Hong Kong Med J 2026;32:Epub 26 Jan 2026
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
PICTORIAL MEDICINE
Third-trimester prenatal brain imaging for early diagnosis of glutaric aciduria type 1 in monochorionic diamniotic twins
Isabella YM Wah, MB, ChB, FRCOG1; Ye Cao, PhD, FACMG1; Natalie KL Wong, MRCOG, FRCOG1; KW Choy, PhD1; TY Leung, FRCOG1; Josephine SC Chong, FHAKM (Paediatrics)1,2; Lo Wong, MCROG1; YH Ting, FRCOG1; Liona C Poon, FRCOG1
1 Department of Obstetrics and Gynaecology, The Chinese University of Hong Kong, Hong Kong SAR, China
2 Department of Paediatrics, The Chinese University of Hong Kong, Hong Kong SAR, China
Corresponding author: Dr Isabella YM Wah (isabellawah@cuhk.edu.hk)
Case presentation
A 33-year-old primiparous Chinese woman presented
for a 12-week ultrasound. Previous early ultrasound
had confirmed monochorionic diamniotic twins.
Her marriage was non-consanguineous. She had
conceived spontaneously and had no family history
of inborn errors of metabolism. The 12-week fetal
scan revealed normal nuchal translucency in both
twins, and non-invasive prenatal testing showed
normal results.
Serial ultrasound examinations were
performed every 2 weeks from 16 weeks onwards
to monitor fetal growth and detect early signs of
twin-twin transfusion syndrome or twin anaemia-polycythaemia
sequence. Both fetuses followed
the 10th percentile growth curve in abdominal
circumference, head circumference, and femur
length. Morphology scan showed no abnormalities,
and there was no evidence of twin-twin transfusion
syndrome or twin anaemia-polycythaemia sequence
throughout the pregnancy. Routine targeted
neurosonography at 32 weeks showed multiple
bilateral germinolytic cysts and temporal cysts in both
fetuses (Fig). Brain findings were almost identical in
both twins. Although abdominal circumference and
femur length remained at the 10th percentile, the
head circumference of Fetus A exceeded more than
two standard deviations above the mean, while that
of Fetus B was at the mean. Fetal magnetic resonance
imaging (MRI) at 33 weeks demonstrated normal
brain structure with cystic findings consistent with the ultrasound findings and no signs of ischaemia.
Amniocentesis at 34 weeks for chromosomal
microarray and cytomegalovirus polymerase chain
reaction tests yielded negative results. Trio whole-genome
sequencing was arranged. At 35 weeks, the
mother developed pre-eclampsia, and an emergency
lower-segment Caesarean section was performed
the following day. Whole-genome sequencing
results, available on the day of delivery, revealed a
homozygous pathogenic variant, c.1244-2A>C, in
the GCDH gene (NM_000159.4) associated with
glutaric aciduria type 1 (GA1). Both parents were
found to be heterozygous carriers of this variant.
Fetus A weighed 2.4 kg with Apgar scores of 7 at 1
minute and 8 at 5 minutes, while Fetus B weighed
2.2 kg and had Apgar scores of 9 at 1 minute and
10 at 5 minutes. Umbilical cord arterial pH was 7.35
for Fetus A and 7.29 for Fetus B. Both neonates were
admitted to the neonatal unit and promptly started
on intravenous L-carnitine supplementation and
a specialised formula diet. No seizures have been
observed to date. Postnatal brain MRI at 1 month of
age showed unchanged cystic findings in both twins,
with no evidence of white matter involvement.
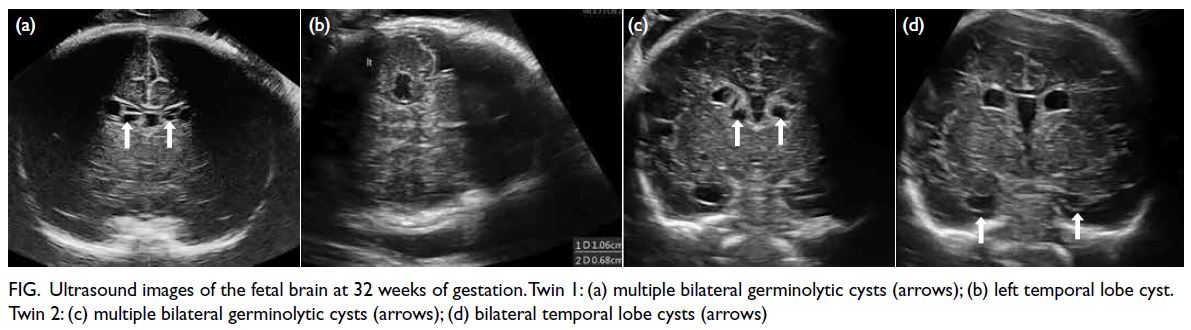
Figure. Ultrasound images of the fetal brain at 32 weeks of gestation. Twin 1: (a) multiple bilateral germinolytic cysts (arrows); (b) left temporal lobe cyst. Twin 2: (c) multiple bilateral germinolytic cysts (arrows); (d) bilateral temporal lobe cysts (arrows)
Discussion
This case highlights the importance of
comprehensive prenatal evaluation, including
detailed neurosonography and fetal brain MRI, when
unusual fetal brain findings are detected. The initial
concern was for transfusion-related complications; however, the absence of typical features prompted
a broader differential diagnosis, ultimately leading
to the diagnosis of GA1. Metabolic crises such as
severe hypoglycaemia, hyperammonaemia, lactic
acidosis, and permanent neurological or systemic
complications can occur in patients diagnosed after
the onset of symptoms. Early identification of GA1
enabled prompt multidisciplinary consultation and
the initiation of appropriate treatment, including
dietary management. Prenatal diagnosis of GA1
based on third-trimester brain features is possible
and facilitates early postnatal management, enabling
prompt treatment at birth and potentially improving
long-term neurological outcomes.
Author contributions
Concept or design: IYM Wah.
Acquisition of data: All authors.
Interpretation of data: All authors.
Drafting of the manuscript: IYM Wah.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: All authors.
Interpretation of data: All authors.
Drafting of the manuscript: IYM Wah.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
The authors thank the Obstetrics and Gynaecology team,
Maternal Fetal Medicine team, midwives, scientists, genetic
counsellor and paediatricians at Prince of Wales Hospital for
their support in managing the case.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration
of Helsinki. Written informed consent was obtained from the
patient for publication of the details of their medical case and
any accompanying images.

