Hong Kong Med J 2026;32:Epub 30 Jan 2026
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE (HEALTHCARE IN CHINA)
Development and optimisation strategies for a
nomogram-based predictive model of malignancy
risk in thyroid nodules
Peng He, MD, PhD1 #; Yu Liang, MD2 #; Yuan Zou, MD1; Zhou Zou, BM3; Bo Ren, MD1; Shan Peng, MD4; Hongmei Yuan, MD, PhD1; Qin Chen, MD2
1 Department of Ultrasound Medicine and Ultrasonic Medical Engineering
Key Laboratory of Nanchong City, Affiliated Hospital of North Sichuan
Medical College, Nanchong, China
2 Department of Ultrasound, Sichuan Academy of Medical Sciences and
Sichuan Provincial People’s Hospital, School of Medicine, University of
Electronic Science and Technology of China, Chengdu, China
3 Department of Orthopedics, Sichuan Academy of Medical Sciences and
Sichuan Provincial People’s Hospital, School of Medicine, University of
Electronic Science and Technology of China, Chengdu, China
4 Department of Rehabilitation, Second Clinical College of North Sichuan Medical College, Nanchong, China
# Equal contribution
Corresponding author: Dr Yuan Zou (zouyuanxiao@163.com)
Abstract
Introduction: This study aimed to develop and
validate a clinical prediction model to assist
radiologists in optimising the diagnostic classification
of the Chinese Thyroid Imaging Reporting and Data
System (C-TIRADS).
Methods: A total of 1659 patients from two hospitals
were included in this study. The derivation cohort
comprised 909 patients for model development
and internal validation, while 750 patients
formed the external validation cohort. A binary
logistic regression model was constructed. Model
performance in the derivation set was evaluated
using receiver operating characteristic (ROC) curves
and visualised with a nomogram. In the external
validation set, ROC and calibration curves were
used to assess discrimination and calibration.
Results: The original C-TIRADS category, abnormal
cervical lymph node sonographic findings,
and changes in thyroid nodule size emerged as
significant predictors of C-TIRADS optimisation.
The optimised nomogram demonstrated an area
under the ROC curve (AUC) of 0.730 (95% confidence
interval=0.697-0.762), with a sensitivity of 63.2%,
specificity of 74.9%, and overall accuracy of 67.7%
for predicting optimisation. Using probability
thresholds of ≥60% to recommend an upgrade and
<30% to recommend a downgrade, the calibration
curve showed good agreement, and decision curve
analysis demonstrated a favourable net clinical
benefit. External validation confirmed excellent discrimination (AUC=0.865; 95% confidence
interval=0.839-0.891).
Conclusion: An optimised C-TIRADS model that
integrates imaging features of thyroid nodules with
clinical risk factors may aid radiologists in improving
the diagnostic efficiency and clinical utility of the
TIRADS classification.
New knowledge added by this study
- This is the first study to integrate clinical risk factors with imaging features to optimise the Chinese Thyroid Imaging Reporting and Data System (C-TIRADS) classification.
- This work established a risk threshold–based decision-making framework to guide C-TIRADS classification adjustments.
- External validation demonstrated the model’s generalisability across diverse clinical settings.
- Our model improved diagnostic precision through the integration of imaging and clinical risk factors.
- This research has the potential to optimise resource allocation and reduce interobserver diagnostic variability.
Introduction
Thyroid nodules are a common clinical finding,
with a prevalence of approximately 4% to 7% in the
general population, and are most often detected by ultrasonography.1 2 Although most thyroid
nodules are benign, distinguishing malignant
from benign nodules remains a clinical priority to
avoid unnecessary procedures and ensure timely intervention.3 To standardise risk stratification,
various Thyroid Imaging Reporting and Data Systems
(TIRADS) have been developed,4 5 including the
ACR-TIRADS (American College of Radiology),6 the
K-TIRADS (Korean Society of Thyroid Radiology),7
and the European Thyroid Association.8 Recognising
the need for a system tailored to the Chinese
healthcare context, the Chinese Artificial Intelligence
Alliance for Thyroid and Breast Ultrasound proposed
the Chinese TIRADS (C-TIRADS) in 2021.2
However, existing TIRADS models primarily focus
on sonographic characteristics and often overlook
relevant clinical risk factors (eg, patient age, sex,
and cervical lymph node [LN] involvement).9 In
clinical practice, radiologists frequently incorporate
such clinical information into their assessments,
contributing to inconsistency and variability in
TIRADS classification.
Papillary thyroid carcinoma accounts for
approximately 80% to 90% of all thyroid cancers and
is typically characterised by indolent behaviour.10 11 A
substantial proportion of new cases involve papillary
thyroid microcarcinoma, defined as tumours
measuring less than 10 mm in diameter, which
generally carry a favourable clinical prognosis.12
Increasing recognition of the indolent nature
of papillary thyroid microcarcinoma has raised
concerns regarding potential overdiagnosis and overtreatment. However, current risk stratification
strategies that rely solely on imaging features may
either overestimate or underestimate malignancy
risk, depending on the patient’s broader clinical
context. Approaches that incorporate clinical risk
factors into TIRADS classification could address
these limitations and enhance diagnostic accuracy,
supporting more individualised patient management.
This study aimed to develop and externally
validate a predictive model that integrates both
imaging characteristics and clinical risk factors to
refine the C-TIRADS classification system. To our
knowledge, this is the first nomogram-based model
to incorporate clinical risk factors into the C-TIRADS
framework. The tool is designed to assist radiologists
in improving diagnostic consistency and supporting
more informed and individualised clinical decision
making in the management of thyroid nodules.
Methods
Study design and population
This retrospective diagnostic study included patients
with thyroid nodules who underwent surgical
resection at two tertiary hospitals in China. The
derivation cohort comprised patients treated at
Sichuan Provincial People’s Hospital from January to
December 2022, while the external validation cohort
was drawn from Affiliated Hospital of North Sichuan
Medical College during the same period. Inclusion
criteria were: (1) thyroid nodules confirmed by
postoperative pathology and (2) preoperative
ultrasonography of the thyroid and cervical LNs with
complete imaging and clinical records. Exclusion
criteria were: (1) unclear pathological diagnosis;
(2) incomplete clinical data; or (3) poor-quality
ultrasound images.
Imaging evaluation and classification
Two junior radiologists, blinded to clinical and
pathological information, independently classified
all nodules according to the C-TIRADS criteria.
Subsequently, two senior radiologists re-evaluated
the cases and adjusted the classifications based on
additional clinical risk factors, including patient
demographics and cervical LN findings. Any
modification from the initial C-TIRADS classification
was defined as ‘classification optimisation’ (*C-TIRADS),
encompassing both upgrades and
downgrades.
Data collection
Structured data collection forms were used to record
clinical and sonographic variables. The collected data
included patient sex, age, nodule size, number of
nodules, C-TIRADS classification, and the presence
of abnormal cervical LNs on ultrasonography.
Predictor variables
Sonographic features that directly determine the
C-TIRADS score (such as solidity, echogenicity,
aspect ratio, microcalcification, and margin
irregularity) were not included independently in the
multivariable analysis to avoid collinearity. Based on
clinical relevance and univariate regression analysis,
six predictors were selected for model development,
namely, patient sex, age-group (≤40, 40-60, and >60
years),13 14 nodule size, number of nodules (single vs
multiple), presence of abnormal cervical LNs, and
original C-TIRADS classification.
Model development and internal validation
A binary logistic regression model was developed
using the derivation cohort from Sichuan Provincial
People’s Hospital (n=909). For categorical variables
with more than two levels, dummy variables were
created. The C-TIRADS category 5 was used as the
reference group as it represents the highest level
of suspicion and the most definitive management
pathway (surgical resection), making it an appropriate
clinical baseline to estimate relative malignancy risk
and the need for reclassification. Model performance
in the derivation cohort was evaluated using the
area under the receiver operating characteristic
(ROC) curve (AUC), and calibration was assessed by
comparing predicted probability (PP) with observed
outcomes using calibration plots.
We emphasise that the primary outcome
variable for model training was the pathological
diagnosis (binary: malignant vs benign). The C-TIRADS
optimisation, defined as upgrading or
downgrading the original category based on PP
thresholds, was a post-model clinical decision rule
applied to the model output, not the outcome used
for model development.
Internal validation was performed using
bootstrap resampling with 1000 samples to obtain
bias-corrected estimates of model performance and
95% confidence intervals (95% CIs). A fixed random
seed was set to ensure reproducibility. The bias-corrected
C-statistic was 0.728, compared with the
original apparent performance of 0.730 (a difference
of 0.002), confirming the model’s stable discriminative
ability (online supplementary Table 1).
External validation
The final model was applied to the external cohort
from Affiliated Hospital of North Sichuan Medical
College (n=750) to evaluate its generalisability.
Model discrimination was evaluated by calculating
the AUC in the validation set, and calibration was
assessed using calibration curves.
Nomogram construction
A nomogram was developed based on the final multivariable regression model to provide a visual
tool for clinical application. Each predictor was
assigned a score, and the total score corresponded
to the PP of C-TIRADS classification optimisation.
Decision curve analysis and risk thresholds
Decision curve analysis and clinical impact curves
were used to evaluate the clinical utility of the
nomogram by quantifying the net benefit across
a range of threshold probabilities. Specifically,
the nomogram generates a PP indicating whether
a nodule’s original C-TIRADS classification
should be modified after integrating clinical
information. For clinical decision making, we pre-specified
probability cut-offs: PP ≥60% (upgrade),
PP <30% (downgrade), and PP ≥30% but <60%
(unchanged). Based on these thresholds, the model’s
recommendations were translated into optimised
C-TIRADS categories, which were then compared
with radiologists’ optimisation decisions and surgical
pathology findings, as appropriate. These thresholds
are reported in the Results section and were applied
consistently across all performance tables
Model performance evaluation
To ensure consistent ROC analysis, all AUCs were calculated using continuous PPs rather than ordinal
risk categories. For the original C-TIRADS system,
the five-level ordinal classification was transformed
into a continuous malignancy probability score using
proportional-odds (ordinal logistic) regression. This
standard statistical method was employed to model
the ordered nature of the C-TIRADS categories and
to derive a continuous probability of malignancy
for each category, enabling fair comparison in ROC
analysis against other models. For the optimised
*C-TIRADS system, PPs were directly obtained
from the final multivariable logistic regression
model. The ROC curves and corresponding
AUCs were constructed using these continuous
predictions.
Statistical analysis
Statistical analyses and data visualisation were
performed using SPSS (Windows version 26.0; IBM
Corp, Armonk [NY], United States) and RStudio
(version 2022). Categorical variables were reported
as number of cases or percentages, with group
comparisons conducted using Chi squared test or
Fisher’s exact test, as appropriate. Multivariable
logistic regression analysis was conducted to identify
independent predictors. Model discrimination
was evaluated using ROC curves, while calibration
curves were used to assess model accuracy. Clinical
decision and impact curves were established to
assess practical clinical utility. A two-tailed P value
of <0.05 was considered statistically significant.
Results
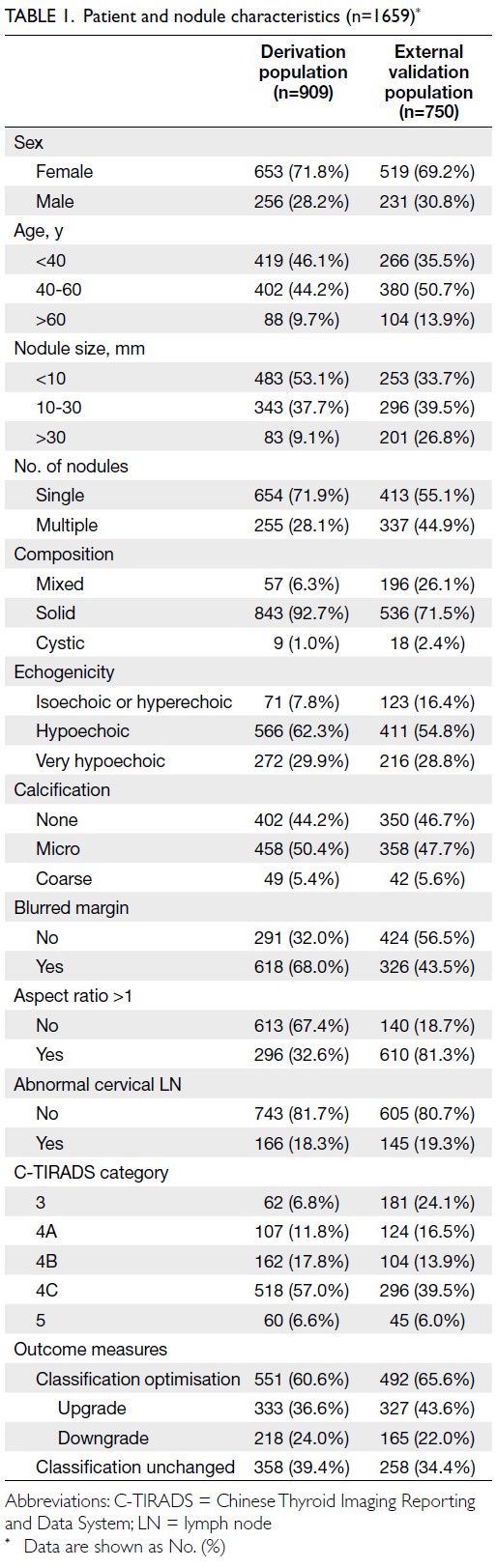
Baseline characteristics
All models were trained to predict pathological
malignancy. The optimised *C-TIRADS
classifications presented here were derived by
applying predefined probability thresholds to the
model’s malignancy predictions.
A total of 1659 patients with thyroid nodules
were included in the study, comprising 909 patients
in the derivation cohort and 750 in the external
validation cohort. In the derivation cohort, 71.8%
of patients were women, and the majority (90.8%)
had nodules measuring ≤30 mm. Approximately
81.7% of patients showed no abnormal cervical
LNs on ultrasonography. The rate of C-TIRADS
optimisation was 60.6%. In the external validation
cohort, similar distributions were observed, with a
higher proportion of nodules >30 mm (Table 1).
Univariate analysis
Univariate binary regression analysis revealed that
several variables were either significantly associated
(P<0.05) or showed a trend towards association
(0.05 < P < 0.1) with C-TIRADS optimisation. These
variables included patient sex, age, nodule size
(10-30 mm), number of nodules, solid composition,
blurred margins, aspect ratio >1, abnormal cervical
LNs, and C-TIRADS category (Table 2 and online supplementary Table 2).
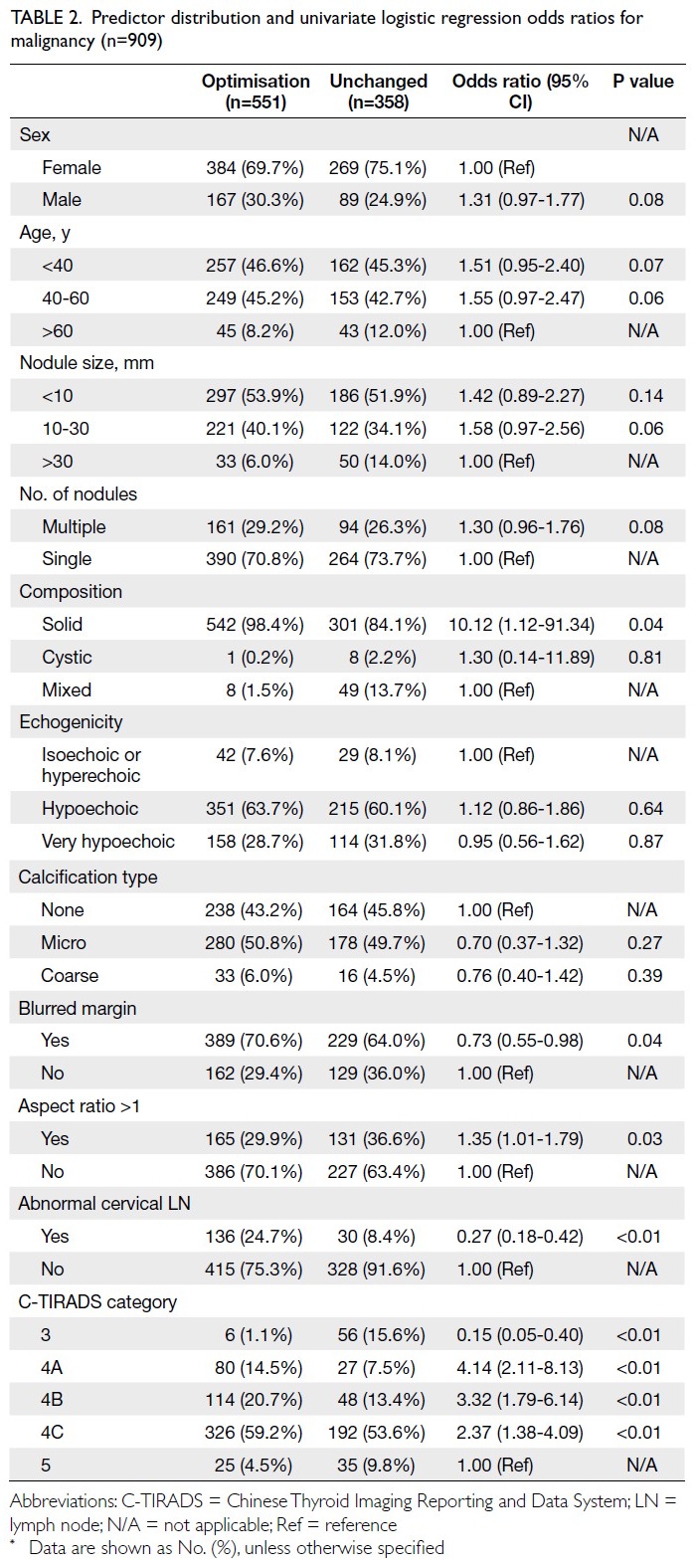
Table 2. Predictor distribution and univariate logistic regression odds ratios for malignancy (n=909)
Multivariable model development
A multivariable binary logistic regression model
was developed to identify independent predictors
associated with C-TIRADS optimisation. Six
predictors were independently associated with
the outcome. The key predictors of C-TIRADS
optimisation were male sex, age 40 to 60 years,
thyroid nodule size (per 1-mm increase), multiple
thyroid nodules, presence of abnormal cervical
LNs, and original C-TIRADS 4A category (online supplementary Table 3). A nomogram model
was constructed based on these six independent
predictors (Fig 1).
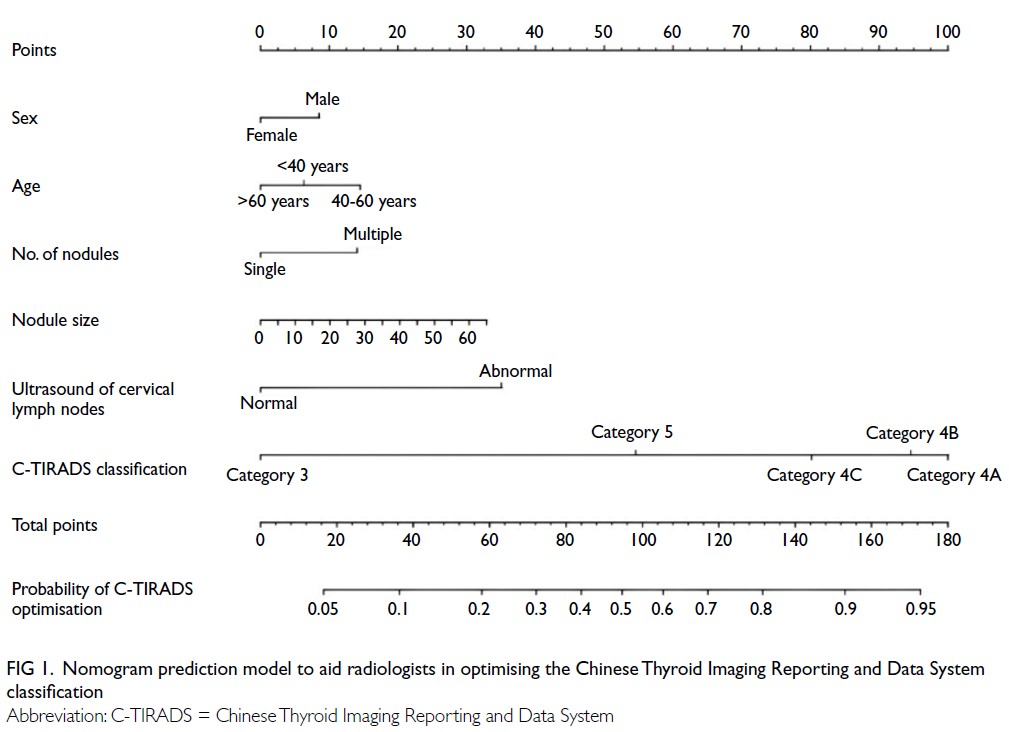
Figure 1. Nomogram prediction model to aid radiologists in optimising the Chinese Thyroid Imaging Reporting and Data System classification
Model performance in the derivation cohort
The model demonstrated good discrimination,
with an AUC of 0.730 (95% CI=0.697-0.762) in
the derivation cohort (online supplementary Fig a). Internal validation using 1000 bootstrap
samples yielded a bias-corrected C-statistic of
0.728, indicating stable model performance (online supplementary Table 1). Calibration curves showed
good agreement between PPs and observed
outcomes (online supplementary Fig b).
Diagnostic thresholds were evaluated to
stratify risk. A PP of ≥60% or <30% was considered indicative of a high likelihood of classification
change: a PP of ≥60% suggested upgrading, while a
PP of <30% suggested downgrading; PPs between
30% and 60% indicated that the classification was
likely to remain unchanged. A detailed summary
of sensitivity, specificity, and overall accuracy
across these thresholds is presented in online supplementary Table 4.
External validation
When applied to the external cohort, the model
achieved an AUC of 0.865 (95% CI=0.839-0.891)
[online supplementary Fig c], demonstrating
excellent generalisability. Calibration plots again
confirmed close agreement between predicted and
observed probabilities (online supplementary Fig d). At the 60% probability threshold, sensitivity was
85.0%, specificity was 69.0%, and overall accuracy
was 79.7% in the external validation cohort.
Diagnostic performance metrics across various
risk thresholds of the final prediction model were
analysed in the external validation population
(online supplementary Table 5).
Clinical utility
Decision curve analysis (Fig 2a) demonstrated that
the nomogram model provided greater net clinical
benefit across a wide range of threshold probabilities
compared with treating all or no patients. The
clinical impact curve (Fig 2b) showed that the
number of true positives closely approximated the
predicted number across relevant thresholds. The
observed distribution of histopathological outcomes
was as follows: in the derivation cohort, 769 nodules
(84.6%) were confirmed malignant and 140 (15.4%)
were benign; in the validation cohort, 434 nodules
(57.9%) were malignant and 316 (42.1%) were benign.
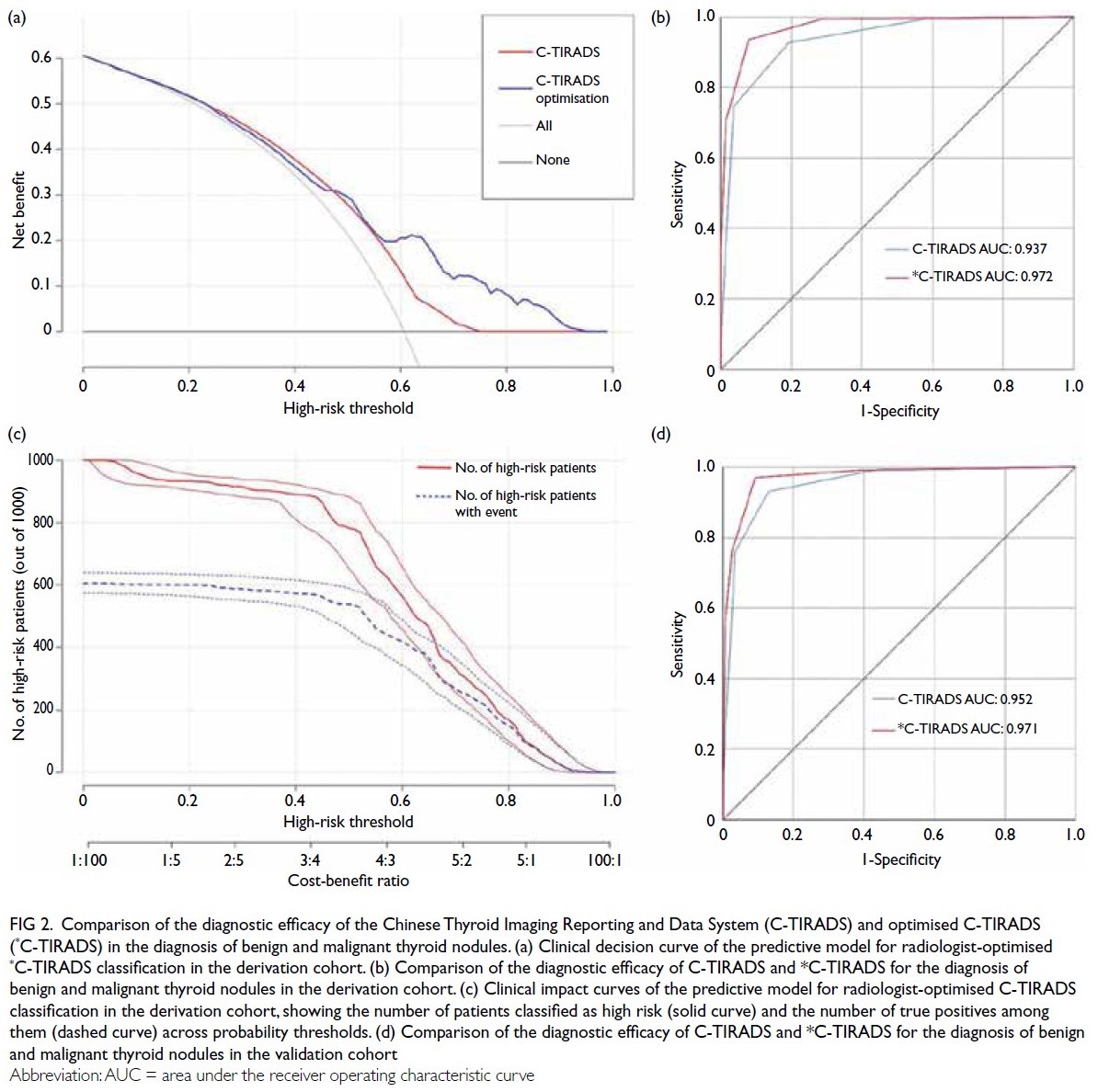
Figure 2. Comparison of the diagnostic efficacy of the Chinese Thyroid Imaging Reporting and Data System (C-TIRADS) and optimised C-TIRADS (*C-TIRADS) in the diagnosis of benign and malignant thyroid nodules. (a) Clinical decision curve of the predictive model for radiologist-optimised *C-TIRADS classification in the derivation cohort. (b) Comparison of the diagnostic efficacy of C-TIRADS and *C-TIRADS for the diagnosis of benign and malignant thyroid nodules in the derivation cohort. (c) Clinical impact curves of the predictive model for radiologist-optimised C-TIRADS classification in the derivation cohort, showing the number of patients classified as high risk (solid curve) and the number of true positives among them (dashed curve) across probability thresholds. (d) Comparison of the diagnostic efficacy of C-TIRADS and *C-TIRADS for the diagnosis of benign and malignant thyroid nodules in the validation cohort
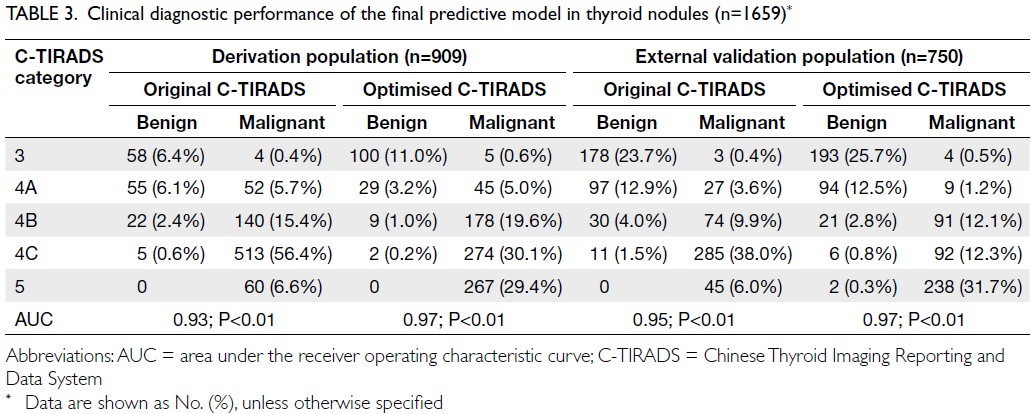
Comparison of diagnostic efficacy between
the original C-TIRADS and optimised C-TIRADS
classifications demonstrated superior performance
of the optimised model in both the derivation and
validation cohorts (Fig 2c and d, respectively).
The optimised classification achieved higher AUC
values for differentiating benign from malignant
nodules (AUC=0.97 vs 0.94 in the derivation cohort;
AUC=0.97 vs 0.95 in the external validation cohort).
The predictive model tended to improve C-TIRADS
classification by upgrading category 4A nodules
to category 4B or 4C, reflecting enhanced clinical
utility (Table 3 and Fig 2).
Application example of the nomogram model
A 55-year-old man underwent ultrasound
examination, which revealed a solid hypoechoic
thyroid nodule in the right lobe measuring
approximately 7.1 × 6.4 mm2 (Fig 3a). Simultaneously,
abnormal LNs were detected on the ipsilateral side of the neck, characterised by indistinct corticomedullary
differentiation and suspected microcalcifications
(Fig 3b). According to the conventional C-TIRADS
system, the nodule was initially classified as
category 4B. However, application of the nomogram
model yielded a cumulative score of 155 points,
corresponding to a malignancy risk of >90%. Based
on this result, the TIRADS category was optimised
and upgraded to category 5 (Fig 3c). Subsequent histopathological examination confirmed the
diagnosis of papillary thyroid microcarcinoma with
cervical LN metastasis.
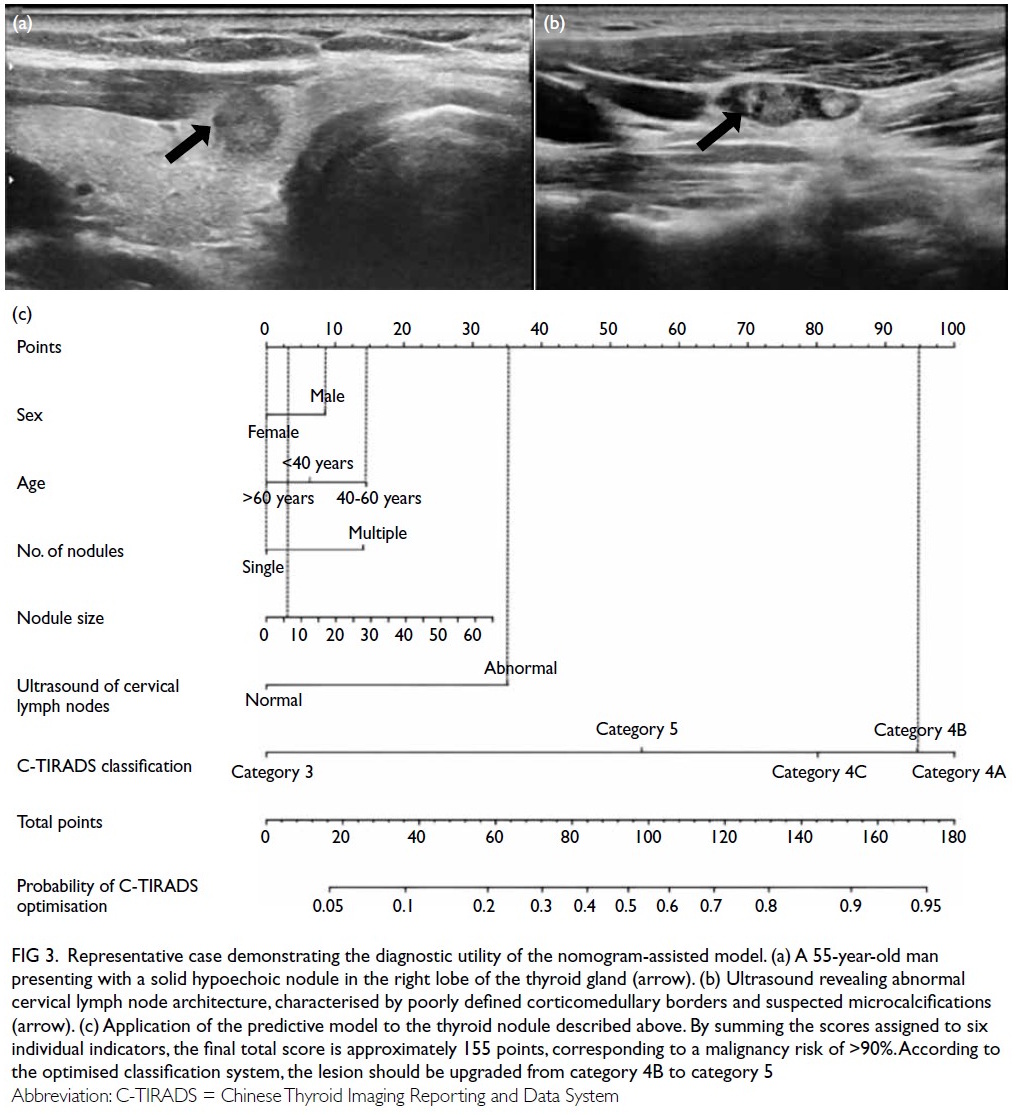
Figure 3. Representative case demonstrating the diagnostic utility of the nomogram-assisted model. (a) A 55-year-old man presenting with a solid hypoechoic nodule in the right lobe of the thyroid gland (arrow). (b) Ultrasound revealing abnormal cervical lymph node architecture, characterised by poorly defined corticomedullary borders and suspected microcalcifications (arrow). (c) Application of the predictive model to the thyroid nodule described above. By summing the scores assigned to six individual indicators, the final total score is approximately 155 points, corresponding to a malignancy risk of >90%. According to the optimised classification system, the lesion should be upgraded from category 4B to category 5
Discussion
This study retrospectively analysed the sonographic
characteristics and clinical risk factors of 1659 thyroid
nodules from two large tertiary hospitals in western China, with the aim of optimising the C-TIRADS
classification. A predictive model integrating clinical
parameters and imaging features was developed and
externally validated, demonstrating high diagnostic
performance (AUC=0.865 in external validation)
and clinical benefit, as evidenced by decision curve
analysis.
Despite the widespread adoption of various
TIRADS frameworks globally,2 4 5 6 7 8 fundamental
methodological limitations persist. Current
models, such as ACR-TIRADS,6 primarily focus on ultrasound features and rely heavily on
consensus-driven rather than statistically validated
risk stratification systems.6 15 Although TIRADS
demonstrates robust sensitivity in clinical settings, its
specificity remains relatively limited.16 Interobserver
variability is another key concern—radiologists’
subjective interpretation of ultrasound features
can result in inconsistent classification outcomes.17
To address these limitations, various strategies
have been proposed, including the integration
of artificial intelligence techniques to reduce observer subjectivity.18 19 20 Artificial intelligence has
shown promise in matching or even surpassing the
specificity achieved by radiologists; however, their
clinical implementation remains constrained by
challenges in interpretability and low acceptance in
routine practice.
Integrating clinical risk factors may enhance
risk stratification for thyroid nodules, as suggested
by a growing body of evidence.21 In alignment
with this, our study incorporated clinical variables
including patient age, sex, number of nodules, and
cervical LN status into the predictive model, thereby
more accurately reflecting routine clinical diagnostic workflows. While previous studies22 23 24 suggested
that male patients with thyroid nodules, particularly
those with indeterminate fine-needle aspiration
cytology undergoing molecular testing, exhibit a
higher malignancy risk,25 our study did not identify
a significant difference in thyroid cancer incidence
between sexes. This discrepancy may be attributable
to methodology differences, as molecular testing was
not performed in our cohort and all diagnoses were
confirmed through postoperative histopathology.
The absence of statistical significance for male sex
may reflect population-specific characteristics,
such as regional variation in risk factor distribution or age composition.26 These methodological and
demographic differences may have attenuated the
observed sex-related effect. Nonetheless, male
patients in our study were assigned higher risk
scores, suggesting an association with malignancy
risk, despite the lack of statistical significance.
Compared with previous models that
primarily focused on intrinsic ultrasound features
of thyroid nodules,27 28 29 our nomogram offers a more
comprehensive assessment. Although the individual
contributions of factors such as sex and age were
relatively modest, they reflected subtle clinical
patterns often considered by radiologists during
decision making. The C-TIRADS optimisation
approach demonstrated clear advantages,
particularly in reducing unnecessary invasive
procedures without compromising diagnostic
accuracy, achieving an AUC of 0.972. Furthermore,
the new model indicated that a risk threshold of
≥60% favoured the recommendation for C-TIRADS
optimisation, whereas a threshold of <30% favoured
exclusion. The integration of complex imaging
data with clinical information represents a core
competency for radiologists.30 With appropriate
standardised training and communication
frameworks in place, radiologists are well positioned
to leverage quantitative metrics generated by the
new model into routine diagnostic workflows. This
advancement holds promise for improving diagnostic
consistency and accuracy in clinical practice.
Limitations
This study has several limitations that should
be acknowledged. First, the optimisation of
the TIRADS classification was influenced by
radiologists’ subjective judgement, which may have
contributed to interobserver variability. Second,
although data collection was conducted by trained
junior radiologists, observer variation and the
subjective nature of ultrasound interpretation may
have affected the model’s performance.31 Third,
internal validation using bootstrap resampling may
have overestimated model performance due to
potential overfitting; therefore, external validation
was essential to confirm generalisability. Fourth,
owing to the retrospective design, only a limited
set of clinical parameters (eg, sex, age, and cervical
LN status) was included. Other relevant factors
such as body mass index, environmental exposures,
nodule location, family history of thyroid cancer, and
radiation exposure history,32 33 were not assessed.
Finally, the study cohort exclusively comprised
cases confirmed by surgical pathology, resulting
in a relatively low proportion of benign lesions,
which may have introduced selection bias. The
exclusion of patients diagnosed solely by fine-needle
aspiration was intentional but may have affected the
generalisability of the findings.
Future directions
To address the limitations of the present study, future
research should aim to standardise the application
of TIRADS by adopting unified classification
frameworks and implementing regular training
programmes to enhance interobserver consistency.
Prospective multicentre studies involving broader
and more diverse populations are warranted,
incorporating a wider range of clinical risk factors
to improve predictive accuracy. In particular,
data regarding family history, radiation exposure,
and other relevant variables across centres would
support more comprehensive risk assessment
and enhance the generalisability of prediction
models. In addition, including patients with fine-needle
aspiration–confirmed benign nodules may
help achieve a more balanced representation of
benign and malignant cases. The development and
application of nomogram-based structured training
programmes for radiologists could also be explored
to further improve diagnostic consistency and
clinical utility. While the widespread adoption of a
revised classification system will require time, we
hope that the findings of this study may contribute
to that transition.
Conclusion
We developed and externally validated a nomogram-based
predictive model that integrates imaging
features and clinical risk factors to optimise
C-TIRADS classification for thyroid nodules. The
model demonstrated good discrimination and
calibration across internal and external cohorts,
offering a practical tool to assist radiologists in
refining diagnostic assessments and improving
clinical decision making. Future research
incorporating additional clinical variables and
prospective validation is warranted to further
strengthen the model’s applicability across diverse
clinical settings.
Author contributions
Concept or design: Y Liang, Y Zou, P He, Q Chen.
Acquisition of data: Y Liang, Y Zou, Z Zou, B Ren.
Analysis or interpretation of data: Y Liang, S Peng, Y Zou.
Drafting of the manuscript: Y Liang, Y Zou, HM Yuan, Z Zou.
Critical revision of the manuscript for important intellectual content: P He, Y Zou.
Acquisition of data: Y Liang, Y Zou, Z Zou, B Ren.
Analysis or interpretation of data: Y Liang, S Peng, Y Zou.
Drafting of the manuscript: Y Liang, Y Zou, HM Yuan, Z Zou.
Critical revision of the manuscript for important intellectual content: P He, Y Zou.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
The authors have disclosed no conflicts of interest.
Declaration
This manuscript was initially posted as a preprint entitled ‘Development and validation of a clinical prediction model
to aid radiologists optimize thyroid C-TIRADS classification’
on Research Square (DOI: 10.21203/rs.3.rs-3831900/v1).
After peer feedback and extensive revisions undertaken
collaboratively by the author team, the current version has
substantially evolved and markedly differs from the preprint
version.
Funding/support
This research was supported by Sichuan Science and
Technology Program (Ref Nos.:2025ZNSFSC1751,
2026YFHZ0039), the University-Industry Collaborative
Education Program (Ref No.: 250505236300920), the
University-level Project of North Sichuan Medical College
(Ref Nos.: CXSY24-06, CBY22-QNA48), and the Hospital-level
Projects of the Affiliated Hospital of North Sichuan
Medical College, China (Ref Nos.: 210930, 2023-2GC013,
2025LC010). The funders had no role in the study design, data
collection/analysis/interpretation, or manuscript preparation.
Ethics approval
This research was approved by the Ethics Committee of
Sichuan Provincial People’s Hospital (Ref No.: ER20210347)
and the Ethics Committee of Affiliated Hospital of North
Sichuan Medical College, China (Ref No.: 2021ER436-1). The
requirement for informed patient consent was waived by both
Committees due to the retrospective nature of the research.
Supplementary material
The supplementary material was provided by the authors, and
some information may not have been peer reviewed. Accepted
supplementary material will be published as submitted by the
authors, without any editing or formatting. Any opinions
or recommendations discussed are solely those of the
author(s) and are not endorsed by the Hong Kong Academy
of Medicine and the Hong Kong Medical Association.
The Hong Kong Academy of Medicine and the Hong Kong
Medical Association disclaim all liability and responsibility
arising from any reliance placed on the content.
References
1. Haugen BR, Alexander EK, Bible KC, et al. 2015 American
Thyroid Association management guidelines for adult
patients with thyroid nodules and differentiated thyroid
cancer: the American Thyroid Association guidelines task
force on thyroid nodules and differentiated thyroid cancer.
Thyroid 2016;26:1-133. Crossref
2. Zhou J, Song Y, Zhan W, et al. Thyroid imaging reporting
and data system (TIRADS) for ultrasound features of
nodules: multicentric retrospective study in China.
Endocrine 2021;72:157-70. Crossref
3. Trimboli P. Complexity in the interpretation and
application of multiple guidelines for thyroid nodules: the
need for coordinated recommendations for “small” lesions.
Rev Endocr Metab Disord 2025;26:223-7. Crossref
4. Park JY, Lee HJ, Jang HW, et al. A proposal for a thyroid
imaging reporting and data system for ultrasound features
of thyroid carcinoma. Thyroid 2009;19:1257-64. Crossref
5. Horvath E, Majlis S, Rossi R, et al. An ultrasonogram reporting system for thyroid nodules stratifying cancer
risk for clinical management. J Clin Endocrinol Metab
2009;94:1748-51. Crossref
6. Tessler FN, Middleton WD, Grant EG, et al. ACR Thyroid
Imaging, Reporting and Data System (TI-RADS): white
paper of the ACR TI-RADS Committee. J Am Coll Radiol
2017;14:587-95. Crossref
7. Shin JH, Baek JH, Chung J, et al. Ultrasonography diagnosis
and imaging-based management of thyroid nodules: revised
Korean Society of Thyroid Radiology consensus statement
and recommendations. Korean J Radiol 2016;17:370-95. Crossref
8. Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R,
Leenhardt L. European Thyroid Association guidelines for
ultrasound malignancy risk stratification of thyroid nodules
in adults: the EU-TIRADS. Eur Thyroid J 2017;6:225-37. Crossref
9. Chen Z, Wang JJ, Du JB, et al. Development and validation
of a dynamic nomogram for predicting central lymph node
metastasis in papillary thyroid carcinoma patients based
on clinical and ultrasound features. Quant Imaging Med
Surg 2025;15:1555-70. Crossref
10. Boucai L, Zafereo M, Cabanillas ME. Thyroid cancer: a
review. JAMA 2024;331:425-35. Crossref
11. Zhang J, Xu S. High aggressiveness of papillary thyroid
cancer: from clinical evidence to regulatory cellular
networks. Cell Death Discov 2024;10:378. Crossref
12. Ma T, Semsarian CR, Barratt A, et al. Rethinking
low-risk papillary thyroid cancers <1 cm (papillary
microcarcinomas): an evidence review for recalibrating
diagnostic thresholds and/or alternative labels. Thyroid
2021;31:1626-38. Crossref
13. Kwong N, Medici M, Angell TE, et al. The influence of
patient age on thyroid nodule formation, multinodularity,
and thyroid cancer risk. J Clin Endocrinol Metab
2015;100:4434-40. Crossref
14. Pizzato M, Li M, Vignat J, et al. The epidemiological
landscape of thyroid cancer worldwide: GLOBOCAN
estimates for incidence and mortality rates in 2020. Lancet
Diabetes Endocrinol 2022;10:264-72. Crossref
15. Tessler FN, Middleton WD, Grant EG, Hoang JK. Re: ACR
Thyroid Imaging, Reporting and Data System (TI-RADS):
white paper of the ACR TI-RADS Committee. J Am Coll
Radiol 2018;15(3 Pt A):381-2. Crossref
16. Angelopoulos N, Goulis DG, Chrisogonidis I, et al.
Diagnostic performance of European and American
College of Radiology Thyroid Imaging Reporting and Data
System classification systems in thyroid nodules over 20
mm in diameter. Endocr Pract 2025;31:72-9. Crossref
17. Jin Z, Pei S, Shen H, et al. Comparative study of C-TIRADS,
ACR-TIRADS, and EU-TIRADS for diagnosis
and management of thyroid nodules. Acad Radiol
2023;30:2181-91. Crossref
18. Wildman-Tobriner B, Buda M, Hoang JK, et al. Using
artificial intelligence to revise ACR TI-RADS risk
stratification of thyroid nodules: diagnostic accuracy and
utility. Radiology 2019;292:112-9. Crossref
19. Wu SH, Li MD, Tong WJ, et al. Adaptive dual-task deep
learning for automated thyroid cancer triaging at screening
US. Radiol Artif Intell 2025;7:e240271. Crossref
20. Trimboli P, Colombo A, Gamarra E, Ruinelli L, Leoncini A.
Performance of computer scientists in the assessment of
thyroid nodules using TIRADS lexicons. J Endocrinol
Invest 2025;48:877-83. Crossref
21. Kobaly K, Kim CS, Mandel SJ. Contemporary management of thyroid nodules. Annu Rev Med 2022;73:517-28. Crossref
22. Xu L, Li G, Wei Q, El-Naggar AK, Sturgis EM. Family
history of cancer and risk of sporadic differentiated thyroid
carcinoma. Cancer 2012;118:1228-35. Crossref
23. Iglesias ML, Schmidt A, Ghuzlan AA, et al. Radiation
exposure and thyroid cancer: a review. Arch Endocrinol
Metab 2017;61:180-7. Crossref
24. Saenko V, Mitsutake N. Radiation-related thyroid cancer.
Endocr Rev 2024;45:1-29. Crossref
25. Figge JJ, Gooding WE, Steward DL, et al. Do ultrasound
patterns and clinical parameters inform the probability of
thyroid cancer predicted by molecular testing in nodules
with indeterminate cytology? Thyroid 2021;31:1673-82. Crossref
26. Li X, Xing M, Tu P, et al. Urinary iodine levels and thyroid
disorder prevalence in the adult population of China: a
large-scale population-based cross-sectional study. Sci Rep
2025;15:14273. Crossref
27. Xiao J, Xiao Q, Cong W, et al. Discriminating malignancy in
thyroid nodules: the nomogram versus the Kwak and ACR
TI-RADS. Otolaryngol Head Neck Surg 2020;163:1156-65. Crossref
28. Xin Y, Liu F, Shi Y, Yan X, Liu L, Zhu J. A scoring system for assessing the risk of malignant partially cystic thyroid
nodules based on ultrasound features. Front Oncol
2021;11:731779. Crossref
29. Zhou T, Hu T, Ni Z, et al. Comparative analysis of machine
learning-based ultrasound radiomics in predicting
malignancy of partially cystic thyroid nodules. Endocrine
2024;83:118-26. Crossref
30. Bluethgen C, Van Veen D, Zakka C, et al. Best practices
for large language models in radiology. Radiology
2025;315:e240528. Crossref
31. He Z, Li Y, Zeng W, et al. Can a computer-aided mass
diagnosis model based on perceptive features learned from
quantitative mammography radiology reports improve
junior radiologists’ diagnosis performance? An observer
study. Front Oncol 2021;11:773389. Crossref
32. Kim Y, Roh J, Song DE, et al. Risk factors for posttreatment
recurrence in patients with intermediate-risk papillary
thyroid carcinoma. Am J Surg 2020;220:642-7. Crossref
33. Zhao J, Wen J, Wang S, Yao J, Liao L, Dong J. Association
between adipokines and thyroid carcinoma: a meta-analysis
of case-control studies. BMC Cancer 2020;20:788. Crossref