DOI: 10.12809/hkmj154803
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE CME
Anticoagulation for stroke prevention in elderly patients with non-valvular atrial fibrillation: what are the obstacles?
CW Wong, FHKCP, FHKAM (Medicine)
Department of Medicine and Geriatrics, Caritas Medical Centre, Shamshuipo, Hong Kong
Corresponding author: Dr CW Wong (chitwaiwong@hotmail.com)
Abstract
The elderly with atrial fibrillation are more prone
to stroke. Oral anticoagulants such as warfarin
are effective in the prevention of atrial fibrillation–associated
stroke and systemic embolism. The
CHADS2 or CHA2DS2-VASc score and HAS-BLED
score were developed to stratify stroke risk associated
with atrial fibrillation and bleeding risk in a patient
with atrial fibrillation, respectively, to facilitate
the decision for and safe use of oral anticoagulant.
Nonetheless, the decision for anticoagulation is
not straightforward and the elderly with non-valvular
atrial fibrillation are often precluded from
anticoagulant prescription. Advanced age and
disadvantages associated with the elderly such
as fall, comorbidities, cognitive impairment, and
polypharmacy contribute to the over-concern of
physicians about bleeding risk. Various treatment
options such as low-intensity warfarin and aspirin
plus clopidogrel have been suggested but are inferior
to dose-adjusted warfarin. Novel oral anticoagulants
with promising efficacy and convenience hold great
appeal. Optimal management of underlying medical
conditions and modifiable stroke risk factors,
together with intervention to improve the safe use of
oral anticoagulants, are useful.
Introduction
Atrial fibrillation (AF) is common in the elderly.
The prevalence tends to increase with age with 1.7% in people aged 60-64
years increasing to 17.8% in those aged ≥85 years.1
A similar trend has been reported in the Chinese
population despite a lower prevalence of 1.3% in the
60-69 years’ age-group and 7.5% in those aged 80-89
years.2
Atrial fibrillation is an independent risk factor
for stroke.3 There is an almost five-fold increase in
age-adjusted incidence of AF-associated stroke if no
anticoagulation therapy is given. The attributable
risk of stroke associated with other cardiovascular
risk factors—such as hypertension, congestive heart
failure, and ischaemic heart disease—decreases
with age. In contrast, the attributable risk for stroke
associated with AF increases with age, rising from
1.5% in people aged 50-59 years to 23.5% in those
aged 80-89 years. In addition, consequent stroke
tends to be more severe with significant disability,
and mortality rate is double that of non-AF stroke,
especially in people ≥75 years.4 Thus, older patients
with AF are particularly prone to stroke and its
adverse effects.
Antithrombotic therapy is effective in reducing
AF stroke risk with oral anticoagulant (OAC) more
efficacious than antiplatelet agents.5 Until recently
and before the advent of novel OAC, the vitamin
K antagonist, warfarin, was the only OAC available
and it is still the most common OAC prescribed
nowadays. Nonetheless, warfarin is inconvenient to use and its
associated bleeding risk is particularly troublesome
for the elderly. As a consequence, it is often
underutilised in the elderly.
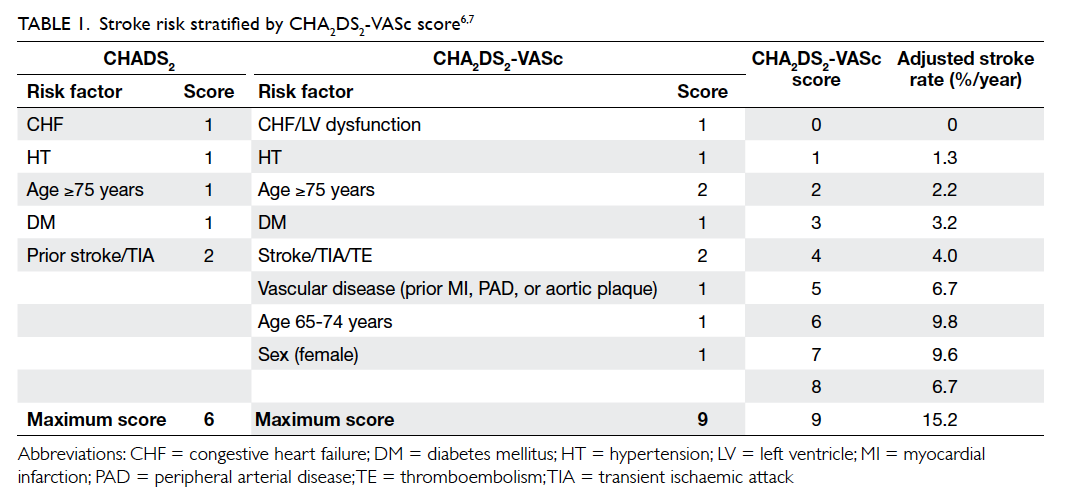
Stroke risk assessment
Stroke risk varies widely in AF patients and depends
on the presence of stroke risk factors. There are
several risk stratification schemes to facilitate the
decision to commence antithrombotic therapy of
which the CHADS2 or CHA2DS2-VASc score is the
most common and easy to use with satisfactory
reliability (Table 1).6 7 The CHA2DS2-VASc score is an extension of the CHADS2 (congestive heart failure,
hypertension, age ≥75 years, diabetes mellitus, and
prior stroke or transient ischaemic attack [TIA])
with the addition of other stroke risk factors
(vascular disease in the form of prior myocardial
infarction, plaque in aorta and peripheral artery
disease, age 65-74 years, and female sex) that enable
a more comprehensive stroke risk assessment. Prior
stroke or TIA, and age ≥75 years are regarded as
major risk factors and score 2 points each while
other risk factors are regarded as non-major risk
factors and score 1 point each. Recommendation for
antithrombotic therapy is based on the presence or
absence of risk factors.
Anticoagulant use in atrial fibrillation for stroke prevention
An anticoagulant that clears clotting factors from
the circulation to prevent blood clot formation is
considered the most effective AF stroke preventive
therapy. The traditional anticoagulant, the vitamin
K antagonist—warfarin—impairs the synthesis
of clotting factors II, VII, IX, and X; novel OACs
selectively inhibit only thrombin (dabigatran) or
factor Xa (rivaroxaban, apixaban, and edoxaban).
Adjusted-dose warfarin (target international
normalised ratio [INR], 2-3) has been shown to
reduce stroke risk by 64% while antiplatelet agents
to reduce the risk by 22%5; high-risk patients showed
larger stroke risk reduction with warfarin. There
was a small increase in major extracranial and
intracranial haemorrhage (ICH) risk (0.2%-0.3% per
year) associated with warfarin but overall mortality
was significantly reduced (26%) by warfarin.
The European Society of Cardiology
incorporated the CHADS2/CHA2DS2-VASc risk
stratification scheme into guidelines to help clinicians
decide the most appropriate antithrombotic therapy.8
It recommends no treatment rather than aspirin for
patients with CHA2DS2-VASc score of 0 (including
female <65 years) because aspirin may not be better
than no treatment in the reduction of stroke risk and
increases the bleeding risk6; OAC is recommended
for those who score ≥1.
Underutilisation of oral anticoagulant in the elderly
The elderly with AF, especially those aged ≥75 years,
are considered to have at least one major risk factor
for stroke with a CHA2DS2-VASc score of 2, thus,
OAC is certainly recommended. Nonetheless, it is
underutilised in the clinical setting. Among older
patients with known AF without contra-indications
to OAC and admitted for ischaemic stroke, only 40%
were prescribed warfarin prior to the stroke event.9
Prescription rate decreased with increasing age,
from 75% in those <70 years to 24% in those aged
≥90 years.10
Overestimation of the bleeding risk and
disadvantages associated with advanced age are
barriers to prescription of OAC in the elderly. The
most commonly cited reason not to anticoagulate
is increased bleeding risk followed by fall risk.11
Advanced age, co-morbidities, and patient compliance
have also been reported to influence physician
decision on anticoagulation. The inconvenience of
frequent monitoring with dose adjustment, and drug
and food interactions further contribute to OAC
underuse. A small survey in Hong Kong showed that
both physician awareness and patient knowledge
of anticoagulation for AF stroke prevention is
insufficient, which is another barrier to OAC use.12
Advancing age
The Birmingham Atrial Fibrillation Treatment of the
Aged Study recruited nearly 1000 patients aged ≥75
years with AF to receive either warfarin (INR, 2-3)
or aspirin 75 mg daily with a mean follow-up of 2.7
years. It revealed a significant reduction in ischaemic
stroke in the warfarin group compared with the
aspirin group (relative risk=0.3; 95% confidence
interval [CI], 0.13-0.63).13 The main benefit was seen
in the reduction of severe or disabling non-fatal
stroke. The efficacy of warfarin did not change with
increasing age. There was no difference between the
two groups in major bleeding rate.
Analysis of the Atrial Fibrillation Investigators
database revealed that the relative benefit of OAC
versus an antiplatelet agent and no antithrombotic
therapy did not vary by age for ischaemic stroke
prevention whilst the benefit of an antiplatelet agent
decreased with age.14
Analysis of local registry data from 2339
non-valvular AF Chinese patients aged ≥80 years
demonstrated a lower rate of ischaemic stroke
and death in patients prescribed warfarin (hazard
ratio=0.53; 95% CI, 0.48-0.58) but a higher ICH rate
than in those without anti-thrombotic therapy after
2.2 years’ follow-up (1.1% per year vs 0.6% per year).15
Overall, net clinical benefits favoured warfarin for all
elderly patients, particularly those at high stroke and
ICH risk.
Therefore, age alone should not be a reason to
exclude anticoagulation.
Bleeding
Elderly patients are prone to anticoagulant-associated
bleeding. The incidence of life-threatening or fatal
bleeding has been shown to be significantly higher
in elderly patients aged ≥80 years than in those aged
<50 years (relative risk=4.6; 95% CI, 1.2-18.1) on
warfarin.16 Major bleeding risk has been shown to
rise with increasing age in AF patients regardless
of anticoagulant use; patients ≥80 years on warfarin
were at particularly high risk of ICH.17 Further, ICH
as a consequence of warfarin intake was associated
with poor outcome; 3-month mortality was double
that of patients not taking warfarin.18 The Chinese
population has a higher background haemorrhagic
stroke rate that accounts for at least 30% of all
strokes.19 Together with a four-fold higher warfarin-associated
ICH risk in Asians compared with
whites,20 concern about warfarin is even greater in
Chinese elderly patients.
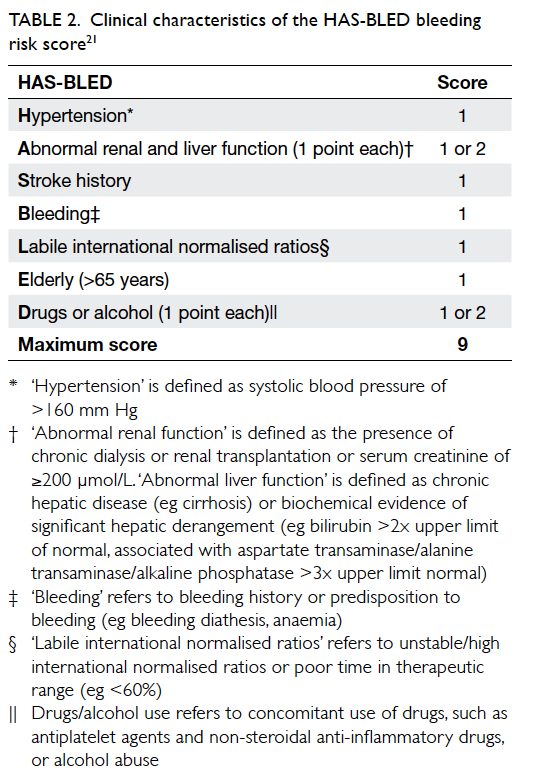
A simple bleeding risk score, HAS-BLED
(hypertension, abnormal renal/liver disease, stroke
history, bleeding history, liable INR, elderly >65
years, drugs/alcohol) [Table 2], has been derived to predict major bleeding risk in AF patients21 and
incorporated into AF management guidelines as an
indicator for bleeding risk.8 A score of 0-1 indicates
‘low risk’ with annual bleeding rate of <2%, a score
of 2-3 indicates ‘moderate risk’ with annual bleeding
rate of 2%-4%, and a score of ≥4 indicates ‘high risk’
with annual bleeding rate of >4%. It is particularly
useful in predicting major bleeding risk in patients
who are receiving an antiplatelet agent alone or no
antithrombotic therapy prior to the initiation of
OAC. Its use is not to exclude patients from OAC
but to identify modifiable bleeding risk factors that
can then be corrected to minimise bleeding risk.
Assessment of both CHA2DS2-VASc and HAS-BLED
can help balance the stroke risk and bleeding
risk in AF patients, but details of how to incorporate
the CHA2DS2-VASc score into the HAS-BLED score
to guide the management of AF needs further study.
Fall
Fall risk and fall-related head injury with ICH
increase with age, which is another concern when
considering anticoagulation.
Using pooled data from major AF trials,
warfarin showed a net benefit of stroke protection in
elderly patients with average stroke and fall risk over
aspirin or no treatment in terms of higher quality-adjusted life-years.22 Regardless of patient age or baseline stroke risk, fall risk was not an important
factor in determining optimal therapy: patients with
average fall risk would need to fall 295 times in a year
for warfarin not to be the optimal therapy.
Another database study of nearly 20 000
elderly AF patients (mean age, 80 years) found that
patients at high fall and stroke (CHADS2 score ≥2) risk
appeared to have a net benefit from OAC despite
an increased baseline ICH risk, in which OAC use
was associated with a 25% relative risk reduction in
the composite outcome of stroke, any haemorrhage,
myocardial infarction and death whilst there was an
insignificant reduction in those at high risk for fall
but with CHADS2 score of 0 or 1.23
A subsequent prospective study showed that
among 515 AF patients discharged on OAC, there
was no significant increase in major bleeding rate
(including fatal haemorrhage and ICH) in patients at
high fall risk compared with those at low fall risk at
12 months.24
These data suggest that in patients with
valid indications for anticoagulation, the benefits
outweigh the risk, and fall risk should not be a sole
reason to withhold anticoagulation.
Narrow therapeutic range, co-morbidities, and polypharmacy
Maintaining INR at 2 to 3 for at least 60% of the time
provides effective stroke prevention and minimises
bleeding risk.8 Nonetheless, maintaining this optimal
range is not easy as it is influenced by both internal
and external factors.
Consistent dietary intake of vitamin K is
important for stable anticoagulation. This is difficult
for elderly patients with poor health, who get sick
frequently, have a poor diet and fluctuating vitamin
K intake, or for patients with cognitive impairment
who cannot comply with a diet with constant vitamin
K content.
Many concurrent diseases can also influence
INR control. It is particularly troublesome during
the exacerbation of disease or if the disease
course is fluctuating. Hepatic dysfunction impairs
synthesis of clotting factors and thus potentiates
the anticoagulation effects. Hepatic congestion as a
result of congestive heart failure can inhibit warfarin
metabolism and lead to accumulation of warfarin
and over-anticoagulation. Hypermetabolic states
such as febrile illness or thyrotoxicosis may increase
catabolism of vitamin K–dependent clotting factors
and may increase INR level.25 On the contrary,
hypothyroidism that decreases the catabolism
of vitamin K–dependent clotting factors may
decrease INR. Patients with AF with concomitant
acute coronary disease and percutaneous coronary
intervention require aspirin-clopidogrel dual
therapy in addition to warfarin, and this further
increases bleeding risk. Patients with severe chronic
kidney disease (estimated glomerular filtration
rate, <30 mL/min/1.73 kg/m2) who are prescribed
warfarin are at higher risk of over-anticoagulation,
which is associated with more than double the
risk for major bleeding compared with patients
with mild or moderate chronic kidney disease.26
Patients with cognitive impairment have difficulty
in managing their warfarin intake, coping with
dosage adjustment, and being aware of drug and
food interactions; all of which may result in over- or
under-anticoagulation.
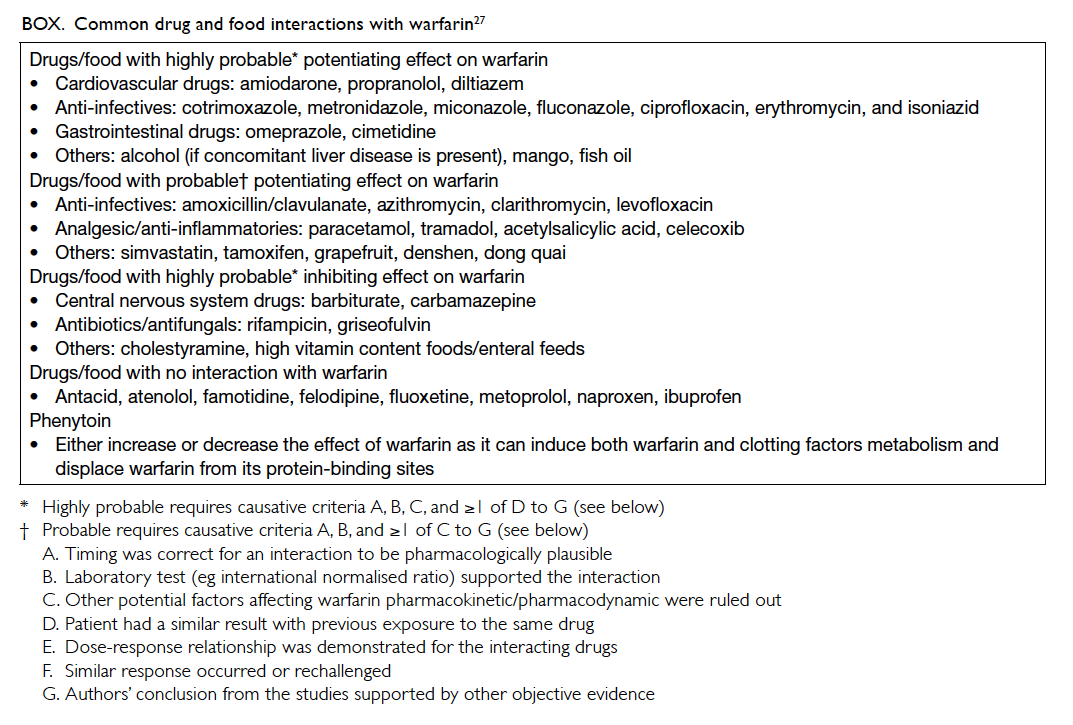
Warfarin can interact with many drugs and
herbal products. This has clinical implications
because polypharmacy and adjustment of
medications due to an acute illness is frequent in the
elderly. Polypharmacy is an independent risk factor
for warfarin-related major bleeding and there is a
12% increase in risk for each additional drug taken.24
It is relatively easier to manage drugs in chronic
use than those prescribed for a short period of
time or when necessary. Warfarin-drug interaction
is through the influence of pharmacokinetics that
reduce gastrointestinal absorption or disrupts
metabolic clearance and pharmacodynamics that
alter the haemostatic response.25 The Box lists the
common drugs and food that interact with warfarin.27
It should be noted that the majority of the data are
from case series or reports because of the scarcity
of randomised controlled trials. Thus, the rate of
harm and its generalisation to all warfarin users
needs continuous review. Nonetheless, physicians
should proceed with caution and frequently monitor
INR when prescribing potential offending drugs.
There is a group of drugs that potentiate bleeding
on their own without alteration of INR that includes
antiplatelet drugs, heparin, non-steroidal anti-inflammatory
drugs (NSAIDs), cyclooxygenase-2
(COX-2) inhibitors, and selective serotonin reuptake
inhibitors.
Pain syndrome and analgesic use are common
in the elderly. Among analgesics, paracetamol is
preferred for occasional use when taking warfarin.28
A dose that exceeds 2 g daily for more than a few
days may raise INR and increase the bleeding risk,
however. The NSAIDs, including COX-2 selective
NSAIDs, should be avoided. Non-pharmacological
methods of pain relief to minimise the use of
analgesics are encouraged.
Undiagnosed/occult atrial fibrillation
It is not uncommon for a patient to have AF first
diagnosed when ischaemic stroke occurs. Very often,
AF is undiagnosed as it is usually asymptomatic;
10% to 40% of AF cases are asymptomatic.29 A population-based
study revealed that 20.1% of AF in patients
aged >60 years was undiagnosed.30 Paroxysmal AF,
which bears a similar stroke risk and benefit from
anticoagulation to sustained AF,31 also easily evades
ordinary electrocardiography (ECG) screening.
Paroxysmal AF is common (up to 16%) in cryptogenic
stroke32 that constitutes 25% of ischaemic stroke.33
Alternatives to warfarin
Low-intensity warfarin
Intensity of anticoagulation correlates directly with
incidence of haemorrhage,34 thus low-intensity
warfarin with a subtherapeutic range of INR, such
as INR of <2, is suggested for the elderly to lower
bleeding risk while still effectively preventing
stroke. The Boston Area Anticoagulation Trial for
Atrial Fibrillation Investigators found that low-dose
warfarin (target INR, 1.5-2.7) was more effective
than placebo in stroke prevention.35 There is a
racial difference in response to warfarin—a lower
INR target of 1.8 to 2.4 appears to be sufficient in
lowering both major bleeding and thromboembolic
events in Chinese.36 Japanese studies and their
registry data also support the lower INR target and
have recommended INR of 1.6 to 2.6 for AF patients
of ≥70 years in their AF management guideline.37
Aspirin plus clopidogrel
Aspirin (75-100 mg daily)–clopidogrel (75 mg daily)
dual therapy has been shown to be better than aspirin
alone in stroke prevention in AF patients, reducing
stroke risk by 28% compared with aspirin alone.38
Nonetheless, it is inferior to OAC in AF patients
with at least one risk factor for stroke; OAC reduced
stroke risk by 42% compared with dual therapy.39
Both dual therapy and OAC were associated with
similar but higher bleeding risk than aspirin.
Therefore, dual therapy may only be considered for
patients in whom OAC is unsuitable or for patient
preference.8
Novel oral anticoagulants
Novel OACs (dabigatran, rivaroxaban, apixaban,
and edoxaban) have been approved by the US Food
and Drug Administration (FDA) for AF stroke
prevention. They have undergone large clinical
trials in AF patients (mean age ≥70 years) with at
least one additional stroke risk factor. Rivaroxaban,
dabigatran 110 mg, and edoxaban were non-inferior
whilst both apixaban and dabigatran 150 mg were
superior to warfarin in stroke or systemic embolism
prevention; overall they significantly reduced the
risk by 19% compared with warfarin, mainly due to
a large reduction in haemorrhagic stroke.40 All-cause
mortality was also significantly reduced by 10%. The
newer anticoagulants appear to be safer with at least
a similar major bleeding rate and consistently lower
ICH rate (>50% fewer) compared with warfarin.
The exception is gastrointestinal bleeding risk in
dabigatran 150 mg, rivaroxaban and edoxaban 60
mg, occurrence of which was 25% more than with
warfarin. The favourable result was sustained across
a wide stratum of patients at high risk of both
ischaemic and bleeding events. The efficacy and
safety of novel OACs are consistent among Asian
patients including Chinese41 42 43 as well as Chinese
elderly aged ≥80 years.44 Because of the favourable
efficacy and safety profile, current guidelines have
recommended novel OACs as an alternative to
warfarin in primary and secondary stroke prevention
in patients with non-valvular AF.8
In addition, novel OACs are convenient because
of their predictable and reliable anticoagulation
properties, with far fewer drug interactions and no
food interactions or dietary restrictions. They can
be administered at a fixed dose and monitoring of
coagulation is unnecessary.
Nonetheless, their use is not without
drawbacks. They are eliminated renally, thus dose
adjustment based on renal function is required and
they are not recommended for patients with severely
impaired renal function (creatinine-clearance, <15
mL/min). Although drug interactions are fewer than
those for warfarin, there is potential interaction with
P-glycoprotein and CY3A4 inhibitors or inducers,
which include common cardiovascular drugs such
as amiodarone, dronedarone and diltiazem (all are
combined P-glycoprotein inhibitors and weak/moderate CYP3A4 inhibitors), and anti-infectives
such as rifampicin (strong CYP3A4 inducers),
clarithromycin and ketoconazole (strong dual
inhibitors of P-glycoprotein and CYP3A4), thus
caution is required. Because they do not affect INR
and there is no readily available measure to monitor
their anticoagulation, it is difficult to confirm if
patients are compliant with therapy. Thus, good
drug compliance is as important as taking warfarin.
Besides, the cost of novel OAC is more expensive
and long-term evidence is not complete. For those
patients already on warfarin who have satisfactory
anticoagulation control, whether there is extra
benefit in switching to novel OAC needs to be
explored.
Left atrial appendage closure
Since >90% non-valvular AF stroke inducing thromboemboli
are from the left atrial appendage (LAA),
LAA closure is considered an alternative to OAC.45
Percutaneous occlusion by placing the WATCHMAN
device in the LAA was approved by the US FDA
for the prevention of LAA thromboembolism in
patients in whom OAC was contra-indicated or
in whom management with an OAC was difficult.
The WATCHMAN device was shown to be non-inferior
to OAC in the composite endpoint of stroke,
cardiovascular death, and systemic embolism.46 Main
adverse effects are procedure-related, eg pericardial
effusion, incomplete LAA closure, dislodgement of
device, and blood clot formation on the device that
requires prolonged OAC.
Decision-making and strategies to improve
The aim of prescribing OAC to AF patients is
to prevent stroke or systemic embolism such
that patients’ health and functional state can be
maintained. The decision to prescribe an OAC in
the elderly is complicated, however. It requires not
only balancing the stroke risk and bleeding risk from
OAC, but also needs to consider the patient’s general
health, functional and cognitive ability, availability
of a caregiver, and patient’s attitude and preference
towards anticoagulation. Elderly patients with AF
who are in good health or have few co-morbidities
and a good functional state will definitely benefit
from OAC; for patients in poor health with multiple co-morbidities
who are functionally dependent, OAC
is not likely to provide additional benefit and there
is a high risk of bleeding. Apart from that, decision
making for other clinical scenarios is not easy.
Careful assessment and discussion with patients
and/or caregivers is essential when deciding whether
to prescribe OAC.
Better preparation of eligible patients for
anticoagulation by optimising their medical
condition to reduce the risk of stroke and bleeding,
together with education of patients and caregiver to
enhance compliance, are also useful. The following
are recommendations to improve the safety and
optimal effect of OAC:
- Co-morbidities: optimise underlying medical conditions, increase frequency of anticoagulation monitoring if medical condition changes or during acute illness
- Cardiovascular risk factors: screening for and proper control of modifiable cardiovascular risk factors
- Cognitive impairment: encourage involvement of caregiver to ensure compliance
- Polypharmacy: review and simplify drug regimen; discontinue unnecessary medications, avoid drugs that interact with OAC or use alternative drugs with less potential for interaction; frequent INR monitoring if offending drugs are prescribed or discontinued
- Bleeding: avoid concomitant medications associated with bleeding such as antiplatelet agent, NSAIDs and alcohol; better control of hypertension to lower ICH risk47; add proton pump inhibitor to lower upper gastrointestinal bleeding risk, especially those at increased risk48
- Fall prevention: fall risk assessment followed by intervention such as exercises for gait, balance, and strength training; education to increase safety awareness, prescription of appropriate walking aids, correction of vision if necessary, environmental modifications, and minimise or avoid offending medications
- Undiagnosed AF: liberal recording of ECGs in the elderly, especially those with cardiovascular risk factors, to capture AF49; for those with cryptogenic stroke, vigorously look for occult AF by prolonged ECG monitoring32
- Regular assessment of patient compliance with OAC, and review of stroke and bleeding risk and adjust the management plan accordingly
- Continued education for physicians about AF and OAC management
- Setting up of a warfarin clinic in Hong Kong with a multidisciplinary approach (physicians, pharmacists, and nurses) to provide patient education, regular INR monitoring with warfarin dosage adjustment, monitoring of drug and food interactions, and signs of bleeding have been shown to improve drug compliance, minimise bleeding risk, and maintain INR within the therapeutic range50
Conclusion
The elderly with AF are more prone to stroke than
younger patients, especially those aged ≥75 years.
Anticoagulation is effective in the prevention of
stroke in the elderly despite the increased bleeding
risk. Age alone should not exclude anticoagulation.
Novel OAC is convenient with comparable efficacy
to warfarin and a lower risk for ICH. This may
improve the prescription, however, long-term
evidence is awaited. The best management of the
elderly with AF depends on careful estimation of
thromboembolic and bleeding risk, patient’s ability
to cope with anticoagulation, and patient preference.
Optimal control of other potential risk factors for
stroke and bleeding is also important.
References
1. Heeringa J, van der Kuip DA, Hofman A, et al. Prevalence,
incidence and lifetime risk of atrial fibrillation: the
Rotterdam study. Eur Heart J 2006;27:949-53. Crossref
2. Zhou Z, Hu D. An epidemiological study on the prevalence
of atrial fibrillation in the Chinese population of mainland
China. J Epidemiol 2008;18:209-16. Crossref
3. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an
independent risk factor for stroke: the Framingham Study.
Stroke 1991;22:983-8. Crossref
4. Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity
in atrial fibrillation. The Framingham Study. Stroke
1996;27:1760-4. Crossref
5. Hart RG, Pearce LA, Aguilar MI. Meta-analysis:
antithrombotic therapy to prevent stroke in patients
who have nonvalvular atrial fibrillation. Ann Intern Med
2007;146:857-67. Crossref
6. Gage BF, Waterman AD, Shannon W, Boechler M, Rich
MW, Radford MJ. Validation of clinical classification
schemes for predicting stroke: results from the National
Registry of Atrial Fibrillation. JAMA 2001;285:2864-70. Crossref
7. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ.
Refining clinical risk stratification for predicting stroke and
thromboembolism in atrial fibrillation using a novel risk
factor–based approach: the Euro Heart Survey on atrial
fibrillation. Chest 2010;137:263-72. Crossref
8. Camm AJ, Lip GY, De Caterina R, et al. 2012 Focused
update of the ESC Guidelines for the management of
atrial fibrillation: an update of the 2010 ESC Guidelines
for the management of atrial fibrillation. Developed with
the special contribution of the European Heart Rhythm
Association. Eur Heart J 2012;33:2719-47. Crossref
9. Gladstone DJ, Bui E, Fang J, et al. Potentially preventable
strokes in high-risk patients with atrial fibrillation who are
not adequately anticoagulated. Stroke 2009;40:235-40. Crossref
10. Hylek EM, D’Antonio J, Evans-Molina C, Shea C, Henault
LE, Regan S. Translating the results of randomized trials
into clinical practice: the challenge of warfarin candidacy
among hospitalized elderly patients with atrial fibrillation.
Stroke 2006;37:1075-80. Crossref
11. Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding
anticoagulation for atrial fibrillation: a systematic review.
Age Ageing 2011;40:675-83. Crossref
12. Lee VW, Tam CS, Yan BP, Yu CM, Lam YY. Barriers to
warfarin use for stroke prevention in patients with atrial
fibrillation in Hong Kong. Clin Cardiol 2013;36:166-71. Crossref
13. Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin
for stroke prevention in an elderly community population
with atrial fibrillation (the Birmingham Atrial Fibrillation
Treatment of the Aged Study, BAFTA): a randomised
controlled trial. Lancet 2007;370:493-503. Crossref
14. van Walraven C, Hart RG, Connolly S, et al. Effect of age on
stroke prevention therapy in patients with atrial fibrillation:
the Atrial Fibrillation Investigators. Stroke 2009;40:1410-6. Crossref
15. Siu CW, Tse HF. Net clinical benefit of warfarin therapy
in elderly Chinese patients with atrial fibrillation. Circ
Arrhythm Electrophysiol 2014;7:300-6. Crossref
16. Fihn SD, Callahan CM, Martin DC, McDonell MB,
Henikoff JG, White RH. The risk for and severity of
bleeding complications in elderly patients treated with
warfarin. The National Consortium of Anticoagulation
Clinics. Ann Intern Med 1996;124:970-9. Crossref
17. Fang MC, Go AS, Hylek EM, et al. Age and the risk of
warfarin-associated hemorrhage: the anticoagulation and
risk factors in atrial fibrillation study. J Am Geriatr Soc
2006;54:1231-6. Crossref
18. Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg
SM. The effect of warfarin and intensity of anticoagulation
on outcome of intracerebral hemorrhage. Arch Intern Med
2004;164:880-4. Crossref
19. Zhang LF, Yang J, Hong Z, et al. Proportion of different
subtypes of stroke in China. Stroke 2003;34:2091-6. Crossref
20. Shen AY, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol
2007;50:309-15. Crossref
21. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ,
Lip GY. A novel user-friendly score (HAS-BLED) to
assess 1-year risk of major bleeding in patients with atrial
fibrillation: the Euro Heart Survey. Chest 2010;138:1093-100. Crossref
22. Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing
antithrombotic therapy for elderly patients with atrial
fibrillation who are at risk for falls. Arch Intern Med
1999;159:677-85. Crossref
23. Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena
DS, Rich MW. Incidence of intracranial hemorrhage in
patients with atrial fibrillation who are prone to fall. Am J
Med 2005;118:612-7. Crossref
24. Donzé J, Clair C, Hug B, et al. Risk of falls and major bleeds
in patients on oral anticoagulation therapy. Am J Med
2012;125:773-8. Crossref
25. Hirsh J, Fuster V, Ansell J, Halperin JL; American Heart
Association/American College of Cardiology Foundation.
American Heart Association/American College of
Cardiology Foundation guide to warfarin therapy.
Circulation 2003;107:1692-711. Crossref
26. Limdi NA, Beasley TM, Baird MF, et al. Kidney function
influences warfarin responsiveness and hemorrhagic
complications. J Am Soc Nephrol 2009;20:912-21. Crossref
27. Holbrook AM, Pereira JA, Labiris R, et al. Systematic
overview of warfarin and its drug and food interactions.
Arch Intern Med 2005;165:1095-106. Crossref
28. NHS choices. Can I take paracetamol if I’m on warfarin?
Available from: http://www.nhs.uk/chq/pages/858.aspx?categoryid=73&subcategoryid=103. Accessed Oct 2016.
29. Rho RW, Page RL. Asymptomatic atrial fibrillation. Prog
Cardiovasc Dis 2005;48:79-87. Crossref
30. Clua-Espuny JL, Lechuga-Duran I, Bosch-Princep R, et al.
Prevalence of undiagnosed atrial fibrillation and of that not
being treated with anticoagulant drugs: the AFABE study.
Rev Esp Cardiol (Engl Ed) 2013;66:545-52. Crossref
31. Hohnloser SH, Pajitnev D, Pogue J, et al. Incidence of
stroke in paroxysmal versus sustained atrial fibrillation
in patients taking oral anticoagulation or combined
antiplatelet therapy: an ACTIVE W Substudy. J Am Coll
Cardiol 2007;50:2156-61. Crossref
32. Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation
in patients with cryptogenic stroke. N Engl J Med
2014;370:2467-77. Crossref
33. Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of
undetermined source: the case for a new clinical construct.
Lancet Neurol 2014;13:429-38. Crossref
34. Levine MN, Hirsh J, Landefeld S, Raskob G. Hemorrhagic
complications of anticoagulant treatment. Chest
1992;102(4 Suppl):352S-363S. Crossref
35. The effect of low-dose warfarin on the risk of stroke
in patients with nonrheumatic atrial fibrillation. The
Boston Area Anticoagulation Trial for Atrial Fibrillation
Investigators. N Engl J Med 1990;323:1505-11. Crossref
36. You JH, Chan FW, Wong RS, Cheng G. Is INR between 2.0
and 3.0 the optimal level for Chinese patients on warfarin
therapy for moderate-intensity anticoagulation? Br J Clin
Pharmacol 2005;59:582-7. Crossref
37. JCS Joint Working Group. Guidelines for pharmacotherapy
of atrial fibrillation (JCS 2008): digest version. Circ J
2010;74:2479-500. Crossref
38. ACTIVE Investigators, Connolly SJ, Pogue J, et al. Effect
of clopidogrel added to aspirin in patients with atrial
fibrillation. N Engl J Med 2009;360:2066-78. Crossref
39. ACTIVE Writing Group of the ACTIVE Investigators,
Connolly S, Pogue J, et al. Clopidogrel plus aspirin versus
oral anticoagulation for atrial fibrillation in the Atrial
fibrillation Clopidogrel Trial with Irbesartan for prevention
of Vascular Events (ACTIVE W): a randomised controlled
trial. Lancet 2006;367:1903-12. Crossref
40. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison
of the efficacy and safety of new oral anticoagulants with
warfarin in patients with atrial fibrillation: a meta-analysis
of randomised trials. Lancet 2014;383:955-62. Crossref
41. Hori M, Connolly SJ, Zhu J, et al. Dabigatran versus
warfarin: effects on ischemic and hemorrhagic strokes and
bleeding in Asians and non-Asians with atrial fibrillation.
Stroke 2013;44:1891-6. Crossref
42. Wong KS, Hu DY, Oomman A, et al. Rivaroxaban for stroke
prevention in East Asian patients from the ROCKET AF
Trial. Stroke 2014;45:1739-47. Crossref
43. Goto S, Zhu J, Liu L, et al. Efficacy and safety of
apixaban compared with warfarin for stroke prevention
in patients with atrial fibrillation from East Asia: a
subanalysis of the Apixaban for Reduction in Stroke and
Other Thromboembolic Events in Atrial Fibrillation
(ARISTOTLE) Trial. Am Heart J 2014;168:303-9. Crossref
44. Chan PH, Huang D, Hai JJ, et al. Stroke prevention
using dabigatran in elderly Chinese patients with atrial
fibrillation. Heart Rhythm 2016;13:366-73. Crossref
45. Blackshear JL, Odell JA. Appendage obliteration to reduce
stroke in cardiac surgical patients with atrial fibrillation.
Ann Thorac Surg 1996;61:755-9. Crossref
46. Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous
closure of the left atrial appendage versus warfarin therapy
for prevention of stroke in patients with atrial fibrillation:
a randomised non-inferiority trial. Lancet 2009;374:534-42. Crossref
47. Hart RG, Tonarelli SB, Pearce LA. Avoiding central
nervous system bleeding during antithrombotic therapy:
recent data and ideas. Stroke 2005;36:1588-93. Crossref
48. Lin KJ, Hernández-Díaz S, García Rodríguez LA. Acid
suppressants reduce risk of gastrointestinal bleeding in
patients on antithrombotic or anti-inflammatory therapy.
Gastroenterology 2011;141:71-9. Crossref
49. Samol A, Masin M, Gellner R, et al. Prevalence of unknown
atrial fibrillation in patients with risk factors. Europace
2013;15:657-62. Crossref
50. Outcome evaluation of warfarin clinic: safe and feasible
care delivery model in primary care setting. Hong
Kong Hospital Authority Convention 2008. Available
from: http://www3.ha.org.hk/haconvention/hac2008/proceedings/pdf/Free%20Paper/SPP6-1.pdf. Accessed Oct
2016.