Hong Kong Med J 2026;32:Epub 2 Feb 2026
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Webbed left atrial septal pouch mimicking septal
abnormality on imaging: a case report
Guoliang Yang, MD1; Shilin Xiao, MD1; Jun Yang, MD1; Ningshan Li, MD, PhD1; Yuan Zou, MD2;
Zheng Liu, MD, PhD1; Yunhua Gao, MD1; Peng He, MD, PhD1,2
1 Department of Ultrasound, Xinqiao Hospital Army Medical University, Chongqing, China
2 Department of Ultrasound Medicine and Ultrasonic Medical Engineering Key Laboratory of Nanchong City, Affiliated Hospital of North
Sichuan Medical College, Nanchong, China
Corresponding author: Dr Peng He (hope18@vip.163.com)
 Three video clips showing the webbed left atrial septal pouch and contrast flow are available at www.hkmj.org
Three video clips showing the webbed left atrial septal pouch and contrast flow are available at www.hkmj.orgCase presentation
A 65-year-old male presented to Xinqiao Hospital
Army Medical University on 29 November 2023 with
a 6-month history of frequent palpitations, fatigue,
and reduced exercise tolerance. His heart rate was
114 bpm and blood pressure was 138/87 mm Hg.
Auscultation revealed irregular heart sounds and
laboratory tests showed an elevated brain natriuretic
peptide level (1411.25 pg/mL). An electrocardiogram
revealed atrial fibrillation with intraventricular
differential conduction (Fig 1a). Transthoracic
echocardiography demonstrated enlargement of
the left atrium and left ventricle, along with mild
mitral and tricuspid valve regurgitation, and slight
thickening of the atrial septum (Fig 1b). The patient
was diagnosed with heart failure with persistent
atrial fibrillation and was initially scheduled for
radiofrequency catheter ablation.
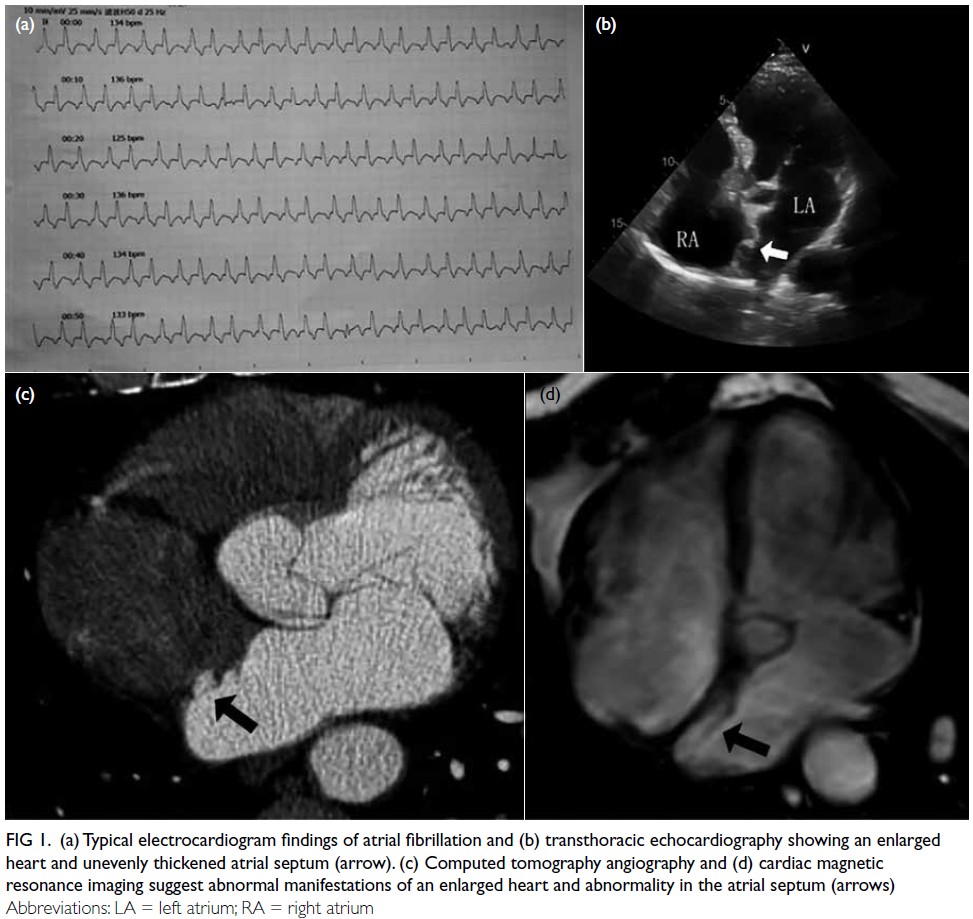
Figure 1. (a) Typical electrocardiogram findings of atrial fibrillation and (b) transthoracic echocardiography showing an enlarged heart and unevenly thickened atrial septum (arrow). (c) Computed tomography angiography and (d) cardiac magnetic resonance imaging suggest abnormal manifestations of an enlarged heart and abnormality in the atrial septum (arrows)
Preoperative transoesophageal echocardiography
(TEE) revealed a sandwiched foramen ovale under
calm breathing conditions, with the depth of the
left atrial surface approximately 7.6 mm and the
height of the open end around 0.13 mm, while the
right atrial surface remained well closed. During
the Valsalva manoeuvre, the opening end of the left
atrial surface widened while the right atrial surface
remained closed, with no evident shunt observed.
Additionally, an irregularly shaped webbed structure
with compartmented echoes was identified in the
middle of the primary septum (Fig 2a and b, Video 1).
These findings were confirmed by three-dimensional
TEE (3D-TEE), which measured the pouch to be
approximately 19.2 × 10.5 × 24.5 mm3, oriented
towards the mitral ring. The pouch exhibited a
cobweb-like appearance, with its largest opening
directed towards the roof, measuring approximately
20.7 × 10.5 mm2. Within the pouch, a small
polycystic division was observed. Colour Doppler
imaging showed low-velocity blood flow within the
septal valve but no flow across the atrial septum
into the right atrium (Fig 2c, Video 2). Right heart
contrast echocardiography confirmed no contrast
images in the left atrium for 30 consecutive cardiac cycles following calm breathing and the Valsalva manoeuvre (Fig 2d, Video 3). Additional diagnostic evaluations with computed tomography angiography (Fig 1c) and cardiac magnetic resonance imaging (CMR) [Fig 1d] revealed a band-like abnormality on the left atrial side of the septum without any abnormal shunt. Following a multidisciplinary team discussion, the patient was diagnosed with a variant atrial septal pouch (ASP). After consulting with the patient, the medical team opted to alter the treatment strategy, discontinuing radiofrequency catheter ablation for atrial fibrillation in favour of conservative management. The patient has been followed up for over 1 year and remains in a stable condition with conservative treatment.
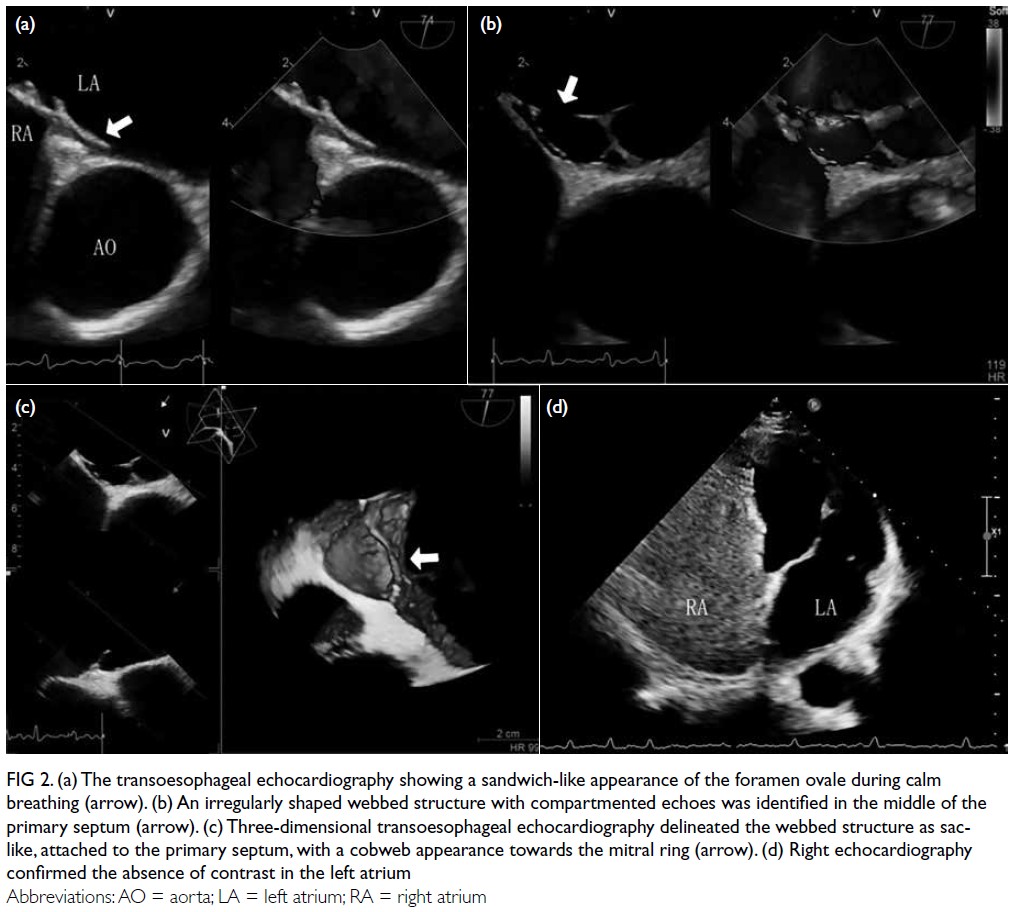
Figure 2. (a) The transoesophageal echocardiography showing a sandwich-like appearance of the foramen ovale during calm breathing (arrow). (b) An irregularly shaped webbed structure with compartmented echoes was identified in the middle of the primary septum (arrow). (c) Three-dimensional transoesophageal echocardiography delineated the webbed structure as sac-like, attached to the primary septum, with a cobweb appearance towards the mitral ring (arrow). (d) Right echocardiography confirmed the absence of contrast in the left atrium
Discussion
An atrial septal anatomical variant known as the
ASP was first described by Krishnan and Salazar
in 2010.1 It is a pouch-like structure resulting from
the incomplete fusion of the primary and secondary
septa, with openings into the left, right, or both
atria. This patient presented with a left ASP with
an accompanying web-like structure that did not
involve the secondary septum, as evidenced by the
absence of microbubbles during right-heart contrast
echocardiography. We hypothesise that this web-like
formation may represent a developmental
variation of the primary septum, independent of the
fusion between the primary and secondary septa.
Nonetheless, this remains speculative due to limited
research on web-like ASP, and we propose referring
to it as a ‘webbed left ASP’.
Left ASP is recognised as a potential risk
factor for cardioembolic stroke and blue toe
syndrome.2 The pouch’s structure can promote
blood stasis, facilitating in situ microthrombus
formation and increasing the risk of embolic events.3
In this case, the additional presence of a web-like
septal structure may further exacerbate the risk of
thrombus formation. A polycystic, web-like septum
over the primary septum with multiple floating
ends was revealed on the 3D-TEE. The rupture of these delicate reticular structures could potentially
result in a stroke. Furthermore, slow blood flow
within the septation contributes to a haemodynamic
environment prone to thrombus formation.
The atrial septum may receive high-velocity
blood flow from the right pulmonary vein, with the
contraction of transverse muscular fibres aiding
in clearing the blood and reducing thrombus risk.
Nonetheless, in conditions such as atrial fibrillation,
heart failure, or mitral stenosis, this protective
mechanism may fail.4 In the present case, atrial
fibrillation impaired this mechanism, increasing the
risk of thrombus formation. Given the high embolic
risk associated with this complex anatomy, the
patient opted for conservative treatment to avoid
complications related to atrial septal puncture.
Multi-slice spiral computed tomography, CMR,
and TEE are primary diagnostic tools for assessing
atrial septal structural variations and thrombi.5
Although multi-slice computed tomography offers high-resolution imaging, it is less effective
in patients with irregular heart rhythms, such as
atrial fibrillation, due to the potential for image
distortion. Detailed 3D morphology can be obtained
using CMR, although it has limited resolution
for thin structures such as the atrial septum.6
Transoesophageal echocardiography, particularly
3D-TEE, offers superior spatial resolution and is less
affected by cardiac rhythm disturbances.7 It enables
real-time visualisation of septal anatomy, variations,
and haemodynamic flow, making it the ideal tool for
diagnosing complex structures such as the webbed
left ASP.
The web-like left ASP must be differentiated
from bronchogenic atrial septal cysts and
hydatid cysts.8 Bronchogenic cysts are benign
congenital cystic masses, most commonly located
in the mediastinum and lungs, with atrial septal
involvement being extremely rare. Ultrasound
typically reveals a thin-walled, well-defined anechoic or hypoechoic area, sometimes with internal
septations and posterior acoustic enhancement,
and no Doppler blood flow signals—features that
differ significantly from this case. Hydatid cysts are
caused by parasitic infections, usually associated
with a history of contact with endemic areas. Their
sonographic features and medical history make
them relatively easy to identify.
This case highlights a novel variant of the
left ASP with a web-like structure. Nonetheless,
our understanding of ASP remains limited, and
further research and observation are needed. Urgent
questions remain regarding risk stratification in
ASP, identification of high-risk cases, the need for
interventional occlusion or surgical resection, and
the optimal treatment approach post-thrombosis.
We hope this case enhances understanding of this
anatomical variation and informs future research.
Author contributions
Concept or design: G Yang, P He.
Acquisition of data: G Yang, J Yang.
Analysis or interpretation of data: Y Zou, S Xiao, J Yang, N Li.
Drafting of the manuscript: G Yang, P He, Y Zou.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: G Yang, J Yang.
Analysis or interpretation of data: Y Zou, S Xiao, J Yang, N Li.
Drafting of the manuscript: G Yang, P He, Y Zou.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This study was funded by Sichuan Science and Technology
Program (Ref Nos.:2025ZNSFSC1751, 2026YFHZ0039), and
North Sichuan Medical College Affiliated Hospital Hospital-level
Projects, China (Ref Nos.: 2025LC010, 210930). The funders
had no role in the study design, data collection/analysis/interpretation, or manuscript preparation.
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki. The patient provided written consent for all treatments, procedures, and consent for publication, including
the publication of the accompanying clinical images.
References
1. Krishnan SC, Salazar M. Septal pouch in the left atrium:
a new anatomical entity with potential for embolic
complications. JACC Cardiovasc Interv 2010;3:98-104. Crossref
2. Strachinaru M, Castro-Rodriguez J, Verbeet T, Gazagnes MD.
The left atrial septal pouch as a risk factor for stroke: a
systematic review. Arch Cardiovasc Dis 2017;110:250-8. Crossref
3. Hołda MK, Krawczyk-Ożóg A, Koziej M, et al. Left-sided
atrial septal pouch is a risk factor for cryptogenic stroke. J
Am Soc Echocardiogr 2018;31:771-6. Crossref
4. Dharshan AC, Joseph J, Goel SK, Tavakoly A, Shenoy MM.
Double interatrial septum. Can J Cardiol 2010;26:e63. Crossref
5. Silvestry FE, Cohen MS, Armsby LB, et al. Guidelines for
the echocardiographic assessment of atrial septal defect
and patent foramen ovale: from the American Society of
Echocardiography and Society for Cardiac Angiography
and Interventions. J Am Soc Echocardiogr 2015;28:910-58. Crossref
6. Rochitte CE. Cardiovascular magnetic resonance
worldwide: a global commitment to cardiovascular care. J
Cardiovasc Magn Reson 2025;27:101842. Crossref
7. Gwak SY, Kim K, Lee HJ, et al. Three-dimensional agitated
saline contrast transesophageal echocardiography for the
diagnosis of patent foramen ovale. Sci Rep 2025;15:29136. Crossref
8. Gross DJ, Briski LM, Wherley EM, Nguyen DM.
Bronchogenic cysts: a narrative review. Mediastinum
2023;7:26. Crossref

