Hong Kong Med J 2026;32:Epub 26 Jan 2026
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
PICTORIAL MEDICINE
Inflammatory myofibroblastic tumour presenting as a gastric submucosal tumour
Huahui Zhang, MD1,2,3; Shouying Li, MD1,2,3, Ling Ren, MD1,2,3
1 Department of Gastroenterology, The Affiliated Lianyungang Hospital of
Xuzhou Medical University/The First People’s Hospital of Lianyungang,
Lianyungang, China
2 Department of Gastroenterology, The First Affiliated Hospital of Kangda
College, Nanjing Medical University/The First People’s Hospital of
Lianyungang, Lianyungang, China
3 Department of Gastroenterology, Lianyungang Clinical College of
Nanjing Medical University/The First People’s Hospital of Lianyungang,
Lianyungang, China
Corresponding author: Dr Ling Ren (ruby804904@126.com)
A 56-year-old male presented to our gastroenterology
department with a 1-month history of abdominal
discomfort. He had a 5-year history of interstitial
lung disease and was regularly taking pirfenidone,
prednisone, cyclosporine, and hydroxychloroquine.
He denied any family history of malignancy.
Laboratory tests, electrocardiography, and physical
examination revealed no significant abnormalities.
Gastroscopy identified a 40-mm submucosal tumour
(SMT) in the gastric angle (Fig 1a). Abdominal
computed tomography revealed an SMT with
intraluminal growth (Fig 1b). Endoscopic ultrasound
gastroscopy showed a well-defined and hypoechoic
lesion originating from the muscularis propria
(Fig 1c). The SMT was completely resected en
bloc via endoscopic submucosal excavation (ESE)
[Fig 1d]. Post-ESE histopathological examination
revealed the tumour to be composed of spindle
fibroblasts arranged in fascicles, with a background
of lymphocytes, plasma cells, and some eosinophilic infiltration (Fig 2a and b). Immunohistochemical
staining showed tumour cells positive for vimentin
(Fig 2c) and CD34 (cluster of differentiation 34) [Fig 2d]. The frequency of Ki-67 positive proliferating cells was very low (1%). CD117, DOG-1, S100, SOX-10,
SMA, desmin, and calponin were all negative.
Histopathological and immunohistochemical
findings confirmed the diagnosis of an inflammatory
myofibroblastic tumour (IMT).
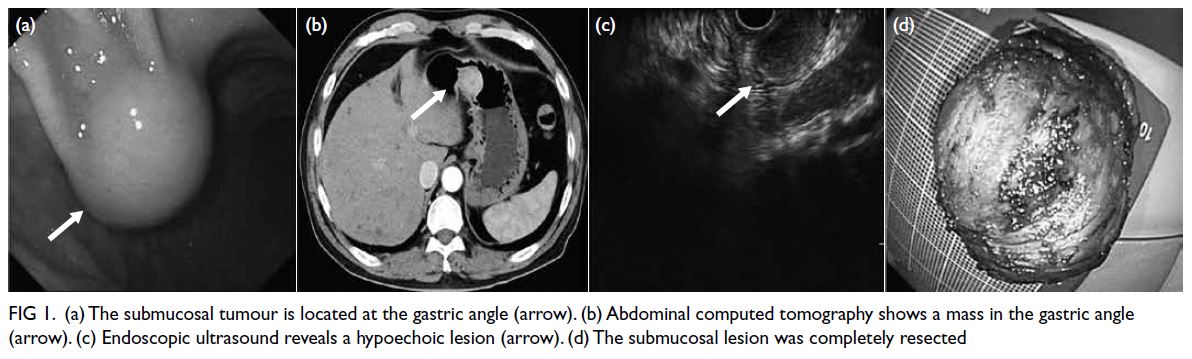
Figure 1. (a) The submucosal tumour is located at the gastric angle (arrow). (b) Abdominal computed tomography shows a mass in the gastric angle (arrow). (c) Endoscopic ultrasound reveals a hypoechoic lesion (arrow). (d) The submucosal lesion was completely resected
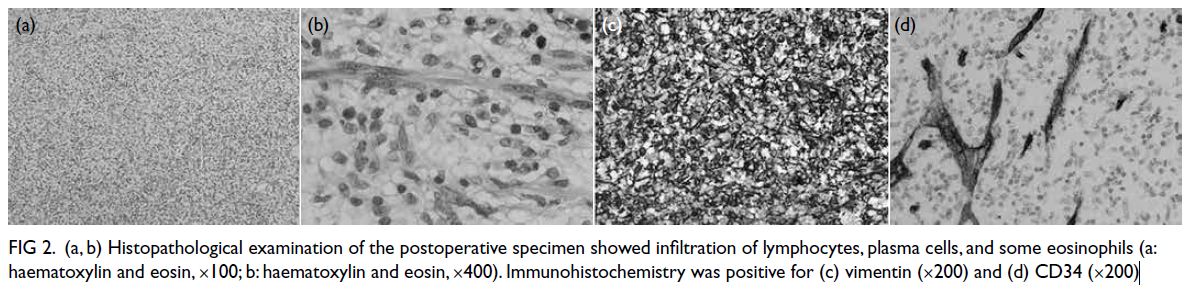
Figure 2. (a, b) Histopathological examination of the postoperative specimen showed infiltration of lymphocytes, plasma cells, and some eosinophils (a: haematoxylin and eosin, ×100; b: haematoxylin and eosin, ×400). Immunohistochemistry was positive for (c) vimentin (×200) and (d) CD34 (×200)
Inflammatory myofibroblastic tumour is a rare
type of mesenchymal tumour, first reported in the
lungs in 1937.1 Primary gastric IMT is extremely
rare and its biological behaviour remains poorly
understood. Due to its non-specific endoscopic and
radiographic features, it is challenging to differentiate
from other SMTs. On immunohistochemistry, IMT
is positive for anaplastic lymphoma kinase (ALK),
vimentin, and CD34, and negative for S-100, DOG1
(discovered on gastrointestinal stromal tumours
[GIST] 1), and CD117 (cluster of differentiation 117). Gastrointestinal stromal tumours, which are also
composed of fascicular spindle cells, can be easily
confused with IMTs. However, GISTs are strongly
positive for CD117, DOG1, and CD34, but negative
for ALK.2 Unlike IMT, an inflammatory background
is not typical in GISTs. Another differential diagnosis
is an inflammatory fibroid polyp, a SMT composed
of spindle cells and inflammatory cell infiltration,
predominantly eosinophils. These lesions are usually
CD34-positive and CD117-negative. Anaplastic
lymphoma kinase positivity is helpful in diagnosing
IMT but is detected in only 50% to 60% of cases.3
Anaplastic lymphoma kinase negativity has been
associated with a higher risk of distant metastasis,4
therefore long-term follow-up is required. Although
our case was ALK-negative, the histological features
were typical of IMT.
Endoscopic submucosal excavation can
completely excavate the tumour in the muscularis
propria along the lesion’s margin.5 The procedure is
comparable to traditional endoscopic submucosal
dissection, with the main difference being the
depth of dissection. No evidence of recurrence was
observed in our patient during 3 years of follow-up.
To the best of our knowledge, this is the first report
of gastric IMT treated with ESE.
Author contributions
Concept or design: L Ren.
Acquisition of data: S Li.
Analysis or interpretation of data: H Zhang.
Drafting of the manuscript: H Zhang, S Li.
Critical revision of the manuscript for important intellectual content: L Ren.
Acquisition of data: S Li.
Analysis or interpretation of data: H Zhang.
Drafting of the manuscript: H Zhang, S Li.
Critical revision of the manuscript for important intellectual content: L Ren.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was conducted in accordance with the Declaration
of Helsinki and was approved by the Ethics Committee of The
First People’s Hospital of Lianyungang, China (Ref No.: LW-
20230612001-01). Informed patient consent was waived by
the Committee due to the retrospective nature of the study,
with the patient anonymised and no identifiable information
included.
References
1. Sanders BM, West KW, Gingalewski C, Engum S, Davis M,
Grosfeld JL. Inflammatory pseudotumor of the alimentary
tract: clinical and surgical experience. J Pediatr Surg
2001;36:169-73. Crossref
2. Jadhav M, Harvi R, Patil R, Kittur S. Inflammatory
myofibroblastic tumor of the stomach presenting as an
exophytic mass—a diagnostic dilemma. Turk Patoloji Derg
2019;35:151-6. Crossref
3. Mahajan P, Casanova M, Ferrari A, Fordham A, Trahair T,
Venkatramani R. Inflammatory myofibroblastic tumor:
molecular landscape, targeted therapeutics, and remaining
challenges. Curr Probl Cancer 2021;45:100768. Crossref
4. Coffin CM, Hornick JL, Fletcher CD. Inflammatory
myofibroblastic tumor: comparison of clinicopathologic,
histologic, and immunohistochemical features including
ALK expression in atypical and aggressive cases. Am J Surg
Pathol 2007;31:509-20. Crossref
5. Qi ZP, Shi Q, Liu JZ, et al. Efficacy and safety of endoscopic
submucosal dissection for submucosal tumors of the colon
and rectum. Gastrointest Endosc 2018;87:540-8.e1. Crossref

