Hong Kong Med J 2026;32:Epub 4 Feb 2026
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
PERSPECTIVE
Diagnostic challenges and treatment outcomes of
primary vitreoretinal lymphoma in Hong Kong
Arnold SH Chee, AFCOphthHK1,2,3; Andrew CY Mak, FCOphthHK1,2,3; KW Kam, FCOphthHK1,2,3; Molly SC Li, FHKCP4; Mary Ho, FCOphthHK1,2,3; Marten E Brelen, PhD1,2; LJ Chen, PhD1,2,3; Wilson WK Yip, FCOphthHK1,2,3; Alvin L Young, FRCSI1,2,3
1 Department of Ophthalmology and Visual Sciences, The Chinese University of Hong Kong, Hong Kong SAR, China
2 Department of Ophthalmology and Visual Sciences, Prince of Wales Hospital, Hong Kong SAR, China
3 Department of Ophthalmology and Visual Sciences, Alice Ho Miu Ling Nethersole Hospital, Hong Kong SAR, China
4 Department of Clinical Oncology, The Chinese University of Hong Kong, Hong Kong SAR, China
Corresponding author: Prof Alvin L Young (youngla@ha.org.hk)
Introduction
Primary vitreoretinal lymphoma (PVRL) is a rare
and aggressive ocular variant of non-Hodgkin
lymphoma (NHL), predominantly of B-cell origin.1
It represents a subset of primary central nervous
system lymphoma (PCNSL), in which malignant
lymphocytic cells primarily affect the vitreous and/or retina, with or without involvement of the brain
and cerebrospinal fluid. Approximately one-fifth
of patients with PCNSL have concurrent ocular
manifestations at presentation, whereas 60% to
90% of patients with PVRL develop central nervous
system (CNS) disease within 16 to 24 months.2
The prognosis for patients with PVRL and
CNS involvement is poor, with a median survival
of 1 to 2 years.3 Thus, early diagnosis is imperative
for timely treatment. However, diagnosis is often
delayed because: (1) PVRL frequently masquerades
as chronic uveitis4; (2) the diagnostic yield of
vitreous samples is often low due to hypocellularity
and fragility of lymphoma cells3; (3) specialised
techniques and experienced cytopathologists are
required; and (4) patients often have reservations
about undergoing invasive diagnostic vitrectomy.
The current first-line treatment for PCNSL
comprises high-dose methotrexate (MTX)–based
polychemotherapy, with or without whole-brain
radiotherapy. Among patients with isolated PVRL,
intravitreal MTX has been shown to achieve ocular
tumour control in multiple studies.4 5 6 However,
there remains no consensus regarding the optimal
treatment regimen.
In Hong Kong, NHL is among the top ten
cancers in terms of both incidence (2.9%) and
mortality (2.6%).7 Diffuse large B-cell lymphoma
(DLBCL) is the most common type of NHL globally
and locally.8 Given that more than 95% of PVRL
cases are DLBCL,9 it is important to examine the
treatment outcomes of this under-reported disease
entity.
Our local experience
We share our experience managing patients
diagnosed with PVRL at Prince of Wales Hospital
and Alice Ho Miu Ling Nethersole Hospital, Hong
Kong, between August 2013 and April 2024.
A case of PVRL was defined by the presence
of characteristic vitreous opacity and/or subretinal
infiltrate, substantiated by a positive tissue biopsy
from the vitreous, brain, or cerebrospinal fluid.
In cases without CNS involvement and negative
vitreous biopsy, the diagnosis was made by
consensus between two vitreoretinal specialists
based on clinical examination. Cases of systemic
NHL (eg, secondary vitreoretinal metastasis from
primary extracranial lymphoma) were excluded.
Visual acuity (VA), ocular examination findings,
and multimodal ocular imaging of the tumours were
recorded. Patient demographics, ocular symptoms,
follow-up duration, oncological treatment details,
complications, and survival data were collected.
Outcomes of interest included initial and final
VA and treatment responses. For the latter, an
international standardised guideline on ocular
responses in PCNSL was utilised10: (1) complete
response (absence of vitreous cells and resolution of
retinal infiltrate [online supplementary Fig a to b of
Patient 2 as reference]); (2) partial response (reduced
but persistent vitreous cells or retinal infiltrate);
(3) progressive disease (increased vitreous cells or
progressive retinal infiltrate); and (4) relapse (new
lesion in patients who had achieved a complete
response).
With ethics approval and waiver of patient
consent (The Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics
Committee, Hong Kong [Ref No.: 2024.175]), 17 eyes
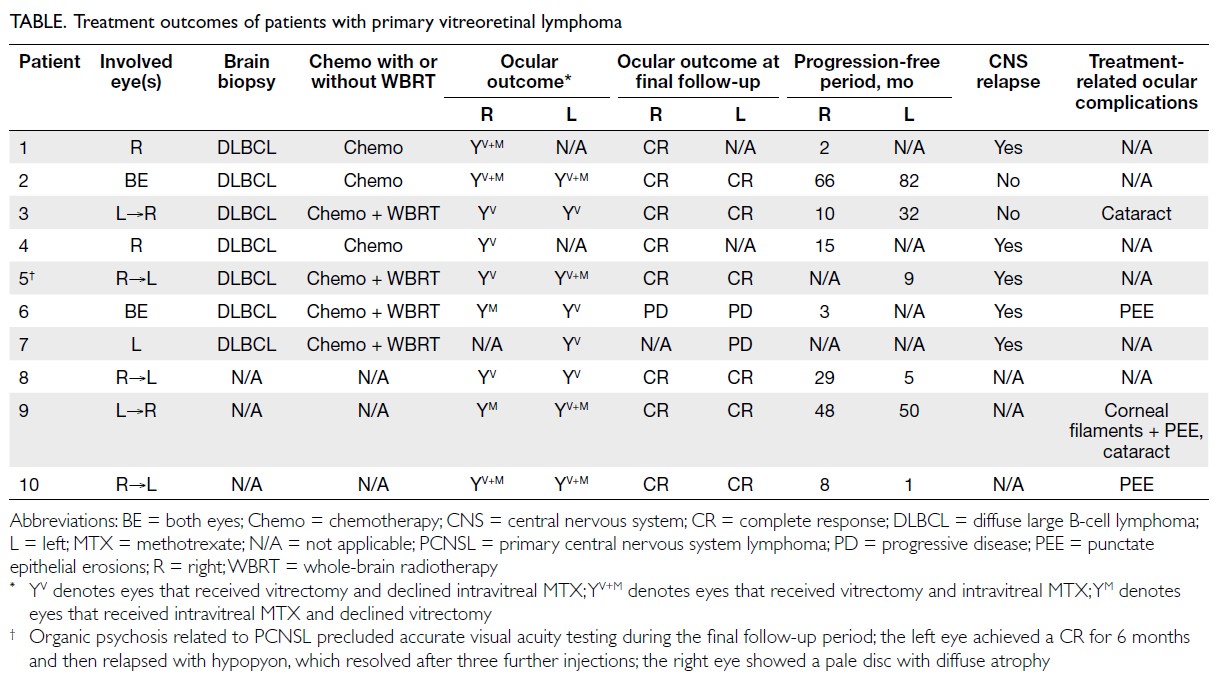
from 10 Chinese patients with PVRL were identified,
with a median follow-up of 32.5 months (range,
4-86). The majority of patients were women (70%)
and the median age at diagnosis was 59 years (range, 52-80; mean, 61.7). Three patients had isolated
ocular involvement, and seven had concurrent CNS
involvement. Among the latter, ocular involvement
preceded CNS disease in four patients (57.1%);
CNS involvement preceded ocular disease in three
patients (42.9%). The median interval between ocular
and CNS involvement was 13 months (range, 4-39).
Seven patients had bilateral PVRL, and all affected
eyes were symptomatic. Blurred vision was the most
common presenting complaint (90%), followed
by floaters (30%). The mean VA at presentation
was 20/100. The most common ophthalmological
finding was vitreous opacity, present in all eyes
(100%), followed by subretinal infiltrate in eight
eyes (47.1%) and secondary neovascular glaucoma
with vitreous haemorrhage in one eye (5.9%) [online
supplementary Table 1].
Diagnostic challenges of primary
vitreoretinal lymphoma
Primary vitreoretinal lymphoma presents ongoing
diagnostic challenges. Its rarity and tendency
to masquerade as other ocular conditions can
delay diagnosis for up to 21 months.3 11 Accurate
cytopathological diagnosis is further hampered by
the intrinsically low volume of vitreous, fragility of
lymphoma cells, and hypocellularity.3 In our series,
all 10 patients (14 of 17 eyes) underwent diagnostic
and therapeutic vitrectomy (online supplementary
Table 2). Among the seven patients with suspicious
brain lesions on magnetic resonance imaging, brain
biopsy confirmed DLBCL and the diagnosis of PVRL
was supported by characteristic vitreous opacity
and/or subretinal infiltrate. For the remaining three
patients without CNS involvement, diagnosis relied
on positive vitreous biopsy findings: (1) cytology
demonstrating atypical lymphoid cells; (2) flow
cytometry identifying CD20+ B lymphocytes;
and (3) polymerase chain reaction revealing
monoclonal immunoglobulin heavy locus (IGH)
gene rearrangement. Two patients fulfilled these
criteria; Patient 10 was diagnosed solely based on
clinical evaluation by vitreoretinal specialists (online
supplementary Fig c to f). Only seven specimens
(50%) yielded positive cytological results with
malignant cells and/or atypical lymphoid cells (online
supplementary Table 2). Negative or equivocal
results do not definitively exclude lymphoma,9 thus
adjunctive cytopathological tests are often required.
These include, in decreasing order of sensitivity (as
ranked by a recent systematic review)3: interleukin
(IL) analysis (IL-10–to–IL-6 ratio >1; 89.4%), flow
cytometry identifying CD20+ B lymphocytes (88.0%),
monoclonal IGH rearrangement via polymerase
chain reaction (85.1%), and myeloid differentiation
primary response 88 (MYD88) mutation analysis
(70%). In our study, flow cytometry was performed
in six eyes (42.8%), with only two (14.2%) showing clonal populations. Polymerase chain reaction
was conducted in two eyes (14.3%); one (7.1%)
demonstrated IGH gene rearrangement. Interleukin
analysis was not performed. Flow cytometry and
gene rearrangement testing are available in the Hong
Kong public healthcare setting; other tests may incur
additional charges.12 A large Chinese case-control
study proposed a six-item diagnostic framework for
DLBCL-associated PVRL9 (online supplementary
Table 3). They reported that 15% of patients were
diagnosed when only criteria 1 to 3 were met.
Requiring criterion 1 plus two positive results from
criteria 4 to 6 increased diagnostic sensitivity to
97.5%, with 100% specificity.9
Several factors may have contributed to
the low diagnostic yield of vitreous biopsy in our
series. First, all patients received corticosteroids
to control ocular inflammation, given that PVRL
frequently masquerades as uveitis. This may
have induced cytolytic effects on lymphoma cells
prior to diagnostic vitrectomy.13 Second, vitreous
biopsy was not repeated in cases with equivocal or
negative cytological results, owing to the absence
of clinically significant vitreous opacities to justify
repeat sampling.3 Third, additional cytopathological
tests require prior arrangement and coordination
with on-duty cytopathologists. Sensitive assays,
such as IL analysis, could have been performed if
preliminary communication had occurred before
the vitreous biopsy. Finally, despite standardisation
of sampling techniques and procedures, sample
hypocellularity limited the yield of clonal lymphoma
cells on flow cytometry (2 of 6 eyes), where definitive
diagnosis requires a substantial number of viable,
intact neoplastic cells.3 To overcome the challenge of
hypocellular vitreoretinal lymphoma tissue samples,
the use of cell-free DNA, rather than cellular DNA, to
detect MYD88 mutations has been proposed. Notably,
detection rates were reportedly 30% higher when
cell-free DNA was used,14 even in aqueous humour
samples, which contain minimal cellular DNA. A
recent report has further validated this technique in
highly diluted (>100-fold) vitreous samples.15
Efficacy and safety of intravitreal
methotrexate in primary vitreoretinal
lymphoma
Before the introduction of intravitreal chemotherapy,
external beam radiation therapy was the primary
treatment for PVRL. Due to its severe adverse
effects, radiation therapy is now generally reserved
for patients with bilateral involvement, advanced
age, or difficulty attending frequent intravitreal
injections.1 Intravitreal MTX is currently considered
the first-line treatment owing to its high efficacy.1
To date, no standardised treatment regimen
for intravitreal MTX in PVRL has been established—the number of injections required to achieve a complete response varies widely.1 While Smith et al16
proposed a protocol of 25 injections, a 10-year
experience reported by Frenkel et al17 indicated
that only 39% of patients were able to complete
the treatment due to frailty or death. Notably, a
median of five injections (range, 2-11) was sufficient
to achieve a complete response. The same group
later reported a complete response rate of 97%
over a mean (± standard deviation) follow-up of
38 months with as few as five (± four) injections,4
which subsequently prompted proposals advocating
for fewer injections.6 16 In our study, intravitreal
MTX was administered at a dose of 400 μg/0.05
mL weekly. The number of injections was titrated
based on clinical response, defined as achievement
of complete response, and patient acceptance and
tolerance. Ultimately, nine eyes (52.9%) received
MTX injections, while eight eyes (47.1%) underwent
vitrectomy alone (Table). Methotrexate was not
administered in cases where patients achieved a
complete response after vitrectomy and declined
invasive treatment, experienced intolerance (Patient
6 developed keratopathy in the fellow treated eye), or
refused treatment for personal reasons (Patient 7).
Among MTX-treated eyes, a complete response
was achieved in seven eyes (77.8%), of which six had
stable vision and one experienced visual improvement
after a mean (± standard deviation) of five (± three)
injections (online supplementary Table 4). This
outcome is comparable to a series from the United
States (n=10), which reported a complete response rate of 80% and visual stability or improvement in
50% of cases following a mean of six MTX injections
(range, 1-10).16 Of the remaining two MTX-treated
eyes, one showed progressive disease and one
experienced ocular relapse. In Patient 6, the right eye
initially improved after two injections, but further
treatment was declined, resulting in worsening
vitritis 3 months later. In Patient 5, the left eye
initially presented with neovascular glaucoma. After
14 injections, a complete response was maintained
for 6 months, followed by relapse 9 months later
with anterior chamber infiltrate. Further injections
were challenging due to PCNSL-related organic
psychosis after three additional doses. Despite a
similar number of injections, the ocular relapse rate
in our study (11.1%) was lower than that reported in
a series from the United States (40%) involving seven
patients with an average of six MTX injections,16 and
was comparable to the largest Chinese cohort, where
patients received an average of five injections (10%).6
Keratopathy, the most common adverse effect of
intravitreal MTX,17 18 was mild in our series and
resolved with preservative-free lubricants, bandage
contact lenses, and oral folic acid. The incidence in
our cohort (33%) was lower than the 100% reported in
other studies.4 18 This difference may be attributable
to the lower number of MTX injections, as well as
our practices of compressing the injection site with
a cotton-tipped applicator, performing thorough
saline rinses to minimise corneal exposure, and pre-emptively
prescribing preservative-free lubricants.
Therapeutic role of vitrectomy alone in
primary vitreoretinal lymphoma
Although vitrectomy is pivotal for the diagnosis of
PVRL, its therapeutic role remains controversial.
The largest Chinese study (n=61) demonstrated
complete clearance of malignant cells in 19.7% of
cases after vitrectomy alone,6 whereas the largest
study in a Western population (n=150)19 found
no difference in outcomes between vitrectomised
and non-vitrectomised eyes. In our study, eight
eyes underwent vitrectomy alone without MTX.
A complete response was observed in six of eight
eyes (75%). One patient with isolated ocular PVRL
(Patient 8) achieved a complete response and visual
improvement (from 20/600 in the right eye and 20/70
in the left eye to 20/30 bilaterally) with vitrectomy
alone; the patient did not receive intravitreal MTX
(due to patient reluctance) or systemic chemotherapy
with or without radiation therapy. This response was
maintained at the latest follow-up, 29 months and 5
months after vitrectomy in the right and left eyes,
respectively.
It is plausible that vitrectomy removed
the vitreous scaffold necessary for lymphocyte
proliferation and concurrently reduced the tumour
burden.19 This process may have enabled effective
tumour control by the host immune system, a
mechanism described in rare reports of spontaneous
regression of PVRL.20 Nevertheless, regular
monitoring is recommended, and treatment should
be initiated if any new chorioretinal infiltrates or
vitreous opacities are detected. Given the favourable
visual improvement observed in patients treated with
vitrectomy alone (62.5%), therapeutic vitrectomy
may be considered in those who are intolerant of, or
unwilling to, undergo weekly MTX injections.
Conclusion and future directions
This study represents the first and largest series
to date describing the diagnosis and treatment
outcomes of PVRL in Hong Kong. To address the
low positivity rate of cytological testing, there is
a need for heightened clinical suspicion, greater
awareness of sensitive adjunctive tests, and
enhanced communication among ophthalmologists,
oncologists, and cytopathologists to improve
diagnostic accuracy in suspected PVRL cases or when
initial results are equivocal. Despite the retrospective
design and limited sample size, attributable to the
rarity of PVRL, our findings align with emerging
evidence suggesting that fewer intravitreal MTX
injections or therapeutic vitrectomy alone, followed
by observation, can be effective, particularly in
patients who are frail or intolerant of intensive
injection regimens. Future research should prioritise
prospective randomised studies to identify optimal
treatment strategies that preserve vision and quality of life in patients with PVRL.
Author contributions
Concept or design: ACY Mak.
Acquisition of data: ASH Chee.
Analysis or interpretation of data: ASH Chee.
Drafting of the manuscript: ASH Chee.
Critical revision of the manuscript for important intellectual content: ACY Mak, KW Kam, MSC Li, M Ho, ME Brelen, LJ Chen, WWK Yip, AL Young.
Acquisition of data: ASH Chee.
Analysis or interpretation of data: ASH Chee.
Drafting of the manuscript: ASH Chee.
Critical revision of the manuscript for important intellectual content: ACY Mak, KW Kam, MSC Li, M Ho, ME Brelen, LJ Chen, WWK Yip, AL Young.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflict of interest.
Funding/support
This study received no specific grant from any funding agency
in public, commercial, or not-for-profit sectors.
Supplementary material
The supplementary material was provided by the authors and
some information may not have been peer reviewed. Accepted
supplementary material will be published as submitted by the
authors, without any editing or formatting. Any opinions
or recommendations discussed are solely those of the
author(s) and are not endorsed by the Hong Kong Academy
of Medicine and the Hong Kong Medical Association.
The Hong Kong Academy of Medicine and the Hong Kong
Medical Association disclaim all liability and responsibility
arising from any reliance placed on the content.
References
1. Raval V, Binkley E, Aronow ME, Valenzuela J, Peereboom DM,
Singh AD. Primary central nervous system lymphoma–ocular variant: an interdisciplinary review on management.
Surv Ophthalmol 2021;66:1009-20. Crossref
2. Riemens A, Bromberg J, Touitou V, et al. Treatment
strategies in primary vitreoretinal lymphoma: a 17-center European collaborative study. JAMA Ophthalmol
2015;133:191-7. Crossref
3. Huang RS, Mihalache A, Popovic MM, et al. Diagnostic
methods for primary vitreoretinal lymphoma: a systematic
review. Surv Ophthalmol 2024;69:456-64. Crossref
4. Habot-Wilner Z, Frenkel S, Pe’er J. Efficacy and safety of
intravitreal methotrexate for vitreo-retinal lymphoma—20
years of experience. Br J Haematol 2021;194:92-100. Crossref
5. Mohammad M, Andrews RM, Plowman PN, et al.
Outcomes of intravitreal methotrexate to salvage eyes with
relapsed primary intraocular lymphoma. Br J Ophthalmol
2022;106:135-40. Crossref
6. Zhou N, Xu X, Liu Y, Wang Y, Wei W. A proposed protocol
of intravitreal injection of methotrexate for treatment of
primary vitreoretinal lymphoma. Eye (Lond) 2022;36:1448-55. Crossref
7. Hospital Authority, Hong Kong. Hong Kong Cancer
Registry, 2021. Available from: https://www3.ha.org.hk/cancereg/pdf/overview/Overview%20of%20HK%20Cancer%20Stat%202021.pdf . Accessed 15 Feb 2025.
8. Lee SF, Evens AM, Ng AK, Luque-Fernandez MA.
Socioeconomic inequalities in treatment and relative
survival among patients with diffuse large B-cell
lymphoma: a Hong Kong population-based study. Sci Rep
2021;11:17950. Crossref
9. Zhang X, Zhang Y, Guan W, et al. Development of
diagnostic recommendations for vitreoretinal lymphoma.
Ocul Immunol Inflamm 2024;32:1142-9. Crossref
10. Abrey LE, Batchelor TT, Ferreri AJ, et al. Report of an
international workshop to standardize baseline evaluation
and response criteria for primary CNS lymphoma. J Clin
Oncol 2005;23:5034-43. Crossref
11. Whitcup SM, de Smet MD, Rubin BI, et al. Intraocular
lymphoma. Clinical and histopathologic diagnosis.
Ophthalmology 1993;100:1399-406. Crossref
12. Hospital Authority, Hong Kong. List of Private Services,
2024. Available from: https://www3.ha.org.hk/fnc/pathology.aspx?lang=ENG. Accessed 15 Feb 2025.
13. Peterson K, Gordon KB, Heinemann MH, DeAngelis LM.
The clinical spectrum of ocular lymphoma. Cancer
1993;72:843-9. Crossref
14. Hiemcke-Jiwa LS, Ten Dam-van Loon NH, Leguit RJ,
et al. Potential diagnosis of vitreoretinal lymphoma by detection of MYD88 mutation in aqueous humor with
ultrasensitive droplet digital polymerase chain reaction.
JAMA Ophthalmol 2018;136:1098-104. Crossref
15. Demirci H, Rao RC, Elner VM, et al. Aqueous humor–derived MYD88 L265P mutation analysis in vitreoretinal
lymphoma: a potential less invasive method for diagnosis
and treatment response assessment. Ophthalmol Retina
2023;7:189-95. Crossref
16. Smith JR, Rosenbaum JT, Wilson DJ, et al. Role of
intravitreal methotrexate in the management of primary
central nervous system lymphoma with ocular involvement.
Ophthalmology 2002;109:1709-16. Crossref
17. Frenkel S, Hendler K, Siegal T, Shalom E, Pe’er J. Intravitreal
methotrexate for treating vitreoretinal lymphoma: 10 years
of experience. Br J Ophthalmol 2008;92:383-8. Crossref
18. Anthony CL, Bavinger JC, Shantha JG, et al. Clinical
outcomes following intravitreal methotrexate for primary
vitreoretinal lymphoma. Int J Retina Vitreous 2021;7:72. Crossref
19. Liberman P, Francis JH, Mehrotra K, et al. Clinical
outcomes in vitrectomized versus non-vitrectomized eyes
in patients with primary vitreoretinal lymphoma. Ocul
Immunol Inflamm 2023;31:496-500. Crossref
20. Kase S, Namba K, Jin XH, Kubota KC, Ishida S. Spontaneous
regression of intraocular lymphoma. Ophthalmology
2012;119:1083-4. Crossref