Hong Kong Med J 2025;31:Epub 27 Nov 2025
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Incidence, risk factors, and clinical outcomes of
peripartum cardiomyopathy in Hong Kong
Liliana SK Law, MB, ChB1; LT Kwong, MB, BS1; KH Siong, MB, BS1; Sani TK Wong, MB, ChB2; WL Chan, MB, ChB3; KY Tse, MB, BS4; Yannie YY Chan, MB, BS5; KS Eu, MB, BS6; CY Chow, MB, ChB7; Joan KO Wai, LMCHK8; HC Mok, MB, BS1; PL So, MB, BS1
1 Department of Obstetrics and Gynaecology, Tuen Mun Hospital, Hong Kong SAR, China
2 Department of Obstetrics and Gynaecology, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong SAR, China
3 Department of Obstetrics and Gynaecology, Kwong Wah Hospital, Hong Kong SAR, China
4 Department of Obstetrics and Gynaecology, Queen Elizabeth Hospital, Hong Kong SAR, China
5 Department of Obstetrics and Gynaecology, Princess Margaret Hospital, Hong Kong SAR, China
6 Department of Obstetrics and Gynaecology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
7 Department of Obstetrics and Gynaecology, United Christian Hospital, Hong Kong SAR, China
8 Department of Obstetrics and Gynaecology, Queen Mary Hospital, The
University of Hong Kong, Hong Kong SAR, China
Corresponding author: Dr Liliana SK Law (lawskliliana@gmail.com)
Abstract
Introduction: Peripartum cardiomyopathy (PPCM)
is an uncommon but serious form of heart failure
affecting women during late pregnancy or early
postpartum. This territory-wide multicentre
retrospective study aimed to evaluate the local
incidence, risk factors, and clinical outcomes,
including subsequent pregnancies, in Hong Kong.
Methods: Medical records were retrospectively
reviewed for women who delivered at all public
hospitals between 1 January 2013 and 31 December
2022 and met the 2010 European Society of
Cardiology Working Group criteria for PPCM.
Regression analysis was performed to investigate
maternal risk factors.
Results: Thirty Asian women were diagnosed with
PPCM, corresponding to an incidence of 1 in 11 179
live births. Eleven (36.7%) had antepartum onset
of symptoms, and 25 (83.3%) were diagnosed after
childbirth, most presenting with severe symptoms
(90%). The median left ventricular ejection fraction
was 30% (range, 10%-44%). Notable complications
included cardiogenic shock (10%), respiratory
failure (23.3%), acute renal failure (23.3%), and
thromboembolism (23.3%). Most women received
guideline-directed heart failure therapy. At 12
months, all-cause mortality was 6.7%, and cardiac
recovery occurred in 60%. Eleven women had 13
subsequent pregnancies (three miscarriages, five
terminations, and five live births). There were no
maternal deaths or cases of recurrent PPCM. Genetic
testing identified potentially pathogenic variants in
at least 10% of women. Antenatal anaemia (adjusted
odds ratio [OR]=13.04; 95% confidence interval
[95% CI]=3.72-45.70) and hypertensive disorders
of pregnancy (adjusted OR=38.00; 95% CI=9.66-149.52) were associated with higher odds of PPCM.
Conclusion: This study highlights the substantial
morbidity and mortality associated with PPCM.
Genetic testing may aid in risk stratification and
prognostication.
New knowledge added by this study
- Peripartum cardiomyopathy (PPCM) is an uncommon but potentially fatal disease in Hong Kong.
- Genetic testing by next-generation sequencing identified 10% of women with PPCM as carriers of potential genetic variants associated with cardiomyopathy.
- Antenatal anaemia and hypertensive disorders of pregnancy are independent clinical risk factors for PPCM.
- Screening for and prevention of anaemia during pregnancy and pre-eclampsia may help reduce the incidence of PPCM.
- The integration of genetic testing in PPCM management may support personalised medical care.
Introduction
Peripartum cardiomyopathy (PPCM) is a rare form
of heart failure that occurs in relation to pregnancy,
resulting in substantial morbidity and mortality.1 In
2010, the Heart Failure Association of the European
Society of Cardiology (ESC) defined PPCM as
“an idiopathic cardiomyopathy presenting with
heart failure secondary to left ventricular systolic
dysfunction towards the end of pregnancy or in the
months following delivery, where no other cause of
heart failure is found”.2 Globally, its incidence varies
widely, ranging from 1 in 100 live births in Nigeria3
to 1 in 20 000 live births in Japan.4
The exact pathogenesis of PPCM is not yet
fully understood; the current hypothesis proposes
a ‘two-hit’ model involving an initial vascular
insult caused by vasculotoxic hormonal effects,
including soluble FMS-like tyrosine kinase-1 and
prolactin, followed by a second hit of underlying
predisposition—such as genetic susceptibility and
other risk factors—that limits some women’s ability
to withstand this vasculotoxic insult.1 Genetic or
familial predisposition to PPCM has been supported
by multiple reports.5 6 7 8 Additionally, well-recognised
risk factors for PPCM include advanced maternal age,
African American ancestry, multiple pregnancies,
hypertension, and pre-eclampsia.9
Peripartum cardiomyopathy is a potentially
life-threatening myocardial disease that affects
women of all ethnic groups10 and can have long-term
health consequences.11 Until now, there has been a
lack of information regarding the clinical phenotype
and outcomes of this disease in Hong Kong. The
present population-based study was conducted to
evaluate the local incidence, clinical presentation,
management, complications, 12-month outcomes,
and subsequent pregnancies in women with PPCM.
Additionally, we examined potential risk factors by
comparing the clinical characteristics of women
with and without PPCM to provide a basis for future
preventive strategies.
Methods
Study design
This was a population-based retrospective study
of all women with PPCM who delivered in public
hospitals in Hong Kong between 1 January 2013 and
31 December 2022. Cases were identified through
the Clinical Data Analysis and Reporting System,
which captures obstetric data and hospitalisation
diagnoses from eight public hospitals providing
obstetric services. First, all women who delivered
during the study period and had a diagnosis code for
heart failure from the third trimester to 6 months
postpartum were identified. Each woman’s medical
record was systematically reviewed by two authors
to determine whether the following criteria for
PPCM were met: development of cardiac failure
(with left ventricular ejection fraction [LVEF] <45%
on echocardiography) during the third trimester or
within 6 months postpartum without an identifiable
cause. Women were excluded if LVEF was ≥45%,
a recognised cause of heart failure was identified,
or there was no physician-confirmed diagnosis of
PPCM.
Clinical variable collection
Baseline characteristics (including socio-demographics,
preexisting health conditions,
and obstetric history) at the time of PPCM
diagnosis were obtained from medical records.
Clinical presentation and initial investigations,
including electrocardiography, chest radiography,
echocardiography, and laboratory results, were
collected. All in-hospital complications and reported
outcomes during follow-up were recorded, including
all-cause mortality and cardiac recovery determined
by echocardiography at 12 months. Management
strategies were documented, including admission
to the intensive care unit or cardiac care unit, use
of mechanical ventilation or circulatory support,
medications prescribed at hospital discharge,
pacemaker insertion, and heart transplantation.
Complete recovery of cardiac function was defined as LVEF ≥50%. Some patients underwent genetic
evaluation, and their reports were analysed.
Obstetric outcomes at the time of the PPCM
event were assessed, including hypertensive
disorders of pregnancy; gestational diabetes;
thyroid disease; antenatal anaemia (defined as a
haemoglobin level <10.5 g/dL); use of tocolytics;
placenta accreta spectrum; placental abruption; fetal
growth restriction; preterm delivery; assisted vaginal
delivery or caesarean section; primary postpartum
haemorrhage (blood loss ≥500 mL); and caesarean
hysterectomy. Neonatal outcomes were examined,
including stillbirth, sex, birth weight, small for
gestational age, Apgar scores, admission to the
neonatal intensive care unit, and death within 28
days of life. Data from the territory-wide electronic
healthcare database were also extracted regarding
outcomes of subsequent pregnancies, including
LVEF before, during, and after pregnancy. The
interval between the PPCM pregnancy and the first
subsequent pregnancy was recorded.
To investigate risk factors for PPCM, women
who gave birth during the same period but did
not develop heart failure were selected as the
control group, with a PPCM-to-control ratio of
1:4. Demographic and clinical characteristics were
compared between women with and without PPCM.
Statistical analysis
Data analysis was conducted using SPSS (Windows
version 26.0; IBM Corp, Armonk [NY], United
States). The incidence rate was calculated by dividing
the total number of PPCM cases by the total number
of live births during the study period. Descriptive data
for continuous variables were presented as mean ±
standard deviation or median (range or interquartile
range), and categorical data were presented as
numbers with percentages. Comparisons between
women with and without PPCM were performed
using Student’s t test or the Mann-Whitney U test
for continuous variables, and the Chi squared test
or Fisher’s exact test for categorical variables. Risk
factors associated with PPCM were assessed using
univariable and multivariable logistic regression
analyses, with results expressed as odds ratios (ORs)
and 95% confidence intervals (95% CIs). A P value
of <0.05 was considered statistically significant.
The STROBE (Strengthening the Reporting of
Observational Studies in Epidemiology) guidelines
were followed in the preparation of this article.
Results
Incidence of peripartum cardiomyopathy in
Hong Kong
During the 10-year study period, 30 women with
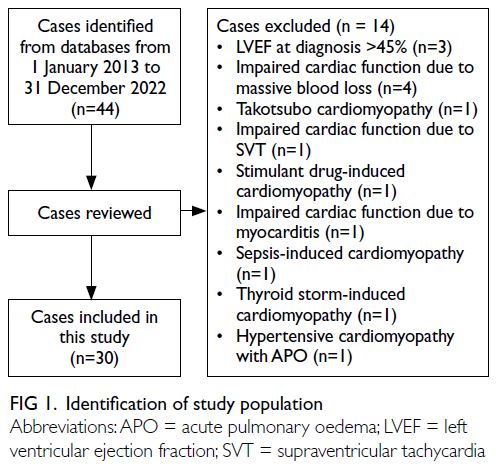
PPCM delivered in public hospitals (Fig 1). Over the
same period, there were 335 376 live births, yielding an estimated PPCM incidence of 1 in 11 179 live births in Hong Kong.
Demographics, clinical characteristics, and
investigations
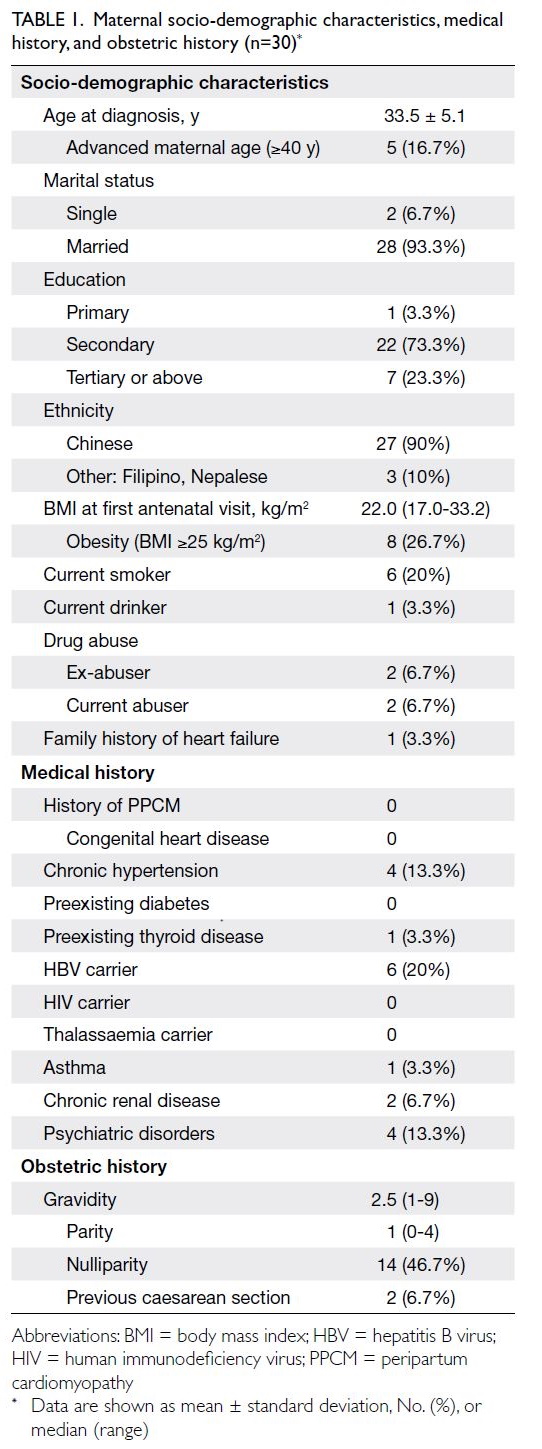
Detailed characteristics are listed in Table 1. All
women in this study were Asian. The mean age was
33.5 years and the median body mass index was
22.0 kg/m2. One woman had a positive family history
of heart failure of unknown cause; no women had a
previous history of PPCM or cardiac disease.
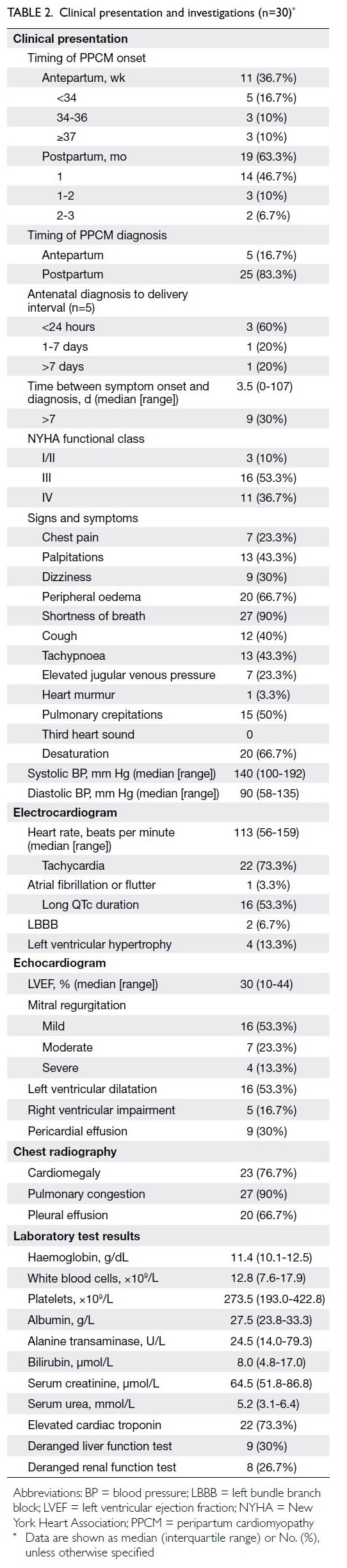
Symptoms began antepartum in 36.7% of
women and postpartum in 63.3%; PPCM was
predominantly diagnosed postpartum (83.3%). The
median time from symptom onset to diagnosis
was 3.5 days (range, 0-107). At diagnosis, 90% of
women had severe symptoms (New York Heart
Association functional class III/IV), most commonly
comprising shortness of breath, peripheral oedema,
and desaturation. Common electrocardiographic
findings included sinus tachycardia and prolonged
QTc interval. At the first echocardiographic
assessment, the median LVEF was 30% (range,
10-44). More than half of the women had abnormal
chest radiographs showing congestive lung fields,
cardiomegaly, and pleural effusion (Table 2).
Complications, management, and cardiac
recovery
Detailed results are presented in Table 3. Of the 30
women with PPCM, 19 (63.3%) were managed in the
intensive care unit or cardiac care unit. Cardiogenic
shock, respiratory failure, and acute renal failure
occurred in 10% to 20% of cases. Inotropic support,
mechanical ventilation, extracorporeal membrane
oxygenation, and renal replacement therapy were
used during acute treatment.
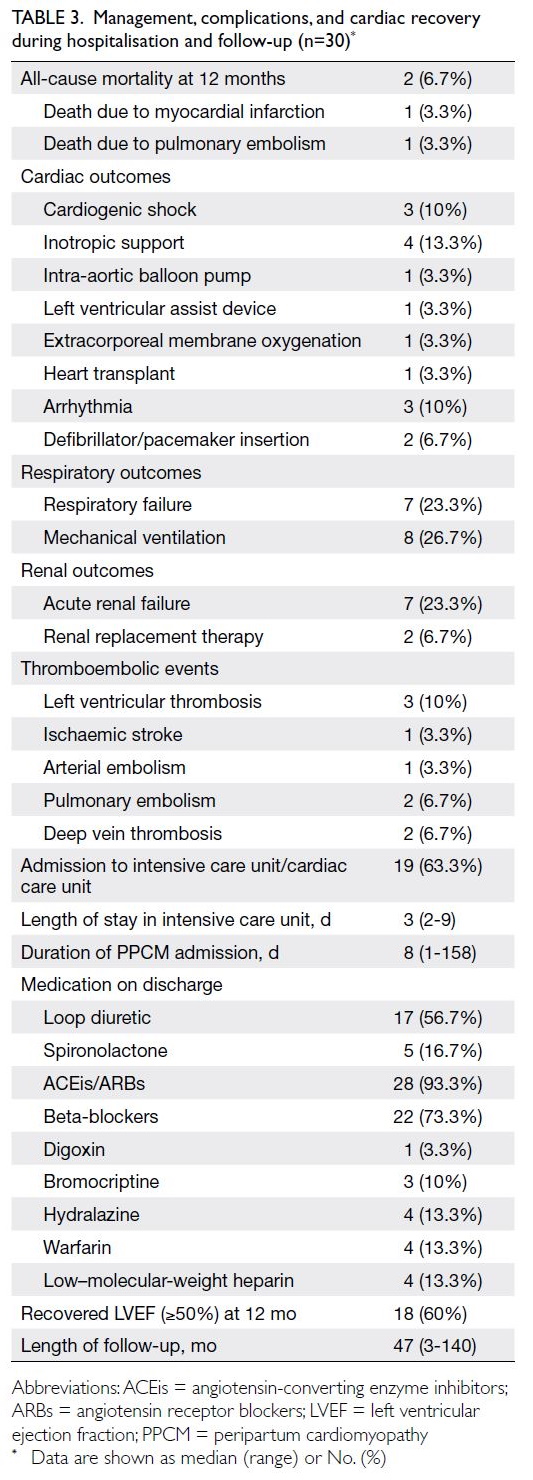
Table 3. Management, complications, and cardiac recovery during hospitalisation and follow-up (n=30)
At hospital discharge, most women were
prescribed angiotensin-converting enzyme
inhibitors (ACEis) or angiotensin receptor blockers (ARBs) and beta-blockers. Four women received
prophylactic low–molecular-weight heparin for
venous thromboembolism prevention after the
event; another four required warfarin for the
treatment of cerebral venous thrombosis, brachial
artery thromboembolism, pulmonary embolism, or
deep vein thrombosis (Table 3).
One woman experienced decompensated
heart failure requiring an intra-aortic balloon
pump and a left ventricular assist device 9 months
after diagnosis, followed by heart transplantation
1 year after the event. Two women underwent
implantable cardioverter-defibrillator insertion due
to symptomatic premature ventricular contractions
and poor LVEF recovery. Seven women (23.3%)
experienced nine thromboembolic events within 1
year of the PPCM episode, including left ventricular
thrombi, ischaemic stroke, and pulmonary embolism.
The median follow-up duration after PPCM was 47
months (range, 3-140). At 12 months, all-cause in-hospital
mortality was 6.7%; causes of death were
myocardial infarction and pulmonary embolism.
Overall, recovery of left ventricular function (LVEF
≥50%) occurred in 60% of women (Table 3).
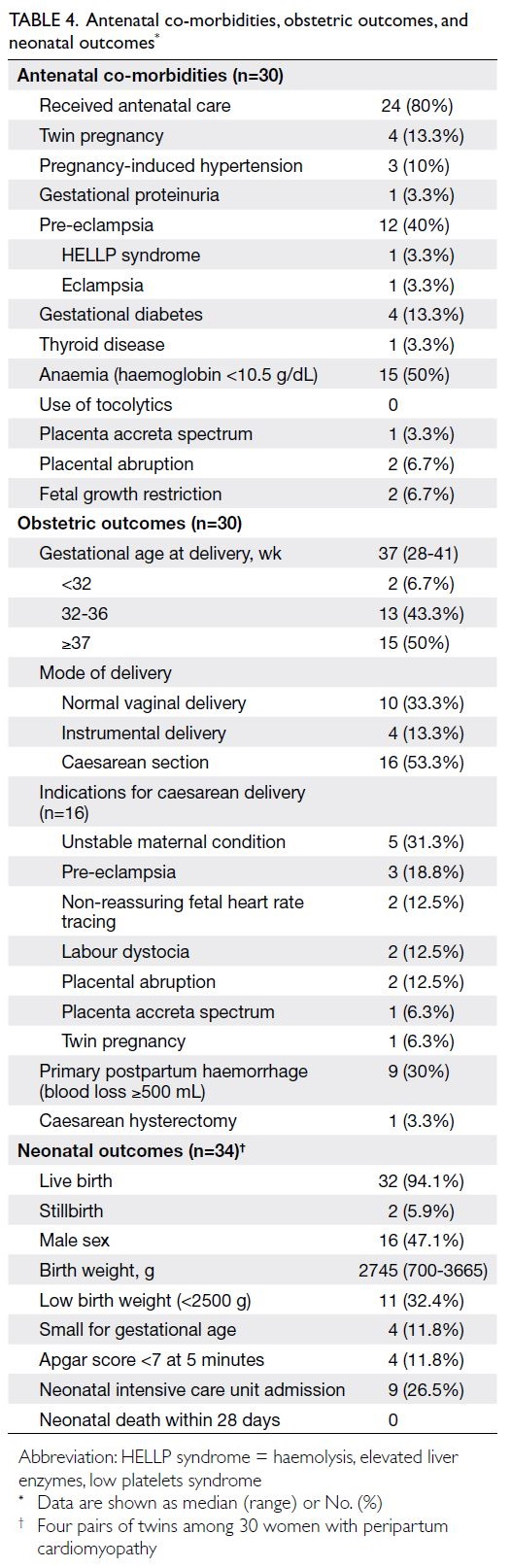
Antenatal co-morbidities, obstetric
outcomes, and neonatal outcomes
Prior to PPCM, 80% of women received antenatal
care. Four women (13.3%) had twin pregnancies.
Antenatal anaemia was present in 50% of women.
Hypertensive disorders of pregnancy occurred
in 56.7%, whereas gestational diabetes was noted
in 13.3%. Complications related to pre-eclampsia
included haemolysis, elevated liver enzymes, and
low platelets syndrome in 3.3%; eclampsia in 3.3%;
and placental abruption in 6.7%. No women received
tocolytics during pregnancy. The median gestational
age at delivery was 37 weeks (range, 28-41). The
caesarean section rate was 53.3%, and the most
frequent indication was unstable maternal condition
(31.3%). Primary postpartum haemorrhage occurred
in 30% of cases; one woman required hysterectomy for
placenta accreta spectrum. Among the 34 newborns,
32 (94.1%) were born alive; two were stillborn in the
third trimester (5.9%) due to placental abruption and
trisomy 18. The median birth weight was 2745 g, and
11.8% of newborns were small for gestational age.
Four newborns (11.8%) had an Apgar score below 7
at 5 minutes, and nine (26.5%) required admission to
a neonatal intensive care unit. There were no cases of
early neonatal death (Table 4).
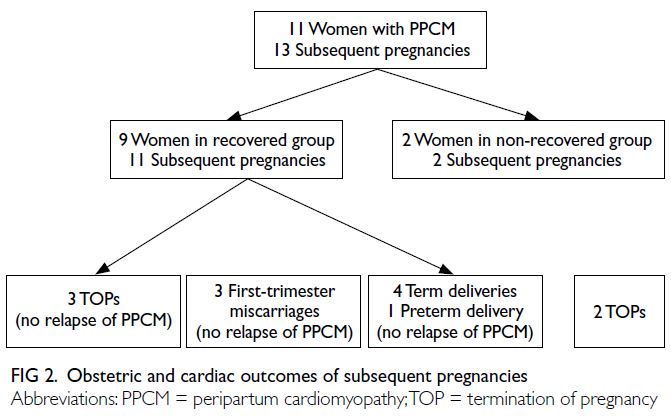
Outcomes of subsequent pregnancies
The obstetric and cardiac outcomes of the 11 women
with subsequent pregnancies are shown in Figure 2. The median interval between the PPCM-affected
pregnancy and the next pregnancy was 17 months (range, 4-60). There were 13 subsequent pregnancies
(three miscarriages, five terminations, and five live
births). Of the five terminations, two were advised
due to poor cardiac condition; the remaining three
were elective for maternal anxiety or social reasons.
There were no maternal deaths or cases of recurrent
PPCM.
Cases with genetic testing
Genetic analysis using a dilated cardiomyopathy
(DCM) panel by next-generation sequencing was
requested by physicians in three cases (online supplementary Table 1). Case 1, involving a woman
with a family history of heart failure, revealed
a pathogenic variant in the FLNC gene. Case 2,
concerning a patient with a history of cancer-related
chemotherapy who developed refractory postpartum
heart failure requiring heart transplantation 1 year
after PPCM diagnosis, had no prior signs of heart
failure before pregnancy. A genetic test identified
two pathogenic variants in the TTN and MYBPC3 genes. Case 3 involved a woman with chronic kidney
disease who exhibited persistent left ventricular
systolic dysfunction 4 years after PPCM diagnosis.
Genetic evaluation was pursued due to her young-onset
multisystem disease, revealing a variant
in the NEXN gene. This variant, associated with
autosomal dominant monogenic DCM, was absent
from population databases but showed conflicting
results on in silico prediction algorithms; therefore,
it was classified as a variant of uncertain significance.
Overall, potentially pathogenic genetic variants were
identified in at least 10% of women with PPCM.
Maternal factors associated with peripartum
cardiomyopathy
Compared with the control group, univariable
logistic regression analysis showed that factors
associated with PPCM included advanced maternal
age (≥40 years), smoking, hypertensive disorders of
pregnancy, and antenatal anaemia. In multivariable
regression analysis, PPCM was independently
associated with hypertensive disorders of pregnancy
(adjusted OR=38.00; 95% CI=9.66-149.52; P<0.001)
and antenatal anaemia (adjusted OR=13.04; 95%
CI=3.72-45.70; P<0.001) [online supplementary Table 2].
Discussion
Time from symptom onset to diagnosis
Over the 10-year study period, we observed a PPCM
incidence of 1 in 11 179 live births in Hong Kong.
Worldwide variation in PPCM incidence may relate
to ethnic and socio-economic factors12; rates are
expected to increase because of advancing maternal
age,13 multiple pregnancies, and obesity. About one-third
of our patients developed symptoms before
delivery, a finding comparable to the Asia-Pacific
group in the ESC EURObservational Research
Programme registry.10 Overall, 30% of women
were diagnosed more than 7 days after symptom
onset. Among those with antepartum-onset
symptoms, 54.5% were diagnosed after delivery. This
diagnostic delay may be attributed to the difficulty
in distinguishing PPCM from normal physiological
changes of pregnancy—its symptoms often mimic
those of late gestation and may only be recognised
postpartum when they become more pronounced.
Delayed diagnosis has been associated with lower
rates of left ventricular recovery.14 Early recognition
and awareness among both pregnant women and
healthcare professionals are crucial to enable
prompt initiation of heart failure therapy, which may
improve cardiac recovery. To support early detection
and facilitate timely specialist referral for diagnostic
evaluation, serum biomarkers can be measured to
rule out heart failure with high probability during
pregnancy or the postpartum period.15
Pre-eclampsia and peripartum
cardiomyopathy
In our study, approximately half of the cases
involved pre-eclampsia, a finding consistent with the
Asia-Pacific cohort in the ESC EURObservational
Research Programme registry.10 A meta-analysis of
22 studies demonstrated a fourfold higher prevalence
of pre-eclampsia among women with PPCM relative
to the general obstetric population (22% vs 5%).16
Our multivariable regression analysis confirmed that
hypertensive disorders of pregnancy constituted an
independent risk factor for PPCM. The association
between pre-eclampsia and PPCM may be explained
by their shared pathophysiological mechanism—systemic vascular angiogenic imbalance.1 15 17 Preeclampsia
and PPCM might represent a single
disease spectrum with substantial overlap.17 Low-dose
aspirin is generally used for the prevention
of pre-eclampsia and its associated morbidity
and mortality.18 Although aspirin use for PPCM
prevention is not supported by evidence-based
guidelines, it could theoretically provide benefit
due to the shared vascular dysfunction pathways.
Consequently, the use of aspirin for pre-eclampsia
prevention may indirectly reduce the risk of PPCM
in high-risk women.
Anaemia and peripartum cardiomyopathy
We found that antenatal anaemia was independently
associated with PPCM. A systematic review and
meta-analysis previously indicated that women
with anaemia had up to fivefold higher odds of
developing PPCM compared with women exhibiting
normal haemoglobin levels.19 The precise nature of
this association remains unclear; iron deficiency
may contribute by impairing myocardial contractile
function.20 Anaemia screening and correction during
pregnancy may help reduce the risk of PPCM.
Management of peripartum cardiomyopathy
A multidisciplinary approach involving
cardiologists, obstetricians, intensivists, cardiac
surgeons, anaesthesiologists, neonatologists, and
nurses is essential for the management of PPCM.21
In severe cases with haemodynamic instability, acute
management—including immediate resuscitation
and mechanical respiratory or circulatory
support—may be required.15 Urgent caesarean
section should be considered for advanced heart
failure that persists despite optimal medical
therapy. According to international consensus, the
main treatment should follow guideline-directed
medical therapy for heart failure with reduced
ejection fraction in non-pregnant patients, while
respecting contraindications for certain drugs
during pregnancy.6 22 23 24 25 Standard therapies include
diuretics, ACEis or ARBs, mineralocorticoid
receptor antagonists, vasodilators (hydralazine/nitrates), digoxin, beta-blockers, and anticoagulants.
A 2022 meta-analysis of global data demonstrated
that frequent prescription of beta-blockers, ACEis/ARBs, and bromocriptine or cabergoline was
associated with lower all-cause mortality and
better left ventricular recovery at 12 months.26 In
our study, most patients received ACEis/ARBs and
beta-blockers; fewer were prescribed bromocriptine
at discharge. The rationale for using dopamine
agonists to inhibit prolactin secretion lies in the
proposed pathophysiological mechanism involving
16-kDa prolactin, an oxidative stress-mediated
cleavage product that damages cardiovascular
tissue.27 Regarding prolactin inhibition in women
with PPCM, a meta-analysis reported that those
treated with bromocriptine had higher odds of
left ventricular recovery, without a significant
difference in all-cause mortality.28 However,
bromocriptine use is associated with an increased
risk of thromboembolic complications. The 2019
ESC–Heart Failure Association position statement
issued a weak recommendation for bromocriptine
use, advising that it should always be accompanied
by at least prophylactic anticoagulation.15 Future
randomised controlled trials and registry data with
longer follow-up are needed to provide stronger
evidence supporting its use. For women who do not
recover from PPCM within 1 year, the American
College of Cardiology/American Heart Association
Joint Committee and the ESC recommend
implantable cardioverter-defibrillator therapy for
the primary prevention of sudden cardiac death
due to ventricular tachyarrhythmia.22 29 30 Cardiac
transplantation may be required for patients with
refractory severe heart failure despite maximal
medical therapy, as occurred in one of our cases.
Cardiac recovery and mortality
Estimates of left ventricular recovery and mortality in PPCM vary considerably across geographic
regions,26 presumably due to differences in medical
therapy, access to healthcare services, and follow-up
duration. A 2022 meta-analysis of 4875 patients
from 60 countries reported overall 12-month rates
of left ventricular recovery and all-cause mortality
of 58.7% and 9.8%, respectively.26 In our cohort, 60%
of women achieved cardiac recovery; two patients
(6.7%) died of myocardial infarction and pulmonary
embolism within 12 months of diagnosis. Both had
poor social support and did not adhere to treatment
or attend follow-up visits, which likely contributed
to their adverse outcomes. These findings highlight
the need for greater public awareness, improved
medication compliance, and stronger social support
systems. We recommend enhanced nursing outreach
and structured patient education, along with post-discharge
monitoring, to optimise outcomes.
Prevention of thromboembolic complications
Thromboembolism, a potentially life-threatening
complication of PPCM, affected 23.3% of women
in our cohort. This high rate may be attributed to
the hypercoagulable state of pregnancy, impaired
circulation, and blood stasis from cardiac failure.
Our incidence was higher than the reported global
rate of 6.1% in a recent international study.26
Therapeutic anticoagulation is recommended for
patients with intracardiac thrombus or systemic
embolism. In our study, 13.3% of patients received
low molecular weight heparin for thromboembolism
prophylaxis. Both the AHA and ESC recommend
anticoagulation in PPCM cases involving severe
left ventricular dysfunction (LVEF <30% to <35%)
during the peripartum period and up to 8 weeks
postpartum.29 31 Despite the high thromboembolic
risk in PPCM, anticoagulation remains a subject of
ongoing debate.32 Our data support prophylactic
anticoagulation for all women with PPCM, given
the high incidence observed. Ultimately, individual
assessment of thromboembolic risk—considering
the extent of left ventricular dysfunction, caesarean
delivery, immobility, and ventricular dilatation—may help identify patients most likely to benefit from
thromboprophylaxis.
Relapse of peripartum cardiomyopathy in
subsequent pregnancies
Relapse of PPCM and associated mortality in
subsequent pregnancies are not uncommon;
rates range from 5.3% to 29.5% and 0% to 55.5%,
respectively.33 In our study, nine of 11 patients
(81.8%) had confirmed recovery of cardiac function
before conception. There were no maternal deaths
or PPCM recurrences during pregnancy. A recent
meta-analysis showed that women with persistent
left ventricular dysfunction prior to a subsequent
pregnancy had a higher risk of mortality and worsening function compared to women whose
cardiac function had recovered.33 However, recovered
left ventricular function does not guarantee an
uncomplicated subsequent pregnancy.34 35 It is
crucial to monitor cardiac function throughout
pregnancy—and up to 6 months postpartum—to
detect subclinical left ventricular dysfunction or
PPCM recurrence. Women with a history of PPCM
should be counselled regarding the risks of future
pregnancies, including irreversible ventricular
deterioration, maternal death, and fetal loss.36
Subsequent pregnancy is not recommended if LVEF
fails to normalise. Contraceptive counselling should
begin early after the acute event; reliable methods
with minimal thromboembolic risk are preferred.37
Genetic assessment
A study has demonstrated a genetic contribution to
PPCM in at least 15% of cases.38 The most commonly
affected gene is TTN, which encodes the large
sarcomeric protein titin.39 The relative prevalence of
truncating variants in these genes is nearly identical
between PPCM and DCM.39 In our study, three of 30
patients (10%) were screened for cardiomyopathy-related
genes (TTN, FLNC, MYBPC3, NEXN), all of
whom were in the non-recovery group, indicating that
at least 10% had a genetic predisposition to PPCM.
The American College of Cardiology/American
Heart Association Joint Committee recommends
that patients with non-ischaemic cardiomyopathy
undergo genetic counselling and testing for inherited
cardiomyopathies to facilitate early cardiac disease
detection and timely initiation of treatments that
reduce heart failure progression and sudden death
risk.22 The identification of pathogenic genetic
variants can provide valuable prognostic information
and clarify associated risks (eg, arrhythmic
complications linked to FLNC and DSP mutations),
thereby guiding decisions on preventive measures,
including implantable defibrillator placement and
exercise recommendations. Furthermore, cascade
genetic testing for relatives enables closer pregnancy
monitoring, informed reproductive decisions
(including prenatal or preimplantation genetic
diagnosis), and lifelong cardiovascular surveillance
to improve outcomes.40 The value of routine
genetic testing remains limited by low penetrance,
variable clinical expression, and uncertain variant
significance. It may also lead to patient anxiety,
potential genetic discrimination, and substantial
resource implications. Careful patient selection with
thorough pre- and post-test counselling is essential.
Because the clinical presentation of PPCM closely
resembles that of DCM, the ESC suggests that
genetic testing be considered in PPCM cases with a
positive family history,15 where clinically actionable
findings are most likely to be identified.
Limitations
This study had several limitations. Because PPCM is
a rare condition, a small sample size was inevitable.
The retrospective nature of data collection over a
10-year period may have resulted in incomplete
information. Outcomes could also have been
influenced by variations in heart failure management
over time and across hospitals. Furthermore, some
PPCM cases managed in the private sector or
outside Hong Kong might not have been captured.
The long-term impact of PPCM on women’s
overall health was not assessed. The establishment
of a local PPCM registry would facilitate a better
understanding of the condition, identification of
outcome determinants, and optimisation of clinical
care in Hong Kong.
Conclusion
Peripartum cardiomyopathy is an uncommon
but potentially life-threatening medical condition
affecting women worldwide. Genetic factors
contribute to disease susceptibility in at least 10%
of cases. Genetic testing may offer a valuable tool to
guide prognosis and management in affected women.
Author contributions
Concept or design: LSK Law, LT Kwong, PL So.
Acquisition of data: LSK Law, KH Siong, HC Mok, STK Wong, JKO Wai, CY Chow, WL Chan, KY Tse, YYY Chan, KS Eu, PL So.
Analysis or interpretation of data: LSK Law, PL So.
Drafting of the manuscript: LSK Law, PL So.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: LSK Law, KH Siong, HC Mok, STK Wong, JKO Wai, CY Chow, WL Chan, KY Tse, YYY Chan, KS Eu, PL So.
Analysis or interpretation of data: LSK Law, PL So.
Drafting of the manuscript: LSK Law, PL So.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
The authors thank all staff in the Statistics Department at
Tuen Mun Hospital for their assistance with data collection.
Funding/support
This research received no specific grant from any funding
agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This research was approved by the Central Institutional Review Board of Hospital Authority, Hong Kong (Ref No.:
CIRB-2023-114-3). The requirement for informed patient
consent was waived by the Board due to the retrospective
nature of the research. All data used in the research were
anomymised and unidentifiable.
Supplementary material
The supplementary material was provided by the authors and
some information may not have been peer reviewed. Accepted
supplementary material will be published as submitted by the
authors, without any editing or formatting. Any opinions
or recommendations discussed are solely those of the
author(s) and are not endorsed by the Hong Kong Academy
of Medicine and the Hong Kong Medical Association.
The Hong Kong Academy of Medicine and the Hong Kong
Medical Association disclaim all liability and responsibility
arising from any reliance placed on the content.
References
1. Davis MB, Arany Z, McNamara DM, Goland S, Elkayam U.
Peripartum cardiomyopathy: JACC state-of-the-art review.
J Am Coll Cardiol 2020;75:207-21. Crossref
2. Sliwa K, Hilfiker-Kleiner D, Petrie MC, et al. Current
state of knowledge on aetiology, diagnosis, management,
and therapy of peripartum cardiomyopathy: a position
statement from the Heart Failure Association of the
European Society of Cardiology Working Group on
peripartum cardiomyopathy. Eur J Heart Fail 2010;12:767-78. Crossref
3. Isezuo SA, Abubakar SA. Epidemiologic profile of
peripartum cardiomyopathy in a tertiary care hospital.
Ethn Dis 2007;17:228-33.
4. Kamiya CA, Kitakaze M, Ishibashi-Ueda H, et al. Different
characteristics of peripartum cardiomyopathy between
patients complicated with and without hypertensive
disorders. -Results from the Japanese Nationwide survey
of peripartum cardiomyopathy-. Circ J 2011;75:1975-81. Crossref
5. Pierce JA, Price BO, Joyce JW. Familial occurrence of
postpartal heart failure. Arch Intern Med 1963;111:651-5. Crossref
6. Morales A, Painter T, Li R, et al. Rare variant mutations
in pregnancy-associated or peripartum cardiomyopathy.
Circulation 2010;121:2176-82. Crossref
7. van Spaendonck-Zwarts KY, van Tintelen JP, van Veldhuisen
DJ, et al. Peripartum cardiomyopathy as a part of familial
dilated cardiomyopathy. Circulation 2010;121:2169-75. Crossref
8. van Spaendonck-Zwarts KY, Posafalvi A, van den Berg MP,
et al. Titin gene mutations are common in families with both
peripartum cardiomyopathy and dilated cardiomyopathy.
Eur Heart J 2014;35:2165-73. Crossref
9. Honigberg MC, Givertz MM. Peripartum cardiomyopathy.
BMJ 2019;364:k5287. Crossref
10. Sliwa K, Petrie MC, van der Meer P, et al. Clinical
presentation, management, and 6-month outcomes in
women with peripartum cardiomyopathy: an ESC EORP
registry. Eur Heart J 2020;41:3787-97. Crossref
11. Koerber D, Khan S, Kirubarajan A, et al. Meta-analysis
of long-term (>1 year) cardiac outcomes of peripartum
cardiomyopathy. Am J Cardiol 2023;194:71-7. Crossref
12. Karaye KM, Ishaq NA, Sai’du H, et al. Disparities in clinical
features and outcomes of peripartum cardiomyopathy in
high versus low prevalent regions in Nigeria. ESC Heart
Fail 2021;8:3257-67. Crossref
13. Kolte D, Khera S, Aronow WS, et al. Temporal trends in
incidence and outcomes of peripartum cardiomyopathy in
the United States: a nationwide population-based study. J
Am Heart Assoc 2014;3:e001056. Crossref
14. Lewey J, Levine LD, Elovitz MA, Irizarry OC, Arany Z.
Importance of early diagnosis in peripartum
cardiomyopathy. Hypertension 2020;75:91-7. Crossref
15. Bauersachs J, König T, van der Meer P, et al. Pathophysiology,
diagnosis and management of peripartum cardiomyopathy:
a position statement from the Heart Failure Association
of the European Society of Cardiology Study Group on
peripartum cardiomyopathy. Eur J Heart Fail 2019;21:827-43. Crossref
16. Bello N, Rendon IS, Arany Z. The relationship between pre-eclampsia
and peripartum cardiomyopathy: a systematic
review and meta-analysis. J Am Coll Cardiol 2013;62:1715-23. Crossref
17. Parikh P, Blauwet L. Peripartum cardiomyopathy and
preeclampsia: overlapping diseases of pregnancy. Curr
Hypertens Rep 2018;20:69. Crossref
18. Henderson JT, Vesco KK, Senger CA, Thomas RG,
Redmond N. Aspirin use to prevent preeclampsia and
related morbidity and mortality: updated evidence report
and systematic review for the US Preventive Services Task
Force. JAMA 2021;326:1192-206. Crossref
19. Cherubin S, Peoples T, Gillard J, Lakhal-Littleton S,
Kurinczuk JJ, Nair M. Systematic review and meta-analysis
of prolactin and iron deficiency in peripartum
cardiomyopathy. Open Heart 2020;7:e001430. Crossref
20. Anand IS, Gupta P. Anemia and iron deficiency in
heart failure: current concepts and emerging therapies.
Circulation 2018;138:80-98. Crossref
21. Sigauke FR, Ntsinjana H, Tsabedze N. Peripartum
cardiomyopathy: a comprehensive and contemporary
review. Heart Fail Rev 2024;29:1261-78. Crossref
22. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart
Failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical
Practice Guidelines. Circulation 2022;145:e895-1032. Crossref
23. Arany Z. Peripartum cardiomyopathy. N Engl J Med
2024;390:154-64. Crossref
24. Azibani F, Sliwa K. Peripartum cardiomyopathy: an update.
Curr Heart Fail Rep 2018;15:297-306. Crossref
25. Maddox TM, Januzzi JL Jr, Allen LA, et al. 2024 ACC Expert
Consensus Decision Pathway for treatment of heart failure
with reduced ejection fraction: a report of the American
College of Cardiology Solution Set Oversight Committee. J
Am Coll Cardiol 2024;83:1444-88. Crossref
26. Hoevelmann J, Engel ME, Muller E, et al. A global
perspective on the management and outcomes of
peripartum cardiomyopathy: a systematic review and
meta-analysis. Eur J Heart Fail 2022;24:1719-36. Crossref
27. Hilfiker-Kleiner D, Kaminski K, Podewski E, et al. A
cathepsin D–cleaved 16 kDa form of prolactin mediates
postpartum cardiomyopathy. Cell 2007;128:589-600. Crossref
28. Kumar A, Ravi R, Sivakumar RK, et al. Prolactin inhibition
in peripartum cardiomyopathy: systematic review and
meta-analysis. Curr Probl Cardiol 2023;48:101461. Crossref
29. Bauersachs J, Arrigo M, Hilfiker-Kleiner D, et al. Current
management of patients with severe acute peripartum
cardiomyopathy: practical guidance from the Heart Failure
Association of the European Society of Cardiology Study
Group on peripartum cardiomyopathy. Eur J Heart Fail
2016;18:1096-105. Crossref
30. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC
Guidelines for the diagnosis and treatment of acute and
chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart
failure of the European Society of Cardiology (ESC). With
the special contribution of the Heart Failure Association
(HFA) of the ESC. Eur J Heart Fail 2022;24:4-131. Crossref
31. Bozkurt B, Colvin M, Cook J, et al. Current diagnostic and
treatment strategies for specific dilated cardiomyopathies: a
scientific statement from the American Heart Association.
Circulation 2016;134:e579-646. Crossref
32. Radakrishnan A, Dokko J, Pastena P, Kalogeropoulos AP.
Thromboembolism in peripartum cardiomyopathy: a
systematic review. J Thorac Dis 2024;16:645-60. Crossref
33. Wijayanto MA, Myrtha R, Lukas GA, et al. Outcomes
of subsequent pregnancy in women with peripartum
cardiomyopathy: a systematic review and meta-analysis.
Open Heart 2024;11:e002626. Crossref
34. Pachariyanon P, Bogabathina H, Jaisingh K, Modi M,
Modi K. Long-term outcomes of women with peripartum
cardiomyopathy having subsequent pregnancies. J Am Coll
Cardiol 2023;82:16-26. Crossref
35. Fett JD, Shah TP, McNamara DM. Why do some recovered
peripartum cardiomyopathy mothers experience heart
failure with a subsequent pregnancy? Curr Treat Options
Cardiovasc Med 2015;17:354. Crossref
36. Sliwa K, van der Meer P, Petrie MC, et al. Corrigendum
to ‘Risk stratification and management of women with
cardiomyopathy/heart failure planning pregnancy or
presenting during/after pregnancy: a position statement
from the Heart Failure Association of the European
Society of Cardiology Study Group on Peripartum
Cardiomyopathy’ [Eur J Heart Fail 2021;23:527-540]. Eur J
Heart Fail 2022;24:733. Crossref
37. Sliwa K, Petrie MC, Hilfiker-Kleiner D, et al. Long-term
prognosis, subsequent pregnancy, contraception and
overall management of peripartum cardiomyopathy:
practical guidance paper from the Heart Failure
Association of the European Society of Cardiology Study
Group on Peripartum Cardiomyopathy. Eur J Heart Fail
2018;20:951-62. Crossref
38. Ware JS, Li J, Mazaika E, et al. Shared genetic predisposition
in peripartum and dilated cardiomyopathies. N Engl J Med
2016;374:233-41. Crossref
39. Goli R, Li J, Brandimarto J, et al. Genetic and phenotypic
landscape of peripartum cardiomyopathy. Circulation
2021;143:1852-62. Crossref
40. Arany Z. It is time to offer genetic testing to women with
peripartum cardiomyopathy. Circulation 2022;146:4-5. Crossref