Hong Kong Med J 2014;20:37–44 | Number 1, February 2014 | Epub 22 Jul 2013
DOI: 10.12809/hkmj133920
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
How can the R.E.N.A.L. nephrometry scoring
system aid management of a solid renal mass?
MH Wong, FHKAM (Surgery)1; KY
Cho, FRCR2; KL Ho, FHKAM (Surgery)1; KW
Wong, MRCS (Surgery)1; CT Lai, MRCS (Surgery)1;
CM Man, MRCS (Surgery)1; MK Yiu, FHKAM (Surgery)1
1 Division of Urology,
Department of Surgery, The University of Hong Kong, Queen Mary
Hospital, Pokfulam, Hong Kong
2 Department of Radiology,
The University of Hong Kong, Queen Mary Hospital, Pokfulam, Hong
Kong
Corresponding author: Dr MH Wong (edwong56@gmail.com)
Abstract
Objectives: To
investigate use of the R.E.N.A.L. nephrometry score in relation
to the choice of treatment and postoperative complications for
renal masses.
Design: Case series.
Setting: A tertiary
referral hospital in Hong Kong.
Patients: Data of
patients undergoing nephrectomy were collected retrospectively
from a clinical database and analysed. A R.E.N.A.L. nephrometry
score was allocated to each renal tumour by a blinded qualified
radiologist, utilising computerised imaging systems. Patient
demographics, choice of surgery (radical vs partial), and
approaches (open vs minimally invasive) were analysed with
respect to their R.E.N.A.L. score.
Results: In all, 74
patients were included during the study period, of which 38
underwent partial nephrectomy and 36 underwent radical
nephrectomy. No differences between the groups were found with
respect to patient demographics. There were significant
differences between the partial and radical nephrectomy groups
in terms of their mean nephrometry score (6.9 vs 9.3,
P<0.001). The mean nephrometry sum was also significantly
different in the open approach versus the minimally invasive
approach in patients having partial nephrectomy (7.8 vs 6.0,
P=0.001). There was no difference in the postoperative 90-day
morbidity and mortality in the partial nephrectomy and radical
nephrectomy groups.
Conclusions: The
R.E.N.A.L. nephrometry score of a renal mass correlated
significantly with our choice of surgery (partial vs radical)
and our approach to surgery (open vs minimally invasive
surgery), particularly in the partial nephrectomy group. It does
not, however, correlate with postoperative complications. The
nephrometry score provides a useful tool for objectively
describing renal mass characteristics and enhancing better
communication for the operative planning directed at renal
masses.
New knowledge added by this
study
- We externally validated the use of the R.E.N.A.L. nephrometry scoring system to differentiate choice of surgery (radical vs partial nephrectomy) and approach (open vs minimally invasive), which was not shown in previous studies.
- We are able to qualify the weighting of individual parameters of the R.E.N.A.L. nephrometry scoring system on decision-making.
- Application of R.E.N.A.L. nephrometry scoring preoperatively may be used as a guide to the complexity and choice of surgery in patients with small solid renal masses. It also serves as a tool for patient counselling, with reference to postoperative outcomes.
- Widespread use of this score may act as communication tools among specialists, such that direct comparisons of data and study results can be achieved.
Introduction
The annual incidence of renal cell
carcinoma (RCC) in Hong Kong has increased steadily over the past
10 years reaching a rate of 5.9 cases per 100 000 inhabitants.1 Surgical management remains the main treatment
modality. With advances and ready availability of imaging,
including screening by ultrasonography, more RCCs are diagnosed at
an early stage (ie T1). The treatment modalities of these
localised renal masses include radical nephrectomy or partial
nephrectomy, in the form of an open or laparoscopic (with or
without robotic-assisted) approach, as well as other form of
ablative therapy. Several large, retrospective studies and the
recently published European Organization for Research and
Treatment of Cancer randomised trial2
have confirmed that the oncological outcomes of partial
nephrectomy and radical nephrectomy are equivalent. The advantages
of radical nephrectomy include better preservation of renal
function and prevention of renal failure, lower cardiovascular
morbidity, and better overall survival.3
Although nephron-sparing surgery has slightly higher complication
rate compared with radical nephrectomy,4
most international guidelines recommend the former as the standard
treatment for solitary renal tumours up to a diameter of 7 cm,
whenever technically feasible.5
6 In the US population,
utilisation of such techniques has recently been reported to be
low, partly due to lack of technical advancements and publicity
about possible adverse long-term consequences.7
Decisions on the choice of surgery mostly
depend on the size and location of the tumour. Other external
factors, such as the surgeon’s training, practice pattern,
operating centre facilities, and hardware available, have a major
impact on the choice of approaches and operation to be performed.
In the presence of multiple treatment options, an objective way to
describe the complexity of renal masses and to accurately assess
the risks of postoperative complications is important for patient
counselling and clinical decision-making. Scoring systems have
therefore been developed and validated, and to date three are
available for clinical use.8
9 10
Herein, we report our investigation into
using the R.E.N.A.L. nephrometry score, as developed by Kutikov
and Uzzo in 2009,8 and its
relationship to the choice of treatment and postoperative
complications.
Methods
Data about patients having renal tumours
treated by total nephrectomy in Queen Mary Hospital during the
period of January 2006 to December 2011 were retrieved
retrospectively from a clinical database and analysed. Patients
who had not had preoperative computed tomography and
three-dimensional reconstruction (available in the Queen Mary
Hospital radiological department) were excluded, so as to
standardise the radiographic characteristic of the renal tumours
under study. This involved allocating a R.E.N.A.L. nephrometry
score to each renal tumour utilising computerised imaging systems
(GE Advantage Workstations; General Electric Healthcare, US) by a
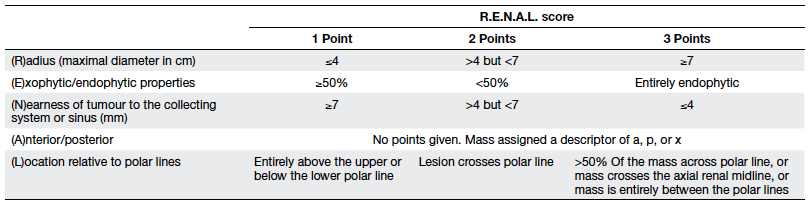
blinded qualified radiologist. The R.E.N.A.L. score was described
in 2009 and includes the assessment of tumour (R)adius (size at
the maximal diameter), (E)xophytic/ endophytic properties,
(N)earness of tumour to the collecting system or sinus,
(A)nterior/posterior descriptor, and (L)ocation relative to polar
lines. Standardised points (1-3 points per descriptor) were
assigned onto each parameter, except the anterior or posterior
component as originally described by Kutikov and Uzzo8 (Table 1). Radius was measured as the
maximum diameter of the tumour in centimetres and points were
allocated as 1 (≤4 cm), 2 (>4 but <7 cm), and 3 (≥7 cm).
Exophytic/endophytic points assigned were 1 when 50% or more of
the tumour was exophytic, 2 when less than 50% was exophytic, and
3 when it was entirely endophytic. For non-spherical or
asymmetrically located tumours, the predominant feature on any
axis (not just the axial or coronal axis) was considered with
reference to the renal cortex. The N component was measured as the
distance of the deepest portion of the tumour to the collecting
system and points were allocated as 1 (≥7 mm), 2 (>4 but <7
mm), and 3 (invading, touching or within 4 mm). Anterior/posterior
location of the tumour was designated as a non-numerical suffix
that describes the location of the tumour with respect to the
kidney midline plane as assessed on axial images. When the mass
was located at the tip of the renal poles or lay on the coronal
plane where a meaningful anterior or posterior designation was not
possible, the suffix “x” was assigned. The location score was
assigned as the position of the mass relative to polar lines. The
polar line was assigned as the plane of the kidney above or below
which the medial lip of parenchyma was interrupted by the renal
sinus fat, vessels or the collecting system and best located in
the coronal plane. Two polar lines were measured for each renal
unit. The position of the renal tumour with respect to the polar
lines was measured and a score allocated as described in Table 1.
Nephrometry classes in terms of complexity were allocated as low
(4-6), moderate (7-9), and high (10-12) based on the sum of scores
allocated to each parameter. Patient demographics, including age,
gender, preoperative renal function, and estimated glomerular
filtration rate (eGFR) as calculated by Chronic Kidney Disease
Epidemiology Collaboration (CKD-EPI) equations were logged.11 In addition, the American Society of
Anesthesiologists (ASA) class,12
chronic kidney disease stage, mode of surgery (radical vs
partial), approaches (open vs minimally invasive surgery [MIS]),
and ischaemic time were analysed with respect to their R.E.N.A.L.
score and classes. The 90-day postoperative morbidity and
mortality were retrieved according to the Clavien-Dindo system.13 Continuous variables
were analysed with Student’s t test and categorical variables by
the Chi squared and Fisher’s exact tests. Any P value of <0.05
was taken as statistically significant. All data were analysed
with the Statistical Package for the Social Sciences (Windows
version 18.0; SPSS Inc, Chicago [IL], US).
Results
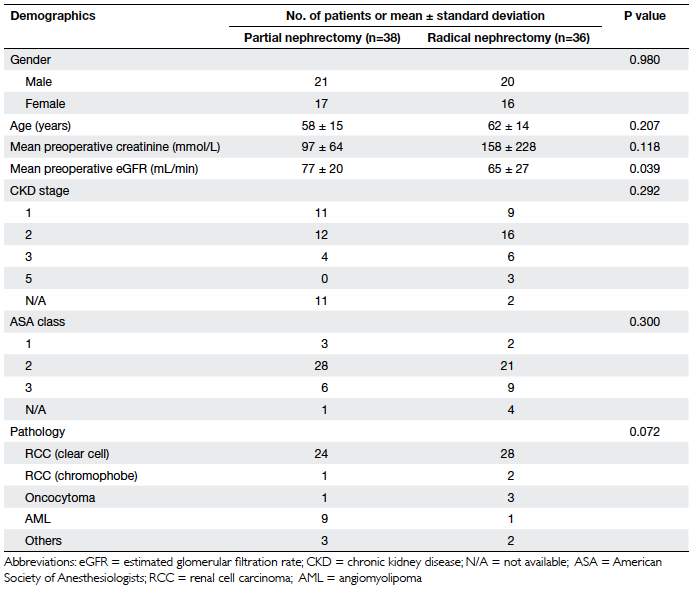
There were 74 patients included during this
study period, of which 38 underwent partial nephrectomy (group 1)
and 36 underwent radical nephrectomy (group 2). There were 41
males and 33 females. No statistical differences were found
between the groups in terms of gender distribution, age,
preoperative creatinine level, ASA class, or chronic kidney
disease stage, although the mean eGFR was significantly lower in
the radical nephrectomy group (65 vs 77 mL/min, P=0.039; Table 2).
The final pathology of the majority of our patients was clear-cell
RCC (n=52), and the remainder suffered from angiomyolipoma (n=10),
oncocytoma (n=4), chromophobe RCC (n=3), and others (n=5). There
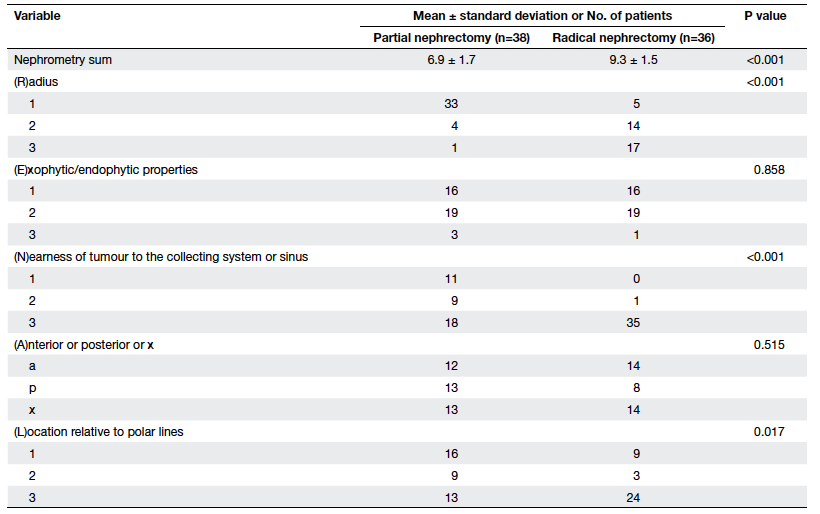
were significant differences between the partial and radical
nephrectomy groups in terms of their mean nephrometry score (6.9
vs 9.3, P<0.001). Individual parameters of the R.E.N.A.L. score
in terms of radius (P<0.001), nearest to the collecting system
(P<0.001), and locations relative to polar lines (P=0.017) were
significantly different in the two groups, but there was no
significant difference in terms of exophytic components or
anterior/posterior location (Table 3).
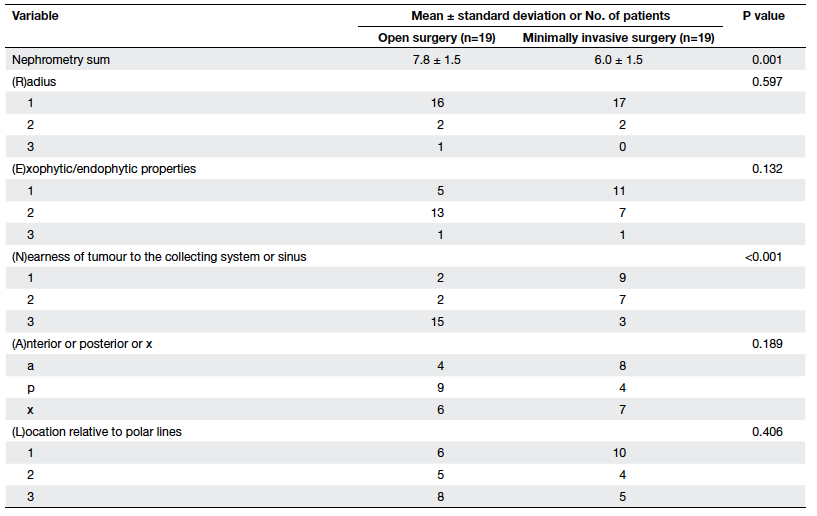
Further analysis of the partial nephrectomy
patients revealed that respective mean nephrometry scores of open
versus MIS were 7.8 vs 6.0 (P=0.001), and in particular the
nearest components were significantly different (P<0.001; Table 4).
Such a difference was evident for the radical nephrectomy group.
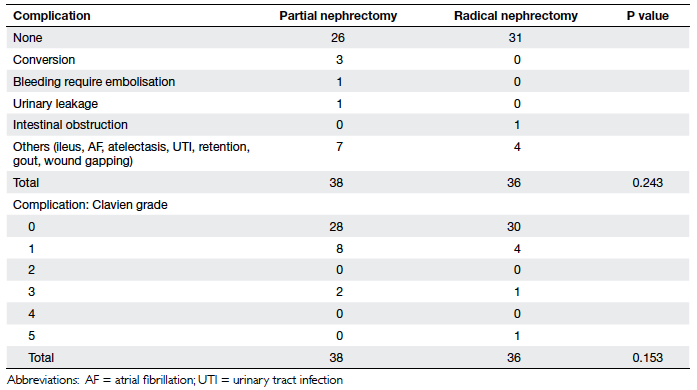
The overall 90-day morbidity in our study cohort was low, and
included urinary leakage (n=1) and bleeding warranting
embolisation (n=1) in the partial nephrectomy group, and
intestinal obstruction (n=1) in the radical nephrectomy group.
None of our patients received a postoperative transfusion.
Mortality at 90 days in the radical nephrectomy group (n=1) was in
a patient with metastatic RCC undergoing cytoreductive
nephrectomy. There was no difference in postoperative 90-day
morbidity and mortality between the two groups, even after
stratification according to mean nephrometry score or with respect
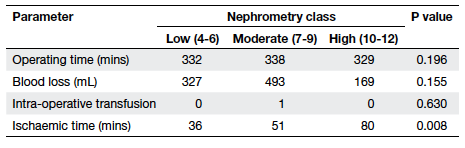
to different classes (Table 5). Ischaemic time was significantly
higher for patients in higher nephrometry classes in the partial
nephrectomy group (36 mins vs 51 mins vs 80 mins, P=0.008; Table 6);
all three patients with high nephrometry scores underwent open
surgery using cold ischaemia with ice sludge surface cooling, thus
explaining the difference in ischaemic time.
Discussion
The standard care of patients with a solid
renal mass is excision. Partial nephrectomy has become the
standard for T1a RCCs and more recent data support its use in
larger tumours of up to 7 cm (ie T1b). Most internationally
recognised guidelines support recourse to partial nephrectomy for
T1a tumours whenever technically feasible,5 6 as
data suggest comparable oncological outcomes with more favourable
outcomes in terms of risk of renal failure warranting dialysis,
cardiovascular morbidity, and even mortality. Approaches to the
management of a solid renal mass include consideration of whether
to remove the whole kidney or resect the tumour only and achieve a
margin clear of pathology. Secondary consideration is given to the
approach of the surgery, be it a traditional open one or MIS
(purely laparoscopic or robotic-assisted laparoscopic). Although
the latter is technically more demanding and has more
postoperative complications (blood loss, recourse to transfusions,
and urinary leakage), many high-volume centres show favourable
results in experienced hand.14
Many factors contribute to the choice of
surgery and mode of approach. They include hospital
infrastructures and patient volume, experience and training
history of the relevant surgeons, patient preference, and most
importantly tumour characteristics. Traditionally, clinical
decisions were based mostly on the first of these factors,
resulting in heterogeneous clinical choices and operative results.
Even when only tumour characteristics were taken into account,
there was wide heterogeneity in definitions, such as centrality or
hilar location, and makes direct comparison of results between
studies difficult and impractical.
The concept of nephrometry was proposed as
a tool to objectively assess the complexity of a solid renal mass.
To date there are three studies of largely nephrometric systems.
They are the R.E.N.A.L nephrometry score proposed by Kutikov and
Uzzo in 2009,8 the preoperative aspects and dimensions used for an
anatomical (PADUA) classification of renal tumours by Ficarra et
al in 2009,9 and the
C-index method proposed by Simmons et al in 2010.10 Most studies utilise the nephrometry scales
in patients undergoing partial nephrectomy. The three methods made
use of different parameters to assess the locations of the tumour
in relation to various important structures of the kidney, and to
predict the technical difficulty that might be encountered during
nephron-sparing surgery of the target lesion. They have been
reviewed as new tools that can guide surgical decision-making to
improve academic reporting, risk assessment of complications, and
prediction of functional outcomes.
The R.E.N.A.L. nephrometry score is one of
the most studied scoring systems with numerous articles describing
its use in clinical practice. The original description of the
score was to set a standard reporting system, and its use
suggested a relationship between renal mass anatomy, pathology,
and prognosis.8 Assessments
of inter-observer variability confirm their reproducibility and
inter-observer agreement was robust across specialties and levels
of training.15 16 17 18 Later studies showed
that high R.E.N.A.L. scores were associated with higher major
complication rates than those with intermediate or low scores.15 19
Moreover, multivariate analysis revealed that prolonged operating
time and high-complexity nephrometry score category were
independent predictors of major complications.19 Other reports demonstrated that the
R.E.N.A.L. score correlated with both tumour grade (P<0.0001)
and histology (P<0.0001), such that as tumour size increases
there would be a greater probability of malignancy, including
high-grade and clear-cell tumour on histology.20 21
Nomograms have been developed based on study results to
preoperatively predict the likelihood of malignant and high-grade
pathology of an enhancing renal mass,20
and such systems have been externally validated.22 Other studies have demonstrated the
association of nephrometry scores with use of ischaemia in partial
nephrectomy,15 warm
ischaemia time,23 choice
of surgery (partial vs radical nephrectomy),17 24 25 need of conversion to
radical nephrectomy,23
changes in the percent functional volume preserved and
perioperative functional decrease,26
long-term renal functional outcome following partial nephrectomy,27 and postoperative
urinary leakage.28 In
particular R.E.N.A.L. scores were higher in patients with partial
nephrectomy who developed complications than in partial
nephrectomy patients who did not (6.9 vs 6.0, P=0.02). No
corresponding differences were found in patients having radical
nephrectomy (P=0.99).29
Other studies investigating their applications on robotic partial
nephrectomy have shown incongruent results. In one study, Mufarrij
et al30 did not show the ability of this scoring system to predict
perioperative outcomes in robotic-assisted partial nephrectomy.
Others found significant correlations of the score with increased
warm ischaemia time, blood loss, complications, and length of
hospital stay31 32 in patients undergoing robotic and
laparoscopic partial nephrectomy. Clinical application of such
anatomical classification systems has gained popularity in
selecting cases suitable for alternative treatment of small renal
masses (such as by thermal ablation).33
Available data so far show more evidence to support the use of
this scoring system to make treatment decision more objective for
renal masses.34 35
The results of our study clearly
demonstrate a positive correlation of R.E.N.A.L. scores with the
choice of nephrectomy (partial vs radical), in terms of the total
summed scores and individual parameters including radius (size),
location nearest to the collecting system, and relationship to polar
lines. These findings support the idea that clinical decisions
based solely on the size of tumours are oversimplified and other
anatomical factors should enter overall considerations. We did not
find significant correlations for other individual parameters,
such as exophytic components and anterior/posterior location. This
was in contrast to a previous study which espoused the relevance
of such components to the choice of ablative therapy
(radiofrequency, cryoablation, or partial nephrectomy) as
originally described by Kutikov and Uzzo.8
Another significant finding was the correlation between the score
and the choice of approach in partial nephrectomy. It was shown
that with an increase in mean nephrometry score or class, there
was a trend towards choosing open rather than a MIS approach. This
signifies that whenever partial nephrectomy is feasible, the open
method is preferred for more complex tumours and that this
practice can be based on an objective scoring system. However,
this was not observed in our radical nephrectomy group, which
echoed a previous study finding and like the original description
aimed at partial nephrectomy (not radical nephrectomy). The
significant correlation of R.E.N.A.L. class with ischaemic time
may be useful to guide the choice of open approaches for partial
nephrectomy in the presence of a renal tumour with a high score.
This could facilitate the safe use of cold ischaemia so as to
maximise preservation of renal function.
Our results were contrary to previous
investigators reporting that the R.E.N.A.L. score was not
associated with presence or severity of complications in both
patient groups in terms of their mean score or class. This could
be explained by the relatively low frequency of major
complications in our study cohort (5.4%) and in the small sample
size. With more prospective data available, we believe similar
correlations of the score with the frequency of postoperative
complications and perioperative outcomes would be revealed.
An inherent limitation of our study was
that it was retrospective with respect to data collection and
analysis. A second limitation was the exclusion of many patients
due to unavailability of satisfactory quality images for the
calculation of scores to make direct comparisons. A third
limitation of the R.E.N.A.L. score per se was that the weight
given to individual components contributed to the total score;
numerical values were allocated arbitrarily and still await
validation. Although ours is one of the few studies that
demonstrate the association of this score and individual
parameters on the choice of surgery rather than sole reliance on
tumour size, we still have to define a single value in this
scoring system below which we can confidently recommend partial
nephrectomy. Moreover, other confounding factors such as the
surgeon’s experience and learning curve data were not available
for analysis, and may heavily influence clinical decisions.
Future directions of studies and clinical
utilisation of such a scoring system will aim to define different
weightings for individual components contributing to the total
score. Other studies may aim at enhancing the reproducibility and
predictability of such tools, so that direct comparison can be
made with other centres. Are we doing better than eyeballing when
managing a solid renal mass? Maybe we are, but the use of the
nephrometry score will enhance communication, documentation, and
education for the coming younger generation of urologists. Lately,
Simmons et al36 have
described the integration of the R.E.N.A.L. and C-index scoring
systems as diameter-axial-polar nephrometry (DAP). Initial results
demonstrate the DAP scoring system to be simpler, to decrease
measurement error, to improve performance characteristic, to make
interpretation easier, and to exhibit a clear association with
volume loss and late function after partial nephrectomy. More
mature data will allow us to choose the best tools for our
patients.
Conclusions
The R.E.N.A.L. nephrometry score of a solid
renal mass shows a significant association with our choice of
surgery (partial vs radical) and our approach to surgery (open vs
MIS), particularly in patients receiving partial nephrectomy. Its
association with postoperative complications was not demonstrated
in this study. The score provides a useful tool to define the
character of a renal mass objectively, aid clinical decision-making, and enhance communication between professionals with
respect to the management of solid renal masses.
References
1. Hospital Authority. Hong Kong
Cancer Registry. Available from:
http://www3.ha.org.hk/cancereg/statistics.html. Accessed Jun 2013.
2. Van Poppel H, Da Pozzo L,
Albrecht W, et al. A prospective randomized EORTC intergroup phase
3 study comparing the oncologic outcome of elective
nephron-sparing surgery and radical nephrectomy for low-stage
renal cell carcinoma. Eur Urol 2011;59:543-52. Crossref
3. Go AS, Chertow GM, Fan D,
McCulloch CE, Hsu CY. Chronic kidney disease and the risks of
death, cardiovascular events, and hospitalization. N Engl J Med
2004;351:1296-305. Crossref
4. Van Poppel H, Da Pozzo L,
Albrecht W, et al. A prospective randomized EORTC intergroup phase
3 study comparing the complications of elective nephron-sparing
surgery and radical nephrectomy for low-stage renal cell
carcinoma. Eur Urol 2007;51:1606-15. Crossref
5. Ljungberg B, Cowan NC, Hanbury
DC, et al. EAU guidelines on renal cell carcinoma: the 2010
update. Eur Urol 2010;58:398-406. Crossref
6. Campbell SC, Novick AC,
Belldegrun A, et al. Guideline for management of the clinical T1
renal mass. J Urol 2009;182:1271-9. Crossref
7. Smaldone MC, Kutikov A, Egleston
B, et al. Assessing performance trends in laparoscopic nephrectomy
and nephron-sparing surgery for localized renal tumors. Urology
2012;80:286-92. Crossref
8. Kutikov A, Uzzo RG. The
R.E.N.A.L. nephrometry score: a comprehensive standardized system
for quantitating renal tumor size, location and depth. J Urol
2009;182:844-53. Crossref
9. Ficarra V, Novara G, Secco S, et
al. Preoperative aspects and dimensions used for an anatomical
(PADUA) classification of renal tumors in patients who are
candidates for nephron-sparing surgery. Eur Urol 2009;56:786-93. Crossref
10. Simmons MN, Ching CB,
Samplaski MK, Park CH, Gill IS. Kidney tumor location measurement
using the C index method. J Urol 2010;183:1708-13. Crossref
11. Levey AS, Stevens LA, Schmid
CH, et al. A new equation to estimate glomerular filtration rate.
Ann Intern Med 2009;150:604-12. Crossref
12. American Society of
Anesthesiologists. New classification of physical status.
Anesthesiology 1963;24:111.
13. Dindo D, Demartines N, Clavien
PA. Classification of surgical complications: a new proposal with
evaluation in a cohort of 6336 patients and results of a survey.
Ann Surg 2004;240:205-13. Crossref
14. Gill IS, Kavoussi LR, Lane BR,
et al. Comparison of 1,800 laparoscopic and open partial
nephrectomies for single renal tumors. J Urol 2007;178:41-6. Crossref
15. Hew MN, Baseskioglu B, Barwari
K, et al. Critical appraisal of the PADUA classification and
assessment of the R.E.N.A.L. nephrometry score in patients
undergoing partial nephrectomy. J Urol 2011;186:42-6. Crossref
16. Kolla SB, Spiess PE, Sexton
WJ. Interobserver reliability of the RENAL nephrometry scoring
system. Urology 2011;78:592-4. Crossref
17. Weight CJ, Atwell TD, Fazzio
RT, et al. A multidisciplinary evaluation of inter-reviewer
agreement of the nephrometry score and the prediction of long-term
outcomes. J Urol 2011;186:1223-8. Crossref
18. Montag S, Waingankar N, Sadek
MA, Rais-Bahrami S, Kavoussi LR, Vira MA. Reproducibility and fidelity of the R.E.N.A.L. nephrometry score. J Endourol
2011;25:1925-8. Crossref
19. Simhan J, Smaldone MC, Tsai
KJ, et al. Objective measures of renal mass anatomic complexity
predicts rates of major complications following partial
nephrectomy. Eur Urol 2011;60:724-30. Crossref
20. Kutikov A, Smaldone MC,
Egleston BL, et al. Anatomic features of enhancing renal masses
predicts malignant and high-grade pathology: a preoperative
nomogram using the RENAL nephrometry score. Eur Urol
2011;60:241-8. Crossref
21. Satasivam P, Sengupta S,
Rajarubendra N, Chia PH, Munshey A, Bolton D. Renal lesions with
low R.E.N.A.L nephrometry score are associated with more indolent
renal cell carcinomas (RCCs) or benign histology: findings in an
Australian cohort. BJU 2012;109 Suppl 3:44-7.
22. Wang HK, Zhu Y, Yao XD, et al.
External validation of a nomogram using RENAL nephrometry score to
predict high grade renal cell carcinoma. J Urol 2012;187:1555-60. Crossref
23. Long JA, Arnoux V, Fiard G, et
al. External validation of the RENAL nephrometry score in renal
tumours treated by partial nephrectomy. BJU Int 2013;111:233-9. Crossref
24. Satasivam P, Rajarubendra N,
Chia PH, Munshey A, Sengupta S, Bolton D. Trends in the use of
nephron-sparing surgery (NSS) at an Australian tertiary referral
centre: an analysis of surgical decision-making using the
R.E.N.A.L. nephrometry scoring system. BJU Int 2012;109:1341-4. Crossref
25. Broughton GJ, Clark PE,
Barocas DA, et al. Tumour size, tumour complexity, and surgical
approach are associated with nephrectomy type in small renal
cortical tumours treated electively. BJU Int 2012;109:1607-13. Crossref
26. Simmons MN, Hillyer SP, Lee
BH, Fergany AF, Kaouk J, Campbell SC. Nephrometry score is
associated with volume loss and functional recovery after partial
nephrectomy. J Urol 2012;188:39-44. Crossref
27. Cha EK, Ng CK, Jeun B, et al.
Preoperative radiographic parameters predict long-term renal
impairment following partial nephrectomy. World J Urol 2013;31:817-22. Crossref
28. Bruner B, Breau RH, Lohse CM,
Leibovich BC, Blute ML. Renal nephrometry score is associated with
urine leak after partial nephrectomy. BJU Int 2011;108:67-72. Crossref
29. Rosevear HM, Gellhaus PT,
Lightfoot AJ, Kresowik TP, Joudi FN, Tracy CR. Utility of the
RENAL nephrometry scoring system in the real world: predicting
surgeon operative preference and complication risk. BJU Int
2012;109:700-5. Crossref
30. Mufarrij PW, Krane LS,
Rajamahanty S, et al. Does nephrometry scoring of renal tumors
predict outcomes in patients selected for robot-assisted partial
nephrectomy? J Endourol 2011;25:1649-53. Crossref
31. White MA, Haber GP, Autorino
R, et al. Outcomes of robotic partial nephrectomy for renal masses
with nephrometry score of ≥7. Urology 2011;77:809-13. Crossref
32. Hayn MH, Schwaab T, Underwood
W, Underood W, Kim HL. RENAL nephrometry score predicts surgical
outcomes of laparoscopic partial nephrectomy. BJU Int
2011;108:876-81.
33. Reyes J, Canter D, Putnam S,
et al. Thermal ablation of the small renal mass: case selection
using the R.E.N.A.L-Nephrometry Score. Urol Oncol 2013;31:1292-7. Crossref
34. Canter D, Kutikov A, Manley B,
et al. Utility of the R.E.N.A.L. nephrometry scoring system in
objectifying treatment decision-making of the enhancing renal
mass. Urology 2011;78:1089-94. Crossref
35. Tobert CM, Kahnoski RJ,
Thompson DE, Anema JG, Kuntzman RS, Lane BR. RENAL nephrometry
score predicts surgery type independent of individual surgeon’s
use of nephron-sparing surgery. Urology 2012;80:157-61. Crossref
36. Simmons MN, Hillyer SP, Lee
BH, Fergany AF, Kaouk J, Campbell SC. Diameter-axial-polar
nephrometry: integration and optimization of R.E.N.A.L. and
centrality index scoring systems. J Urol 2012;188:384-90. Crossref