et al. Comparative study of thoracoscopy versus
thoracotomy in esophageal atresia [in Spanish]. Cir Pediatr
2009;22:177-80.
29. Huang J, Tao J, Chen K, et al.
Thoracoscopic repair of oesophageal atresia: experience of 33
patients from two tertiary referral centres. J Pediatr Surg
2012;47:2224-7. CrossRef
30. Lansdale N, Alam S, Losty PD,
Jesudason EC. Neonatal endosurgical congenital diaphragmatic
hernia repair: a systematic review and meta-analysis. Ann Surg
2010;252:20-6. CrossRef
31. Aziz A, Healey JM, Qureshi F,
et al. Comparative analysis of chest tube thoracostomy and
video-assisted thoracoscopic surgery in empyema and parapneumonic
effusion associated with pneumonia in children. Surg Infect
(Larchmt) 2008;9:317-23. CrossRef
32. Chiu CY, Wong KS, Huang YC,
Lai SH, Lin TY. Echo-guided management of complicated
parapneumonic effusion in children. Pediatr Pulmonol
2006;41:1226-32. CrossRef
33. Freitas S, Fraga JC, Canani F.
Thoracoscopy in children with complicated parapneumonic pleural
effusion at the fibrinopurulent stage: a multi-institutional study
[in English, Portuguese]. J Bras Pneumol 2009;35:660-8. CrossRef
34. Gates RL, Hogan M, Weinstein
S, Arca MJ. Drainage, fibrinolytics, or surgery: a comparison of
treatment options in pediatric empyema. J Pediatr Surg
2004;39:1638-42. CrossRef
35. Kurt BA, Winterhalter KM,
Connors RH, Betz BW, Winters JW. Therapy of parapneumonic
effusions in children: video-assisted thoracoscopic surgery versus
conventional thoracostomy drainage. Pediatrics 2006;118:e547-53. CrossRef
36. Padman R, King KA, Iqbal S,
Wolfson PJ. Parapneumonic effusion and empyema in children:
retrospective review of the duPont experience. Clin Pediatr
(Phila) 2007;46:518-22. CrossRef
37. St Peter SD, Tsao K, Spilde
TL, et al. Thoracoscopic decortication vs tube thoracostomy with
fibrinolysis for empyema in children: a prospective, randomized
trial. J Pediatr Surg 2009;44:106-11; discussion 111. CrossRef
38. Tsao K, St Peter SD, Sharp SW,
et al. Current application of thoracoscopy in children. J
Laparoendosc Adv Surg Tech A 2008;18:131-5. CrossRef
39. Wong KS, Lin TY, Huang YC,
Chang LY, Lai SH. Scoring system for empyema thoracis and help in
management. Indian J Pediatr 2005;72:1025-8. CrossRef
40. Bialas RC, Weiner TM, Phillips
JD. Video-assisted thoracic surgery for primary spontaneous
pneumothorax in children: is there an optimal technique? J Pediatr
Surg 2008;43:2151-5. CrossRef
41. Choi SY, Kim YH, Jo KH, et al.
Video-assisted thoracoscopic surgery for primary spontaneous
pneumothorax in children. Pediatr Surg Int 2013;29:505-9. CrossRef
42. Ozcan C, McGahren ED, Rodgers
BM. Thoracoscopic treatment of spontaneous pneumothorax in
children. J Pediatr Surg 2003;38:1459-64. CrossRef
43. Qureshi FG, Sandulache VC,
Richardson W, Ergun O, Ford HR, Hackam DJ. Primary vs delayed
surgery for spontaneous pneumothorax in children: which is better?
J Pediatr Surg 2005;40:166-9. CrossRef
44. Bonnard A, Malbezin S,
Ferkdadji L, Luton D, Aigrain Y, de Lagauise P. Pulmonary
sequestration children: is the thoracoscopic approach a good
option? Surg Endosc 2004;18:1364-7. CrossRef
45. Bratu I, Laberge JM, Flageole
H, Bouchard S. Foregut duplications: is there an advantage to
thoracoscopic resection? J Pediatr Surg 2005;40:138-41. CrossRef
46. Diamond IR, Herrera P, Langer
JC, Kim PC. Thoracoscopic versus open resection of congenital lung
lesions: a case-matched study. J Pediatr Surg 2007;42:1057-61. CrossRef
47. Kunisaki SM, Powelson IA,
Haydar B, et al. Thoracoscopic vs open lobectomy in infants and
young children with congenital lung malformations. J Am Coll Surg
2014;218:261-70. CrossRef
48. Rahman N, Lakhoo K. Comparison
between open and thoracoscopic resection of congenital lung
lesions. J Pediatr Surg 2009;44:333-6. CrossRef
49. Rothenberg SS, Kuenzler KA,
Middlesworth W, et al. Thoracoscopic lobectomy in infants less
than 10 kg with prenatally diagnosed cystic lung disease. J
Laparoendosc Adv Surg Tech A 2011;21:181-4. CrossRef
50. Tölg C, Abelin K, Laudenbach
V, et al. Open vs thoracoscopic surgical management of
bronchogenic cysts. Surg Endosc 2005;19:77-80. CrossRef
51. Vu LT, Farmer DL, Nobuhara KK,
Miniati D, Lee H. Thoracoscopic versus open resection for
congenital cystic adenomatoid malformations of the lung. J Pediatr
Surg 2008;43:35-9. CrossRef
52. Al Tokhais T, Zamakhshary M,
Aldekhayel S, et al. Thoracoscopic repair of tracheoesophageal
fistulas: a case-control matched study. J Pediatr Surg
2008;43:805-9. CrossRef
53. Holcomb GW 3rd, Rothenberg SS,
Bax KM, et al. Thoracoscopic repair of esophageal atresia and
tracheoesophageal fistula: a multi-institutional analysis. Ann
Surg 2005;242:422-8; discussion 428-30.
54. Lugo B, Malhotra A, Guner Y,
Nguyen T, Ford H, Nguyen NX. Thoracoscopic versus open repair of
tracheoesophageal fistula and esophageal atresia. J Laparoendosc
Adv Surg Tech A 2008;18:753-6. CrossRef
55. MacKinlay GA. Esophageal
atresia surgery in the 21st century. Semin Pediatr Surg
2009;18:20-2. CrossRef
56. Nguyen T, Zainabadi K, Bui T,
Emil S, Gelfand D, Nguyen N. Thoracoscopic repair of esophageal
atresia and tracheoesophageal fistula: lessons learned. J
Laparoendosc Adv Surg Tech A 2006;16:174-8. CrossRef
57. Rothenberg SS. Thoracoscopic
repair of esophageal atresia and tracheoesophageal fistula in
neonates, first decade’s experience. Dis Esophagus 2013;26:359-64. CrossRef
58. van der Zee DC, Tytgat SH,
Zwaveling S, van Herwaarden MY, Vieira-Travassos D. Learning curve
of thoracoscopic repair of esophageal atresia. World J Surg
2012;36:2093-7. CrossRef
59. Arca MJ, Barnhart DC, Lelli JL
Jr, et al. Early experience with minimally invasive repair of
congenital diaphragmatic hernias: results and lessons learned. J
Pediatr Surg 2003;38:1563-8. CrossRef
60. Becmeur F, Reinberg O,
Dimitriu C, Moog R, Philippe P. Thoracoscopic repair of congenital
diaphragmatic hernia in children. Semin Pediatr Surg
2007;16:238-44. CrossRef
61. Cho SD, Krishnaswami S, Mckee
JC, Zallen G, Silen ML, Bliss DW. Analysis of 29 consecutive
thoracoscopic repairs of congenital diaphragmatic hernia in
neonates compared to historical controls. J Pediatr Surg
2009;44:80-6; discussion 86. CrossRef
62. Gander JW, Fisher JC, Gross
ER, et al. Early recurrence of congenital diaphragmatic hernia is
higher after thoracoscopic than open repair: a single
institutional study. J Pediatr Surg 2011;46:1303-8. CrossRef
63. Gomes Ferreira C, Reinberg O,
Becmeur F, et al. Neonatal minimally invasive surgery for
congenital diaphragmatic hernias: a multicenter study using
thoracoscopy or laparoscopy. Surg Endosc 2009;23:1650-9. CrossRef
64. Gourlay DM, Cassidy LD, Sato
TT, Lal DR, Arca MJ. Beyond feasibility: a comparison of newborns
undergoing thoracoscopic and open repair of congenital
diaphragmatic hernias. J Pediatr Surg 2009;44:1702-7. CrossRef
65. Keijzer R, van de Ven C, Vlot
J, et al. Thoracoscopic repair in congenital diaphragmatic hernia:
patching is safe and reduces the recurrence rate. J Pediatr Surg
2010;45:953-7. CrossRef
66. Kim AC, Bryner BS, Akay B,
Geiger JD, Hirschl RB, Mychaliska GB. Thoracoscopic repair of
congenital diaphragmatic hernia in neonates: lessons learned. J
Laparoendosc Adv Surg Tech A 2009;19:575-80. CrossRef
67. Lao OB, Crouthamel MR, Goldin
AB, Sawin RS, Waldhausen JH, Kim SS. Thoracoscopic repair of
congenital diaphragmatic hernia in infancy. J Laparoendosc Adv
Surg Tech A 2010;20:271-6. CrossRef
68. Okazaki T, Nishimura K,
Takahashi T, et al. Indications for thoracoscopic repair of
congenital diaphragmatic hernia in neonates. Pediatr Surg Int
2011;27:35-8. CrossRef
69. Szavay PO, Obermayr F, Maas C,
Luenig H, Blumenstock G, Fuchs J. Perioperative outcome of
patients with congenital diaphragmatic hernia undergoing open
versus minimally invasive surgery. J Laparoendosc Adv Surg Tech A
2012;22:285-9. CrossRef
70. Yang EY, Allmendinger N,
Johnson SM, Chen C, Wilson JM, Fishman SJ. Neonatal thoracoscopic
repair of congenital diaphragmatic hernia: selection criteria for
successful outcome. J Pediatr Surg 2005;40:1369-75. CrossRef